Introduction
Tumor-related leukocytosis, which is occasionally
encountered in patients with malignant tumors, is a paraneoplastic
syndrome involving high leukocyte counts without underlying
infection, bone marrow metastasis, or corticosteroid administration
(1,2).
Tumor-related leukocytosis is observed in 10% of patients with
solid tumors and is associated with poor clinical outcome (3). This condition is caused, in part, by
granulocyte colony-stimulating factor (G-CSF) synthesized by
neoplastic cells and has therefore been regarded as potentially
indicative of the autocrine stimulation of tumor growth by G-CSF
(4,5).
However, little is known regarding the precise mechanisms of
aggressive behavior and poor outcome in G-CSF-producing tumors.
G-CSF production by tumors has been reported for various
non-hematopoietic malignancies, predominantly lung or pancreatic
cancer (6,7). On the other hand, G-CSF-producing cancer
originated from upper gastrointestinal tract including esophagus,
esophagogastric junction (EGJ), stomach is extremely rare, and the
clinical characteristics and outcomes of this entity remain
unclear.
Here, we present a patient with a rapidly enlarged
liver metastasis from EGJ cancer accompanied by progressive
leukocytosis and high levels of serum G-CSF, although her leukocyte
count had been normal two years earlier at the first surgery for
the primary lesion. Additionally, we review 21 cases of
G-CSF-producing upper gastrointestinal tract cancer, including 20
previously published cases and the present case, to elucidate the
clinicopathological features of this entity.
Case report
A 72-year-old woman had undergone laparoscopic total
gastrectomy with regional lymph node dissection for EJG cancer
[pT3N1M0, stage IIB according to the Union for International Cancer
Control (UICC) TNM classification (8)]. Histopathological examination revealed
well to moderately differentiated adenocarcinoma with a 3+ score
for human epidermal growth factor receptor 2 (HER2) expression
(9). Atrophic gastritis positive for
H. pylori infection was identified in the pyloric gland
area. The patient received adjuvant chemotherapy with S-1 for a
year and did not experience tumor recurrence or metastasis prior to
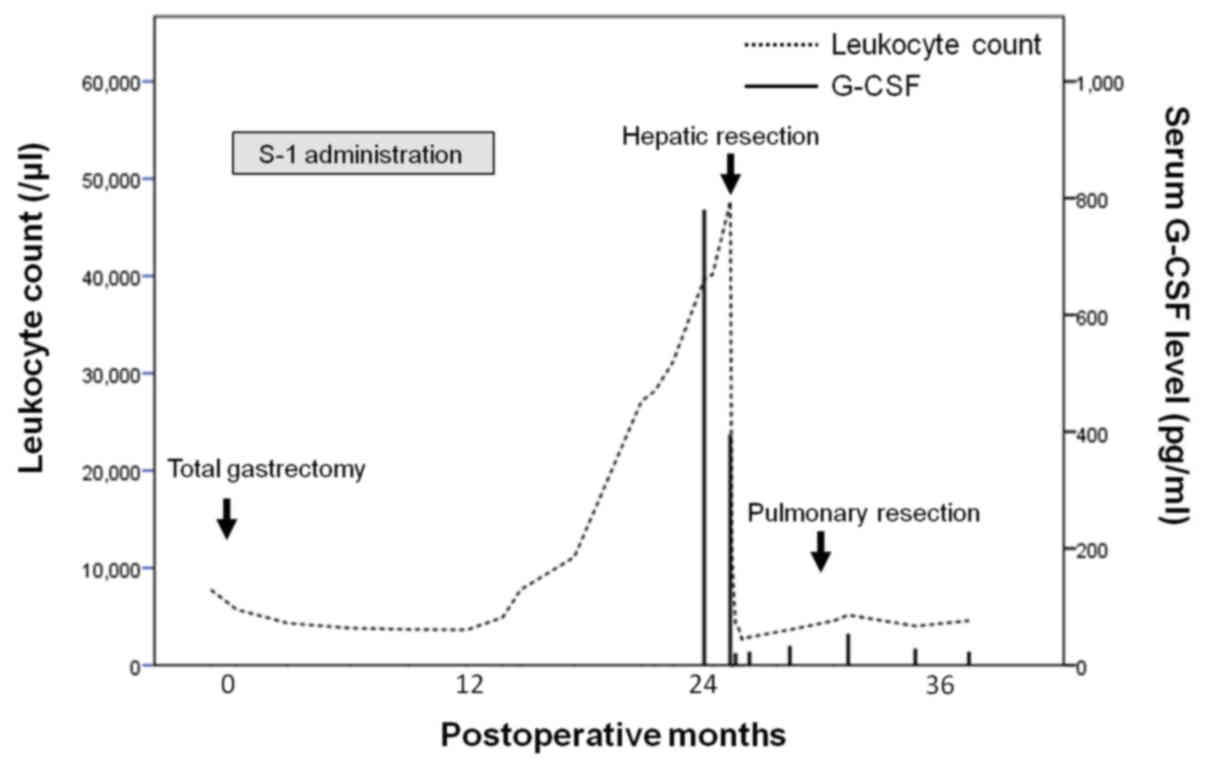
the events described here. Before gastrectomy, her leukocyte count
was 7,770/µl, with 68.8% neutrophils. During follow-up, the
leukocyte count increased to 27,150/µl with 87.0% neutrophils at
the 21st month after gastrectomy, but the serum C-reactive protein
concentration was within a normal range (0.07 mg/dl). Bone marrow
aspiration biopsy revealed a hypercellular marrow with
predominantly granulocytic mature cells. Molecular analyses
revealed neither a BCR/ABL fusion gene nor the JAK2-V617F mutation.
