Introduction
In the last decade, the incidence and mortality of
lung cancer have markedly increased globally (1). Of the diagnosed histological types of
lung cancer, >80% are non-small cell lung cancer (NSCLC), which
is frequently diagnosed at an advanced stage (2). The efficacy of traditional treatment,
including surgery, radiotherapy and chemotherapy, is limited, and
effective treatments that inhibit tumor recurrence and lower the
mortality rate among patients with late-stage lung cancer and
distant metastases are still inadequate (3). With recent developments in the molecular
etiology and gene therapy of lung cancer, molecular targeted
therapies are considered the most promising approach to overcome
difficulties with the treatment of lung cancer (4).
The suicide gene therapy caused by herpes simplex
virus-thymidine kinase (HSV-TK) has received significant attention
due to its direct killing effect and bystander effect (BSE)
(5); however, the efficacy of
HSV-TK/GCV treatment in treating cancer is limited, due to
insufficient gene transfection and the insufficient induction of
host immunity (6–8). Previous studies have demonstrated that
the combination therapy of HSV-TK and immune genes has an
improved response when compared with therapy using the simple
HSV-TK gene; additionally, combination therapy has been
demonstrated to improve survival time in animals and to promote
tumor regression (9,10).
Interleukin (IL)-12 is produced by monocytes,
macrophages and B cells. It has multiple physiological and
pathological functions and can be used for treating infection,
autoimmune disease and tumors (11).
A previous study reported that the systemic administration of
recombinant IL-2 in combination with HSV-tk gene therapy exhibited
an enhanced antitumor effect in a murine bladder cancer model
(MBT-2) (12). Also, combination gene
therapy with HSV-tk+GCV and IL-12 increased the expression of
interferon-γ-dependent Fas and FasL, contributing to
tumor cell apoptosis in murine prostate cancer models (RM-1 and
RM-9) (13,14); however, the majority of studies
regarding the combined gene therapies of HSV-TK and
IL-12 have only applied to the mouse (m)IL-12
gene (13,14). Therefore, there are a limited number
of studies on the co-operative antitumor effect of the human
(h)IL-12 gene (15). The
present study further explored the co-operative antitumor effect of
the HSV-TK and hIL-12 genes.
The cytomegalovirus (CMV) promoter is frequently
used in tumor gene therapy due to its efficient transcription
activity in mammalian cells; however, its lack of tumor specificity
is a limitation (16,17). In vivo host cells transfected
with the CMV promoter can be killed using the metabolites of
precursor drugs, which have side effects in normal tissues
(16,17); therefore, further studies are required
in order to understand how to improve the specificity of the
TK gene, thus reducing the side effects.
The human secretory leukocyte protease inhibitor
(hSLPI), an 11.7 kDa non-glycosylated, serine protease
inhibitor with 107 amino acids (18),
is highly active in human cancer cells, including hNSCLC (19), pancreatic (20) and ovarian cancer (21), but not in normal differentiated human
cells, including the liver, the endocrine glands and the blood
system (22); therefore, hSLPI
was selected as a tumor-specific promoter.
In the present study, the hSLPI promoter was
cloned, which regulated the HSV-TK/hIL-12
gene-targeted expression in hNSCLC. To the best of our knowledge,
the present study was the first to establish a eukaryotic
expression vector containing HSV-TK/hIL-12 and hSLPI,
and to transfect this into target cells by liposome-mediated gene
transfection. The aim of the present study was to investigate the
killing effect of the HSV-TK/hIL-12 gene regulated by
hSLPI, in order to explore a novel strategy for the
molecular targeted therapy of hNSCLC.
Materials and methods
Cell lines and plasmids
Human lung adenocarcinoma cell lines (A549 and
SPC-A1) and a hepatoblastoma cell line (HepG2) (23) were obtained from the Jilin Tumor
Research Institute (Changchun, China) and the Academy of Military
Medical Sciences (Changchun, China), respectively. Plasmid
pcDNA3.1(+) was procured from the Central Laboratory of China-Japan
Union Hospital of Jilin University (Changchun, China).