The patient's serum G-CSF level was significantly elevated (779
pg/ml; normal, <39 pg/ml). Contrast-enhanced computed tomography
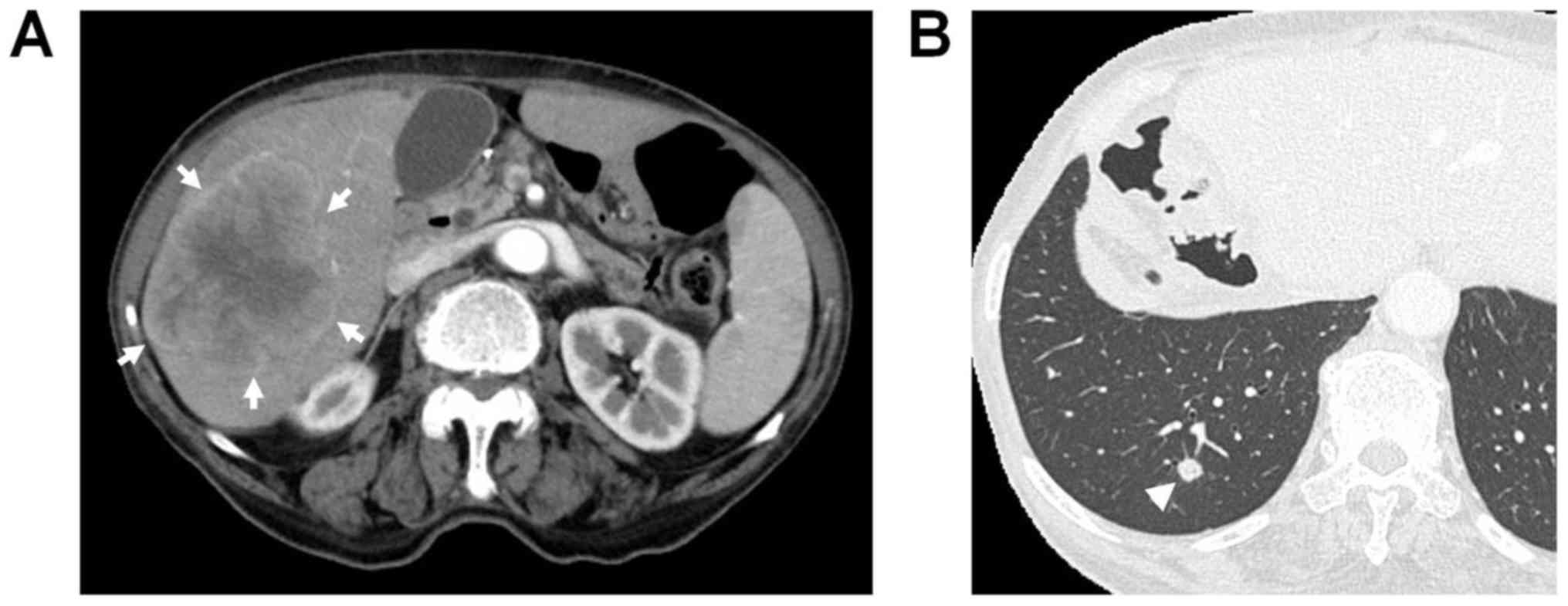
(CT) during the 24th postoperative month revealed a low-density
mass measuring approximately 80 mm in diameter in the right lobe of
the liver, with irregular and peripheral enhancement of the lesion
in the arterial phase and wash-out in the portal and delayed phases
(Fig. 1). Furthermore, a small nodule
with a size of 6 mm was identified in the right lower lobe of the
lung. The hepatic mass had not been detected on abdominal CT
performed 6 months previously, suggesting rapid tumor growth.
Although the patient did not exhibit symptoms, her leukocyte count
continued to increase, reaching 47,680/µl during the 26th
postoperative month. As other potential causes of leukocytosis were
excluded, we hypothesized that this condition was a paraneoplastic
manifestation induced by G-CSF production by the liver tumor. Based
on appropriate radiological findings for hepatocellular carcinoma,
the lesion was diagnosed as G-CSF-producing hepatocellular
carcinoma, with liver metastasis from EGJ cancer included as a
differential diagnosis, and the patient underwent right
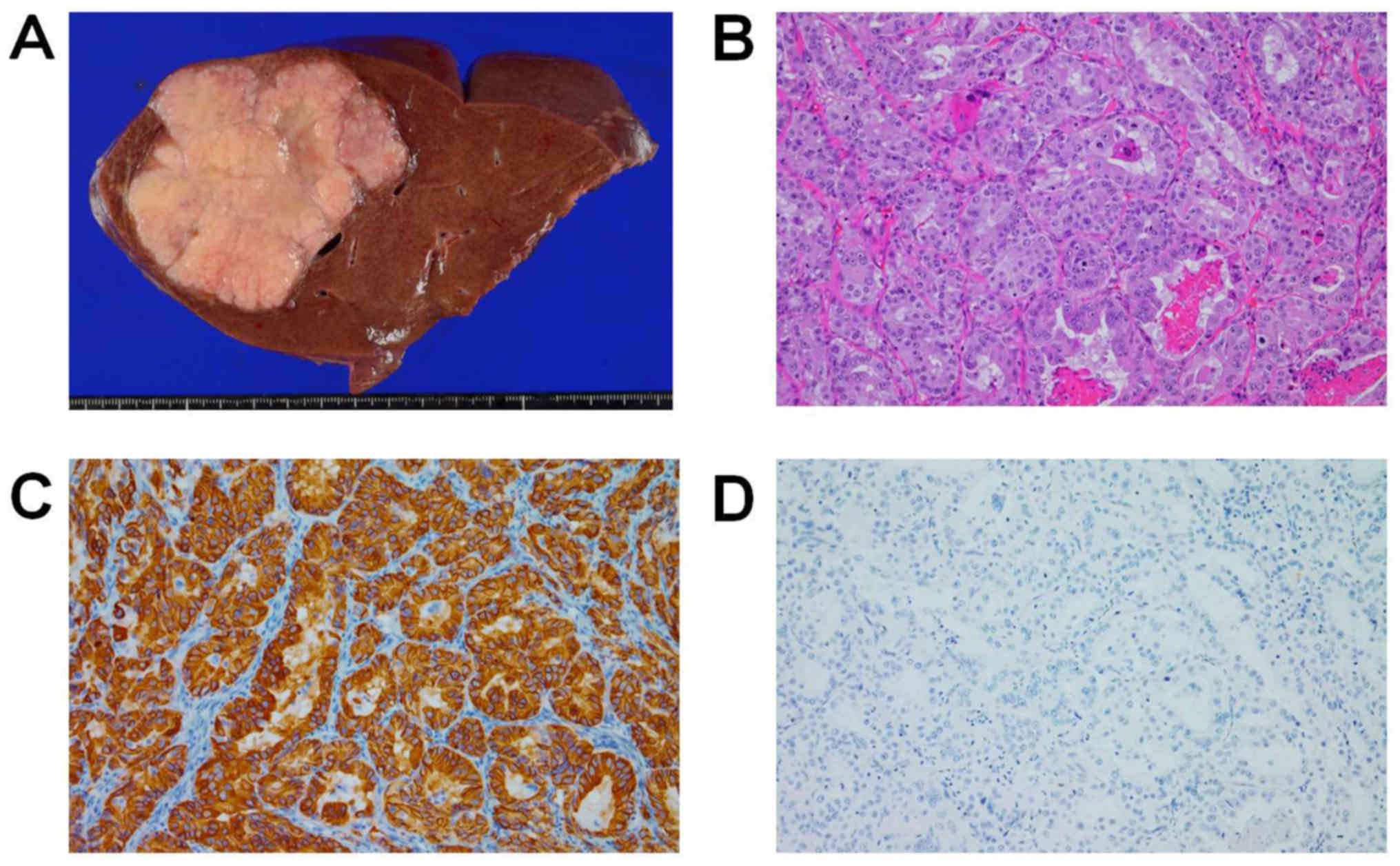
hemihepatectomy 26 months after gastrectomy. The resected specimen
included a hard, solid, whitish tumor measuring 99×79×63 mm in size
in the right lobe of the liver (Fig.
2A). Histopathological examination revealed a well to
moderately differentiated adenocarcinoma (Fig. 2B). The tumor in the liver and the
primary lesion had similar histology and identical
immunohistochemical (IHC) patterns, including reactivity to
cytokeratin (CK)7, CK19, CK20, and carcinoembryonic antigen (CEA).
The HER2 expression score (9) of the
liver tumor was 3+, as was that of the primary lesion (Fig. 2C). IHC examination using an anti-G-CSF
monoclonal antibody revealed negative G-CSF expression (Fig. 2D). However, the patient's leukocyte
count and serum G-CSF level had dramatically decreased to 4,280/µl
and ≤19.5 pg/ml, respectively, on the seventh postoperative day.