Cell culture
A549, SPC-A1 and HepG2 cell lines were cultured in
10-cm plastic culture dishes using Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), supplemented with 10% Gibco fetal bovine serum (FBS), 100
U/ml penicillin and 100 µg/ml streptomycin. The culture conditions
were: 37°C, 5% CO2 and saturated humidity. The medium
was changed every two days, and the cells were passaged using
trypsin/ethylenediaminetetraacetic acid medium (Gen-View
Scientific, Inc., El Monte, CA, USA) at 37°C for 5 min when the
confluence was ~100%.
Construction of vectors
The hSLPI promoter gene sequence was amplified by
polymerase chain reaction (PCR) using the forward primer,
5′-TTCGACGCGTCTCACTGCAGCCTCAAAC-3′; and the reverse primer,
5′-TTCTAGCTAGCGGTGAAGGCAGGAGTGAC-3′; and the two restriction
enzymes MluI and NheI, which were added to each of
the primers. After replacing the CMV sequence of pcDNA3.1(+) with
hSLPI using the MluI and NheI enzymes, the
pcDNA3.1-hSLPI vector was obtained. HSV-TK-IRES and IL-12 sequences
were also cloned from the pGT60-hIL-12 vector, provided by
Professor Sun Ying from the King's College London (London, UK). The
sequences of the primers were as follows: HSV-TK-IRES forward,
5′-AAACCGGAATTCGCCATCATGGCCTCGTAC-3′; and reverse,
5′-TATGCGGCCGCGGTATTATCGTGTTTTTC-3′; and two restriction sites
EcoRI and NotI were added to each primer.
hIL-12 forward, 5′-AAATATGCGGCCGCTAAGCCACCATGGGTCAC-3′; and
reverse, 5′-CCGCTCGAGCGTTAGGAAGCATTCAGATAG-3′; and two restriction
sites NotI and Xhol were added to each primer.
Vectors containing HSV-TK-IRES and hIL-12 sequences, under
the transcription control of the hSLPI promoter, were
constructed by connecting the HSV-TK-IRES and hIL-12
sequences to the pcDNA3.1-PSLPI vector, particularly the fusion
gene eukaryotic expression vector pcDNA3.1-phSLPI-TK/hIL-12.
Vectors containing the fusion gene HSV-TK/hIL-12 regulated
by the CMV promoter were also constructed by connecting the
HSV-TK-IRES and hIL-12 sequences to the pcDNA3.1(+) vector,
particularly pcDNA3.1-CMV-TK/hIL-12. Similarly, by connecting the
HSV-TK-IRES to the pcDNA3.1-PSLPI or the pcDNA3.1(+) vectors, a
single gene eukaryotic expression vector was produced, either
pcDNA3.1-phSLP-TK or pcDNA3.1-CMV-TK.
Liposome-mediated gene
transfection
Plasmid pcDNA3.1(+), pcDNA3.1-CMV-TK/hIL-12,
pcDNA3.1-CMV-TK, pcDNA3.1-phSLP-TK/hIL-12 and pcDNA3.1-phSLP-TK
were extracted using a Endo-Free Plasmid Mini kit (Promega
Corporation, Madison, WI, USA). The content and purity of the
extracted plasmids were determined using a NanoDrop 2000 (Thermo
Fisher Scientific, Inc.). Logarithmic growth phase cells of the
A549, SPC-A1 and HepG2 cell lines were seeded into 24-well plates
at a density of 5×104 cells/well and cultured for 18–24
h, at 37°C in an atmosphere containing 5% CO2, until the
cells reached ~80% confluency.
Following this, cells were transfected using
Lipofectamine® 2000, according to the manufacturer's
instructions (Invitrogen; Thermo Fisher Scientific, Inc.). Solution
A was prepared by diluting 0.4 µg plasmids with 25 µl DMEM without
FBS. Solution B was prepared by diluting 1 µl liposomes with 25 µl
DMEM without FBS. A mixture of solutions A and B was incubated for
30–45 min at room temperature, and then DMEM without FBS was added
to attain a volume of 400 µl. The cells were incubated with the
prepared mixture for 5 h; after adding 400 µl DMEM with 20% FBS,
the mixture was incubated again. The transfected cells were
selected using G418 (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
at different concentrations (800 mg/l for A549 and SPC-A1; 400 mg/l
for HepG2).