Therefore, clinically, the tumor was diagnosed as G-CSF-producing
liver metastasis from EGJ cancer, although IHC staining for G-CSF
was negative. The right pulmonary mass had slightly expanded to 8
mm on chest CT performed during the 28th postoperative month
(Fig. 1B). However, the patient's
leukocyte count (3,650/µl) and serum G-CSF level (32.0 pg/ml) were
in the normal range. Thoracoscopic partial pulmonary resection was
performed four months after hepatectomy. Histological examination
of the pulmonary mass revealed similar findings to those obtained
for the primary lesion and the liver metastasis, and the HER2
expression score (9) of the pulmonary
tumor was 3+. Thus, this mass was diagnosed as pulmonary
metastasis. We decided not to proceed with adjuvant chemotherapy
after pulmonary resection because no other obvious recurrence was
identified. The patient's leukocyte count and serum G-CSF have
continued to remain at normal levels during follow-up (Fig. 3). She has experienced no tumor
recurrence and has survived 38 and 12 months after gastrectomy and
hepatectomy, respectively.
Discussion
In general, the following criteria are used as the
diagnostic standard for detecting a G-CSF-producing tumor:
(1) an increased number of
leukocytes, primarily mature neutrophils, with no other explanatory
factors; (2) elevated serum G-CSF;
(3) a reduction in the leukocyte
count following tumor resection; and (4) confirmation of G-CSF production via
immunostaining (10). However,
clinically, positive immunostaining is not a definitive finding for
the diagnosis of G-CSF-producing tumors (11). It has been suggested that the rapid
secretion of G-CSF without intracellular retention is the reason
why such tumors may exhibit negative staining with an anti-G-CSF
monoclonal antibody (12). In fact,
Yokoyama et al (13), reported
a case involving G-CSF-producing gastric cancer that exhibited
negative IHC staining for G-CSF but highly increased G-CSF mRNA
levels detected using real-time reverse transcription polymerase
chain reaction. In the present case, postoperative observations
indicated that criteria (1) to
(3) were fulfilled, and the lesion
satisfied the criteria for a G-CSF-producing tumor.
In the present case, a normal leukocyte count was
initially detected at the first surgery for the primary EGJ cancer,
and the clinical course varied for different metastatic sites.
Interestingly, the liver metastasis, which was accompanied by
progressive leukocytosis and elevated serum G-CSF, showed rapid
tumor growth during a six-month period, whereas the subsequent
pulmonary metastasis, which was associated with a normal leukocyte
count and normal serum G-CSF, grew slowly during the same time
period. These findings indicate the existence of tumor cell
heterogeneity in different metastatic sites or a subset of cancer
cells acquiring the ability to produce G-CSF that could respond to
G-CSF with enhanced proliferation, a phenomenon suggestive of
autocrine mechanisms and of more aggressive biological activities
for such cells compared with that of cancer cells that do not
produce G-CSF.
In a PubMed search, 14 case reports (13–26) and 6
cited publications (11,27–31)
regarding G-CSF-producing upper gastrointestinal tract cancers,
including esophageal cancer (12 cases), EGJ cancer (1 case), and
gastric cancer (7 cases), were retrieved from the English-language
literature. Demographic and clinical characteristics for all of the
described cases of such cancers, including the present case, are
summarized in Table I. A male
predominance was identified in these 21 cases (90% vs. 10%).
Interestingly, male predilection was also observed in
G-CSF-producing pancreatic cancer (85% vs. 15%) (7). However, the association of sex
predominance with G-CSF production in cancer tissue is unclear.
Pyrexia was observed in 7 of these 21 cases, although the patient
described here remained asymptomatic despite her large tumor
burden. Two cases and one case of G-CSF-producing gastric cancer
were histologically diagnosed as adenosquamous carcinoma and
undifferentiated carcinoma, respectively. Among the 21 described
cases, neutrophil infiltration in the tumor was observed in only
two cases of G-CSF-producing esophageal cancer (16,25).
However, no specific morphological features were found. In
addition, this is the first reported case of G-CSF-producing upper
gastrointestinal tract cancer with HER2 expression. However, to the
best of our knowledge, no studies have demonstrated correlations or
interactions between HER2 and G-CSF. Among the 21 described cases,
14 cases involved surgical resection, whereas the remaining 7 cases
involved chemo- and/or radiation therapy or the best supportive
care due to advanced disease or deterioration of the patient's
general condition. Overall survival in these 21 cases ranged from 2
to 24 months. For the 13 cases involving resection, excluding a
case of gastric cancer that exhibited G-CSF production only after
recurrence, resection of the G-CSF-producing tumor was followed by
a decrease in leukocyte count, with normal leukocyte levels reached
in 10 of these 13 cases. However, in 8 of 13 cases, an increase in
leukocyte count and recurrence were observed during the early
postoperative period, and 6 patients died of their disease within 2
years after surgical resection. In addition, chemotherapy and/or
radiotherapy decreased the leukocyte counts and serum G-CSF levels
in accordance with the remission of tumors in five cases (15,21,28,30,31).
These findings suggest that leukocyte count or serum G-CSF level
would be useful for monitoring disease in cases involving
G-CSF-producing tumors, even if conventional biomarkers are
negative. The patient described here is the first reported case of
GCSF-producing upper gastrointestinal tract cancer that underwent
surgical resection of distant organ metastasis. The patient has
survived for 38 months and 12 months after surgical resection of
the non-G-CSF-producing primary lesion and the G-CSF-producing
liver metastasis, respectively. Little is known about surgical
indications for patients with EGJ cancer who undergo surgery for
the primary lesion and experience recurrence. Depypere et al
(32), analyzed 1754 patients
surgically treated with curative resection for esophageal cancer
and EGJ cancer and reported a 49.9% 5-year overall survival rate in
patients who underwent surgical resection of isolated local
recurrence or solitary solid organ metastasis with or without
systemic therapy. However, a multidisciplinary team is needed for
the optimal management of the recurrence of EGJ cancer, and surgery
should be limited to selected patients, especially when considering
the surgical indication for patients with G-CSF-producing EGJ
cancer given its rapid progression and unfavorable prognosis. In
the present case, hepatectomy was planned because the liver tumor
was initially diagnosed as G-CSF-producing hepatocellular carcinoma
based on radiological findings. In addition, pulmonary resection
was performed because the lung tumor grew slowly without
leukocytosis, unlike the liver tumor, and primary lung cancer was
included as a differential diagnosis.
 | Table I.Characteristics of reported cases of
G-CSF-producing upper gastrointestinal cancer. |
Table I.
Characteristics of reported cases of
G-CSF-producing upper gastrointestinal cancer.
|
|
|
|
|
| Histology |
|
|
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Cancer location | First author | Age (yrs) | Sex | Pyrexia | Type | Grade | IHC staining for
G-CSF | Leukocyte count at
diagnosis (per µl) | G-CSF level at
diagnosis (pg/ml) | Treatment | Postoperative
leukocyte count (per µl) | Postoperative G-CSF
level (pg/ml) | OS (mos) | Status | (Refs.) |
|---|
| Esophagus | Ichiishi | 66 | M | − | SCC | G2-G3 | Positive | 33,900 | NM | BSC | NA | NA | 2 | DE | (16) |
|
| Matsumoto | 66 | M | − | SCC | G2 | Positive | 41,500 | 154 | Resection | 15,300 | 52.0 | 16 | DE | (27) |
|
| Kato | 54 | M | − | SCC | G2 | Positive | 16,900 | 150 | CT | NA | NA | 3 | DE | (28) |
|
| Nakata | 56 | M | + | SCC | G2 | Positive | 24,300 | 78 | Resection | 9,400 | 6.5 | 18 | Alive | (29) |
|
| Mimatsu | 69 | M | + | SCC | G3 | Positive | 19,600 | 113 | RT | NA | NA | 7 | DE | (30) |
|
| Tanabe | 76 | M | − | SCC | G2 | Focally
positive | 24,260 | 134 | Resection | 10,450 | NM | 10 | DE | (31) |
|
| Mayanagi | 30 | M | + | SCC | G1 | Positive | 19,020 | 53.7 | Resection following
CRT | 6,130 | 21.4 | 3 | Recurrent | (20) |
|
| Shimakawa | 70 | M | − | SCC | G2 | Positive | 16,700 | NM | Resection following
CT | Normalized | NM | 12 | DE | (24) |
|
| Oshikiri | 65 | M | + | SCC | G1 | Focally
positive | 15,900 | 140 | Resection | Normalized | 19.0 | 3 | Alive | (11) |
|
| Kitani | 92 | F | − | SCC | G2 | Positive | 23,500 | 131 | Resection | 5,000 | <19.5 | 18 | Alive | (18) |
|
| Fukuda | 50 | M | − | SCC | G3 | Positive | 27,100 | 60.2 | CRT | NA | NA | 3 | DE | (15) |
|
| Yamaguchi | 60s | M | − | SCC | G3 | Positive | 25,100 | 292 | BSC | NA | NA | 3 | DE | (25) |
| EGJ | Komatsu | 73 | M | − | SCC | G2 | Positive | 45,710 | 231 | Resection | 6,870 | 12.0 | 19 | Alive | (19) |
|
| Present case | 72 | F | − | AC | G1-G2 | Negative | 47,680a | 779a | Resection | 4,280 | <19.5 | 12 (38b) | Alive |
| Stomach | Obara | 78 | M | − | AC | G3 | NM | 58,200 | NM | Resection | Normalized | NM | 8 | DE | (23) |
|
| Endo | 55 | M | + | ASC | NM | Positive | 35,000 | 105 | Resection | Normalized | 11.0 | 24 | DE | (14) |
|
| Yokoyama | 57 | M | + | UND | G4 | Negative | 34,000 | 107 | Resection | 13,000 | 35.6 | 4 | DE | (13) |
|
| Yamano | 77 | M | + | AC | G3 | Positive | 38,590 | NM | BSC | NA | NA | 3 | DE | (26) |
|
| Kawaguchi | 62 | M | − | AC | G3 | Negative | 54,300a | 28a | Resection | NA | NA | 2 | DE | (17) |
|
| Mori | 72 | M | − | AC | G2 | Positive | 34,900 | 293 | CT | NA | NA | 23 | DE | (21) |
|
| Moro | 66 | M | − | ASC | NM | Focally
positive | 12,900 | NM | Resection | Normalized | NM | 8 | Recurrent | (22) |
There have been a few reported cases of
G-CSF-producing gastric cancer similar to the present case in that
metastatic disease or local recurrence was present when
paraneoplastic leukocytosis was detected. Kawaguchi et al
(17), reported a case of gastric
cancer with an aggressive course after recurrence in the liver and
lymph nodes as a G-CSF-producing tumor, despite the fact that the
primary lesion did not exhibit G-CSF production; the patient in
question died of the disease only two months after surgery.