The cells were sorted into groups as follows:
Control group, cells without transfection; pcDNA3.1(+) group, cells
with pcDNA3.1(+); Pcmv-TK-hIL-12 group, cells with
pcDNA3.1-CMV-TK/hIL-12; Pcmv-TK group, cells with pcDNA3.1-CMV-TK;
PSLPI-TK-hIL-12 group, cells with pcDNA3.1-phSLP-TK/hIL-12; and
PSLPI-TK group, cells with pcDNA3.1-phSLP-TK.
HSV-TK/hIL-12 fusion gene expression
analysis using reverse transcription (RT)-PCR
Total RNA was extracted from three cell lines in the
control, Pcmv-TK-hIL-12 and PSLPI-TK-hIL-12 groups using an RNA
Purification kit (Promega Corporation). Total RNA was subjected to
cDNA synthesis using a Reverse Transcription System (Promega
Corporation). The primers of human β-actin were as follows:
Forward, 5′-GAGCTACGAGCTGCCTGACG-3′; and reverse,
5′-CCTAGAAGCATTTGCGGTGG-3′. The primers of HSV-TK and
IL-12 are as aforementioned. The RT-PCR performed comprised
35 thermal cycles at 95°C for 5 min, 94°C for 30 sec, 55°C for 45
sec, 72°C for 100 sec and 72°C for 10 min. The PCR product of 5
µl/lane was visualized with ethidium bromide dye on 1% agarose gel
and the imaging was observed by JS 680D gel imaging system
(Shanghai Peiqing Science and Technology Co., Inc., Shanghai,
China). The negative control used was water. The gray value of DNA
per lane was analyzed by ImageJ 1.43b (National Institutes of
Health, Bethesda, MD, USA).
hIL-12 gene expression levels analysis
using an ELISA
The cells in the logarithmic growth phase from the
control, pcDNA3.1(+), Pcmv-TK-hIL-12 and PSLPI-TK-hIL-12 groups
were seeded in 100-ml culture flasks, with each containing
6×106 cells. The supernatant (0.5 ml) was collected
after 24, 48 and 72 h. The IL-12 concentration of the supernatant
was determined using the Human IL-12 ELISA kit (cat. no. DRE10282;
Solarbio, Beijing, China). Each group underwent three
repetitions.
Lymphocyte proliferation assay
The supernatants of the control, pcDNA3.1(+),
Pcmv-TK-hIL-12 and PSLPI-TK-hIL-12 group A549 cells, after culture
for 72 h, were collected and added to the lymphocytes isolated from
healthy human peripheral blood samples obtained at the Second
Hospital of Jilin University in May 2014. The present study was
conducted with the approval of the Institutional Ethics Committee
of The Second Hospital of Jilin University (approval no. 139).
Based on the type of supernatant, the lymphocytes were grouped as
follows: Control group, pcDNA3.1(+) group, Pcmv-TK-hIL-12 group and
PSLPI-TK-hIL-12 group. Each group comprised two subgroups: The
experimental well and the control well. Human lymphocytes were
plated in a 96-well plate at a density of 1×105 cells/well. The
corresponding supernatant (100 µl) and phytohaemagglutinin (PHA, 2
µl, 5 mg/ml; Sigma-Aldrich; Merck KGaA) were added to the cells
within the experimental wells to generate a final concentration of
50 µg/ml, and 200 µl of the corresponding supernatant without PHA
was added to the cells within the control wells. Each well had a
total volume of 200 µl. Following this, lymphocytes were cultured
in DMEM containing 10% FBS with 100 U/ml penicillin and 100 µg/ml
streptomycin, at 37°C in 5% CO2 humidified atmosphere
and an MTT assay was performed after 24, 48 and 72 h. Dimethyl
sulfoxide (Sigma-Aldrich; Merck KGaA) was used to dissolve the
purple formazan and the wavelength used to measure the formazan was
490 nm. With OD indicating the optical density, the lymphocyte
proliferation rate was calculated as follows: Cell proliferation
rate (%) = (OD value of experimental well - OD value of control
well)/(OD value of control well) × 100%. Each group underwent three
repetitions.