Moreover, Yamano et al (26),
reported a case of G-SCF-producing gastric cancer in which rapid
progression of the residual tumor was observed after endoscopic
mucosal resection. In that case, histological analysis of the tumor
resected via endoscopic mucosal resection revealed a
well-differentiated adenocarcinoma with negative immunoreactivity
for G-CSF, whereas the residual tumor, which showed rapid tumor
growth, presented as a poorly differentiated adenocarcinoma with
positive immunoreactivity for G-CSF; this finding suggested that
histological change in the tumor may have influenced G-CSF
production and induced rapid progression. However, in the present
case, histological evaluation of the liver metastasis revealed a
well to moderately differentiated adenocarcinoma similar to the
primary lesion, and there was no evidence of dedifferentiation or
anaplastic transformation. In addition, we assessed the tumor
proliferative ability of each lesion by immunostaining for Ki-67,
as previously described (33).
Notably, the Ki-67 index of the liver metastasis (58.4%) was
similar to the Ki-67 indices of the primary lesion and the
pulmonary metastasis (65.0 and 73.6%, respectively). In addition,
the presence of tumor-infiltrating lymphocytes in liver and lung
metastasis was similar, whereas abundant stromal myofibroblasts
with elevated expression of α-smooth muscle actin was observed in
only liver metastasis. No differences in CD3+ lymphocyte and CD20+
lymphocyte counts were noted between normal liver and lung tissue
surrounding the tumor. These findings suggest that morphological
and biological characteristics remained unchanged throughout the
clinical course of the described case, even though rapid tumor
growth and elevated G-CSF were only observed during the course of
the liver metastasis.
G-CSF has been reported to promote tumor progression
in different tumor models. A prior study demonstrated that G-CSF
could promote the survival and activation of myeloid-derived
suppressor cells via the signal transducer and activator of
transcription 3 (STAT3) signaling pathway, resulting in the
induction of immune suppression (34). Furthermore, G-CSF is known to regulate
epithelial to mesenchymal transition (EMT) via recruitment of the
c-jun proto-oncogene. Moreover, in previous studies, G-CSF
increased the proliferation and migration of tumor cells in a
manner dependent on ERK1/2 and RSK1 phosphorylation (4) and stimulated tumor angiogenesis
(35,36). However, the precise mechanisms of
G-CSF-dependent autocrine growth and proliferation are not well
known.
Clinically, a more aggressive course is sometimes
observed in the metastatic site than in the primary lesion. The
case described here suggests that one of the mechanisms of this
biological change might be due to tumor cell heterogeneity in
different metastatic sites or to a subset of cancer cells acquiring
the ability to produce G-CSF. The present case involved successful
treatment with radical surgery for a G-CSF-producing liver
metastasis and was distinct from previously described cases in that
the patient exhibited long-term survival. Given the unfavorable
prognosis and relatively high probability of treatment failure
associated with G-CSF-producing tumors, optimal diagnostic and
therapeutic approaches and careful monitoring for the early
detection of recurrence should be considered for patients with such
tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no
datasets were generated or analyzed during the current study.
Authors' contributions
SoH and NH designed the report. MT, TF, HS, IO, ShH
and YO treated the patient and contributed to the collection of the
clinical data. SoH analyzed the data and wrote the manuscript. NH,
ShH and IO reviewed and edited the manuscript. NH, SW, SaH and KH
reviewed the pathological findings, performed immunohistochemical
staining and prepared the pathological images.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
The patients provided written informed consent for
the publication of their clinical data and images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bahar B, Iota Acedil Ayc B, Çoşkun U,
Büyükberber S, Benekli M and Yildiz R: Granulocyte colony
stimulating factor (G-CSF) and macrophage colony stimulating factor
(M-CSF) as potential tumor markers in non small cell lung cancer
diagnosis. Asian Pac J Cancer Prev. 11:709–712. 2010.PubMed/NCBI
|
|
2
|
Kawano M, Mabuchi S, Matsumoto Y, Sasano
T, Takahashi R, Kuroda H, Kozasa K, Hashimoto K, Isobe A, Sawada K,
et al: The significance of G-CSF expression and myeloid-derived
suppressor cells in the chemoresistance of uterine cervical cancer.
Sci Rep. 5:182172015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Granger JM and Kontoyiannis DP: Etiology
and outcome of extreme leukocytosis in 758 nonhematologic cancer
patients: A retrospective, single-institution study. Cancer.
115:3919–3923. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Morris KT, Khan H, Ahmad A, Weston LL,
Nofchissey RA, Pinchuk IV and Beswick EJ: G-CSF and G-CSFR are
highly expressed in human gastric and colon cancers and promote
carcinoma cell proliferation and migration. Br J Cancer.
110:1211–1220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimamura K, Fujimoto J, Hata J, Akatsuka
A, Ueyama Y, Watanabe T and Tamaoki N: Establishment of specific
monoclonal antibodies against recombinant human granulocyte
colony-stimulating factor (hG-CSF) and their application for
immunoperoxidase staining of paraffin-embedded sections. J
Histochem Cytochem. 38:283–286. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aliper AM, Frieden-Korovkina VP, Buzdin A,
Roumiantsev SA and Zhavoronkov A: A role for G-CSF and GM-CSF in
nonmyeloid cancers. Cancer Med. 3:737–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vinzens S, Zindel J, Zweifel M, Rau T,
Gloor B and Wochner A: Granulocyte colony-stimulating factor
producing anaplastic carcinoma of the pancreas: Case report and
review of the literature. Anticancer Res. 37:223–228. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin LH, Gospodarowicz MK and Wittekind
Ch: TNM classification of malignant tumours. Wiley-Blackwell;
Hoboken, NJ: 2010
|
|
9
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hanaoka T, Jingu K, Tochigi T, Hoshino I,
Uematu T and Matsubara H: A case of G-CSF-producing histiocytic
sarcoma of the stomach. Int Surg. 100:568–573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oshikiri T, Yasuda T, Harada H, Ohyama M,
Hasegawa H, Ohara T, Sendo H, Sugimoto T, Fujino Y, Tominaga M and
Takahash Y: G-CSF-producing esophageal cancer with induction of
intense bone marrow FDG uptake. Esophagus. 12:pp258–262. 2015.
View Article : Google Scholar
|
|
12
|
Katoh Y, Nakamura M, Ohnishi Y, Shimamura
K, Ueyama Y and Tamaoki N: Autonomous production of
granulocyte-colony stimulating factor in tumour xenografts
associated with leukocytosis. Br J Cancer. 68:715–719. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yokoyama T, Hyodo M, Hosoya Y, Koinuma K,
Kurashina K, Saitoh S, Hirashima Y, Arai W, Zuiki T, Yasuda Y, et
al: Aggressive G-CSF-producing gastric cancer complicated by lung
and brain abscesses, mimicking metastases. Gastric Cancer.
8:198–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Endo K, Kohnoe S, Okamura T, Haraguchi M,
Adachi E, Toh Y, Baba H and Maehara Y: Gastric adenosquamous
carcinoma producing granulocyte-colony stimulating factor. Gastric
Cancer. 8:173–177. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukuda S, Fujiwara Y, Mishima H, Wakasa T,
Hanamoto H, Inoue K, Kitani K, Ishikawa H, Tsujie M, Yukawa M, et
al: Choroidal metastasis from granulocyte colony-stimulating
factor-producing esophageal squamous cell carcinoma: A case report.
Clin Case Rep. 5:419–424. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ichiishi E, Yoshikawa T, Kogawa T, Yoshida
N and Kondo M: Possible paracrine growth of adenocarcinoma of the
stomach induced by granulocyte colony stimulating factor produced
by squamous cell carcinoma of the oesophagus. Gut. 46:432–434.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kawaguchi M, Asada Y, Terada T, Takehara
A, Munemoto Y, Fujisawa K, Mitsui T, Iida Y, Miura S and Sudo Y:
Aggressive recurrence of gastric cancer as a
granulocyte-colony-stimulating factor-producing tumor. Int J Clin
Oncol. 15:191–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitani M, Yamagata Y, Tanabe A, Yagi K,
Aikou S, Kiyokawa T, Nishida M, Yamashita H, Mori K, Nomura S and
Seto Y: Radical esophagectomy for a 92-year-old woman with
granulocyte colony-stimulating factor-producing esophageal squamous
cell carcinoma: A case report. World J Surg Oncol. 14:2642016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Komatsu D, Sakurai M, Nakafuji H, Koide N,
Morishita H and Nakamura T: Granulocyte colony stimulating
factor-producing collision tumor of the gastric cardia. J
Gastroenterol. 38:1013–1015. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayanagi S, Niihara M, Goto H, Yokota T,
Tabuse H, Yasui H, Ogawa H, Nishimura T, Kusafuka K and Tsubosa Y:
Granulocyte colony-stimulating factor-producing esophageal squamous
cell carcinoma following chemoradiotherapy and bone marrow
transplantation for acute lymphoblastic leukemia. Esophagus.