Analysis of the direct antitumor
effect of the fusion gene using an MTT assay
Three types of cells were divided into six groups,
as follows: Control group, pcDNA3.1(+) group, Pcmv-TK-hIL-12 group,
Pcmv-TK group, PSLPI-TK-hIL-12 group and PSLPI-TK group. The cells
in the logarithmic phase were collected and plated at a density of
5×103 cells/well in a 96-well plate. GCV at a
concentration of 10 µg/ml, and lymphocytes at an effector-target
ratio of 20:1 were added into the experimental wells, and the DMEM
medium (200 µl/well) without GCV or any lymphocytes, was added into
the control wells. Each well had a total volume of 200 µl, and each
group underwent three repetitions of the assay. The MTT assay was
performed after culturing for 72 h at 37°C with an atmosphere
containing 5% CO2. The cell survival rate was calculated
as follows: Cell survival rate (%) = OD value of experimental
well/OD value of control well × 100%.
BSE of fusion gene analysis using an
MTT assay
TK+ cells obtained from the Pcmv-TK,
PLPI-TK, Pcmv-TK-hIL-12 and PSLPI-TK-hIL-12 groups were cultured
together with TK-cells where TK+ cells were in the
proportion of 0, 10, 25, 50, 75 and 100% of the total cells. Mixed
cells were plated in 96-well plates at a density of
5×103 cells/well, with GCV at a final concentration of
10 µg/ml and lymphocytes at a 20:1 effector-target ratio for the
experimental wells, and DMEM medium (200 µl/well) without GCV or
lymphocytes for the control wells. Each well had a total volume of
200 µl, and each group had three repetitions. The MTT assay was
performed 72 h later. The cell survival rate was calculated as
follows: Cell survival rate (%) = OD value of experimental well/OD
value of control well × 100%.
Statistical analysis
The SPSS version 13.0 software (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis. The quantitative
data are expressed as the mean ± standard deviation. Student's
t-test was used to analyze differences in gene expression and cell
survival rate between two groups. A one-way ANOVA followed with
least significant difference for post hoc were performed to analyze
intergroup differences for hIL-12 gene expression, lymphocyte
proliferation rate and cell survival rate. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
The HSV-TK/hIL-12 fusion gene
eukaryotic expression vectors regulated by hSLPI are constructed
successfully
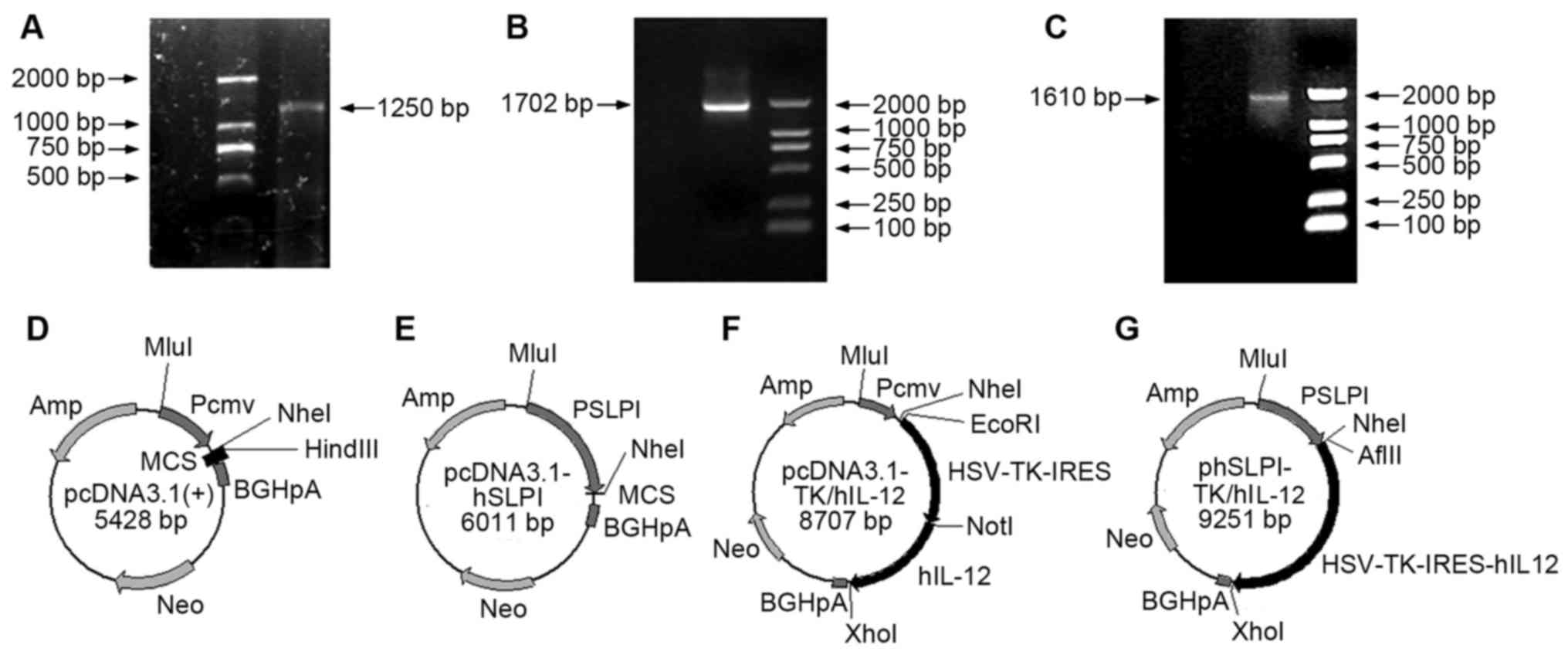
The hSLPI promoter gene sequence (1,250 bp)
was amplified from the genome DNA of human mononuclear cells in the
peripheral blood using PCR (Fig. 1A).
HSV-TK-IRES (Fig. 1B) and IL-12
(Fig. 1C) sequences were cloned from
the pGT60-hIL-12 vector. Replacing the CMV sequence of pcDNA3.1(+)
with hSLPI, using MluI and NheI enzymes,
enabled the pcDNA3.1-PSLPI vector to be obtained (Fig. 1D and E). After connecting the
HSV-TK-IRES and hIL-12 sequences with the pcDNA3.1(+)
vector, pcDNA3.1-CMV-TK/hIL-12 was obtained (Fig. 1F). Following connecting the
HSV-TK-IRES and hIL-12 sequences with the pcDNA3.1-PSLPI
vector, pcDNA3.1-phSLPI-TK/hIL-12 was obtained (Fig. 1G).
HSV-TK/hIL-12 fusion gene regulated by
hSLPI promoter is successfully expressed in hNSCLC
HSV-TK/hIL-12 fusion gene expression
is demonstrated using RT-PCR
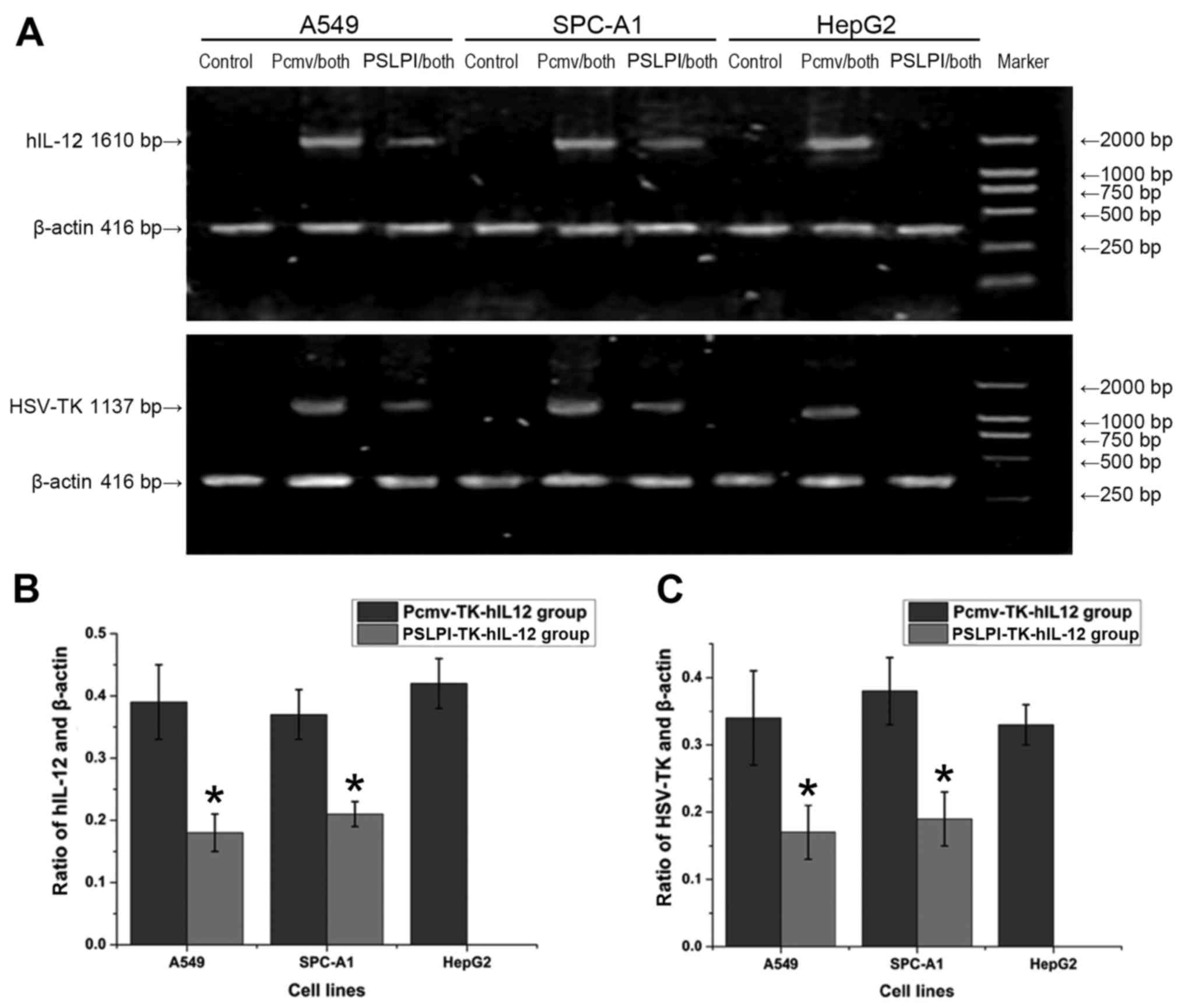
The expression of HSV-TK/hIL-12 mRNA among
various groups and three different cell lines was analyzed by
RT-PCR. Three electrophoresis bands were observed: β-actin