10:258–263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mori H, Shibuya T, Osada T, Kodani T,
Higashihara Y, Serizawa N, Kato J, Nagahara A, Ogihara T and
Watanabe S: Response to chemotherapy in a case of gastric
adenocarcinoma producing granulocyte colony-stimulating factor. Med
Sci Monit. 16:CS119–CS123. 2010.PubMed/NCBI
|
|
22
|
Moro K, Nagahashi M, Naito T, Nagai Y,
Katada T, Minagawa M, Hasegawa J, Tani T, Shimakage N, Usuda H, et
al: Gastric adenosquamous carcinoma producing granulocyte-colony
stimulating factor: A case of a rare malignancy. Surg Case Rep.
3:672017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Obara T, Ito Y, Kodama T, Fujimoto Y,
Mizoguchi H, Oshimi K, Takahashi M and Hirayama A: A case of
gastric carcinoma associated with excessive granulocytosis.
Production of a colony-stimulating factor by the tumor. Cancer.
56:782–788. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shimakawa T, Asaka S, Usuda A, Yamaguchi
K, Yoshimatsu K, Shiozawa S, Katsube T and Naritaka Y:
Granulocyte-colony stimulating factor (G-CSF)-producing esophageal
squamous cell carcinoma: A case report. Int Surg. 99:280–285. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamaguchi S, Kanetaka K, Kobayashi S,
Nagata Y, Kinosita N, Fukuoka J, Murakami S, Fujita F, Takatsuki M
and Eguchi S: Severe neutrophilic leukocytosis as a progression
marker in granulocyte colony-stimulating factor-producing squamous
cell carcinoma of the esophagus. Clin Case Rep. 5:688–693. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamano T, Morii E, Ikeda J and Aozasa K:
Granulocyte colony-stimulating factor production and rapid
progression of gastric cancer after histological change in the
tumor. Jpn J Clin Oncol. 37:793–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsumoto G, Ise H, Kimura Y, Inoue H,
Suzuki N, Ohtani H, Ogawa H, Fukushima K and Matsuno S:
Granulocyte-colony stimulating factor-producing esophageal
carcinoma: Serum level as a marker for monitoring the effects of
treatment. Int J Clin Oncol. 5:pp328–333. 2000. View Article : Google Scholar
|
|
28
|
Kato M, Osawa H, Usui N and Hano H: An
autopsy case of esophageal squamous cell carcinoma associated with
granulocyte colony-stimulating factor production (case report).
Jikeikai Med J. 49:191–195. 2002.
|
|
29
|
Nakata K, Ohtsuka T, Sato S, Tanaka M,
Shimonishi T, Mori D, Nakafusa Y and Miyazaki K: Esophageal
carcinoma with humoral hypercalcemia and leukocytosis successfully
treated by a two-stage operation: Report of a case. Esophagus.
3:pp13–17. 2006. View Article : Google Scholar
|
|
30
|
Mimatsu K, Oida T, Kano H, Kawasaki A and
Amano S: Aggressive progression of granulocyte colony-stimulating
factor producing squamous cell carcinoma of the esophagus: Case
report and literature review. Esophagus. 5:pp205–209. 2008.
View Article : Google Scholar
|
|
31
|
Tanabe T, Kanda T, Ishihara N, Kosugi S-I,
Matsuki A, Watanabe G, Sasamoto R and Hatakeyama K: An esophageal
squamous cell carcinoma patient with high serum granulocyte-colony
stimulating factor level: Report of a case. Esophagus.
6:2532009.https://doi.org/10.1007/s10388-009-0206-z
View Article : Google Scholar
|
|
32
|
Depypere L, Lerut T, Moons J, Coosemans W,
Decker G, Van Veer H, De Leyn P and Nafteux P: Isolated local
recurrence or solitary solid organ metastasis after esophagectomy
for cancer is not the end of the road. Dis Esophagus. 30:1–8.
2017.PubMed/NCBI
|
|
33
|
Hoshimoto S, Hoshi S, Hishinuma S,
Tomikawa M, Shirakawa H, Ozawa I, Wakamatsu S, Hoshi N, Hirabayashi
K and Ogata Y: Adenosquamous carcinoma in the biliary tract:
Association of the proliferative ability of the squamous component
with its proportion and tumor progression. Scand J Gastroenterol.
52:425–430. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng
Q, Wang Y, Yuan W and Ma J: G-CSF is a key modulator of MDSC and
could be a potential therapeutic target in colitis-associated
colorectal cancers. Protein Cell. 7:130–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Natori T, Sata M, Washida M, Hirata Y,
Nagai R and Makuuchi M: G-CSF stimulates angiogenesis and promotes
tumor growth: Potential contribution of bone marrow-derived
endothelial progenitor cells. Biochem Biophys Res Commun.
297:1058–1061. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Voloshin T, Gingis-Velitski S, Bril R,
Benayoun L, Munster M, Milsom C, Man S, Kerbel RS and Shaked Y:
G-CSF supplementation with chemotherapy can promote
revascularization and subsequent tumor regrowth: Prevention by a
CXCR4 antagonist. Blood. 118:3426–3435. 2011. View Article : Google Scholar : PubMed/NCBI
|