(416 bp), hIL-12 (1,610 bp) and HSV-TK (1,137 bp).
The control groups for all three cell lines, and the
PSLPI-TK-hIL-12 group for HepG2 cells did not express the
hIL-12 or HSV-TK gene. On the contrary, the
PSLPI-TK-hIL-12 group for the A549 and SPC-A1 cell lines, and the
Pcmv-TK-hIL-12 group for all three cell lines expressed the
hIL-12 and HSV-TK genes (Fig. 2A). Evaluation of the OD of the
electrophoresis bands indicated no significant differences in the
hIL-12 to β-actin ratio in the Pcmv-TK-hIL-12 group
among the A549, SPC-A1 and HepG2 cells (0.39±0.06 vs. 0.37±0.04 vs.
0.42±0.04; P>0.05). No significant differences were observed in
the PSLPI-TK-hIL-12 group between the A549 and SPC-A1 cells
(0.18±0.03 vs. 0.21±0.02; P>0.05) either; however, the ratio in
the PSLPI-TK-hIL-12 group was lower than that calculated for the
Pcmv-TK-hIL-12 group Between A549 and SPC-A1 cells (P<0.01;
Fig. 2B). For the HSV-TK and
β-actin ratio, no significant differences were determined
between A549, SPC-A1 and HepG2 cells within the Pcmv-TK-hIL-12
group (0.34±0.07 vs. 0.38±0.05 vs. 0.33±0.03; P>0.05). No
significant differences in the HSV-TK and β-actin
ratio were demonstrated in the PSLPI-TK-hIL-12 group between A549
and SPC-A1 cells (0.17±0.04 vs. 0.19±0.04; P>0.05); however, the
ratio was lower in the PSLPI-TK-hIL-12 group than in the
Pcmv-TK-hIL-12 group between A549 and SPC-A1 cells (P<0.01;
Fig. 2C). The results indicated that
the genes regulated by the CMV promoter were expressed in three
types of tumor cell lines at the same expression level, without
cell specificity. The genes regulated by the hSLPI promoter
were expressed in two types of lung cancer cell lines in a targeted
manner, the exception being the HepG2 cells.
hIL-12 gene expression in protein
level is demonstrated using an ELISA
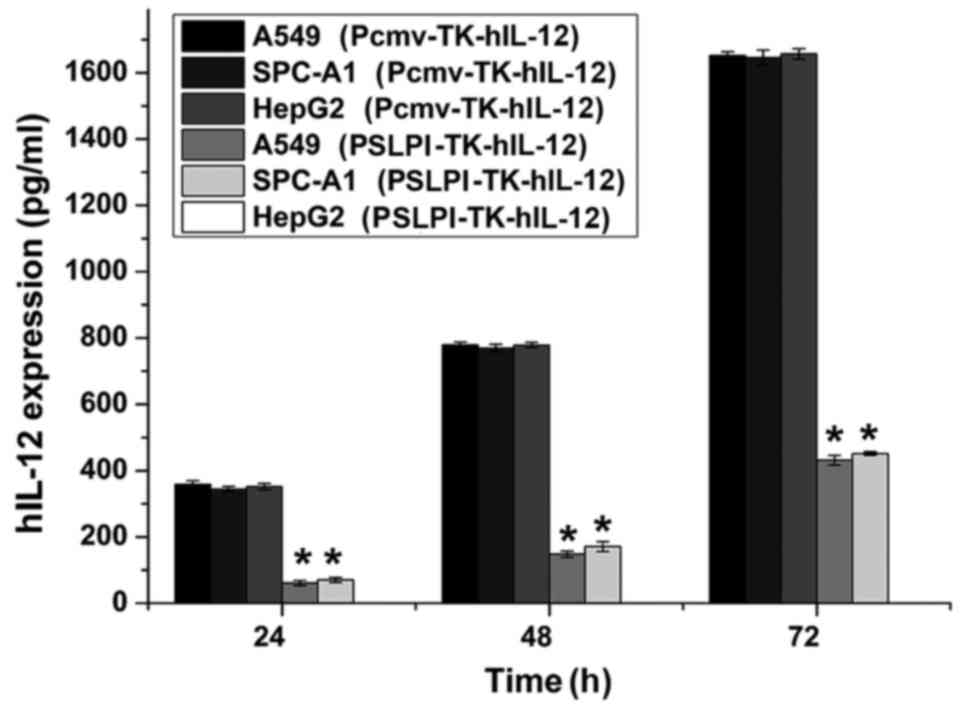
An ELISA was performed to detect hIL-12 gene
expression. The results indicated that all three cell lines
infected with Pcmv-TK-hIL-12 expressed hIL-12, and that no
significant differences were determined among the A549 [(16.52±0.11
ng)/106/72 h], SPC-A1 [(16.45±0.22 ng)/106/72
h] and HepG2 cells [(16.57±0.14 ng)/106/72 h]
(P>0.05). No hIL-12 expression was observed in the
PSLPI-TK-hIL-12 group of the HepG2 cells. The expression level of
hIL-12 within A549 [(4.32±0.15 ng)/106/72 h] and
SPC-A1 cells [(4.52±0.28 ng)/106/72 h] infected with
PSLPI-TK-hIL-12 was significantly lower than that in the
Pcmv-TK-hIL-12 group (P<0.01). The expression levels of each
group increased as the culture time increased (P<0.01; Fig. 3). These results indicated that the
gene regulated by the CMV promoter expressed hIL-12 in all
three cell lines at the same level, and without cell specificity.
The gene regulated by the hSLPI promoter expressed
hIL-12 in the two types of lung cancer cell lines, and not
in HepG2 cells, with specificity for lung cancer tissue
(P<0.01).
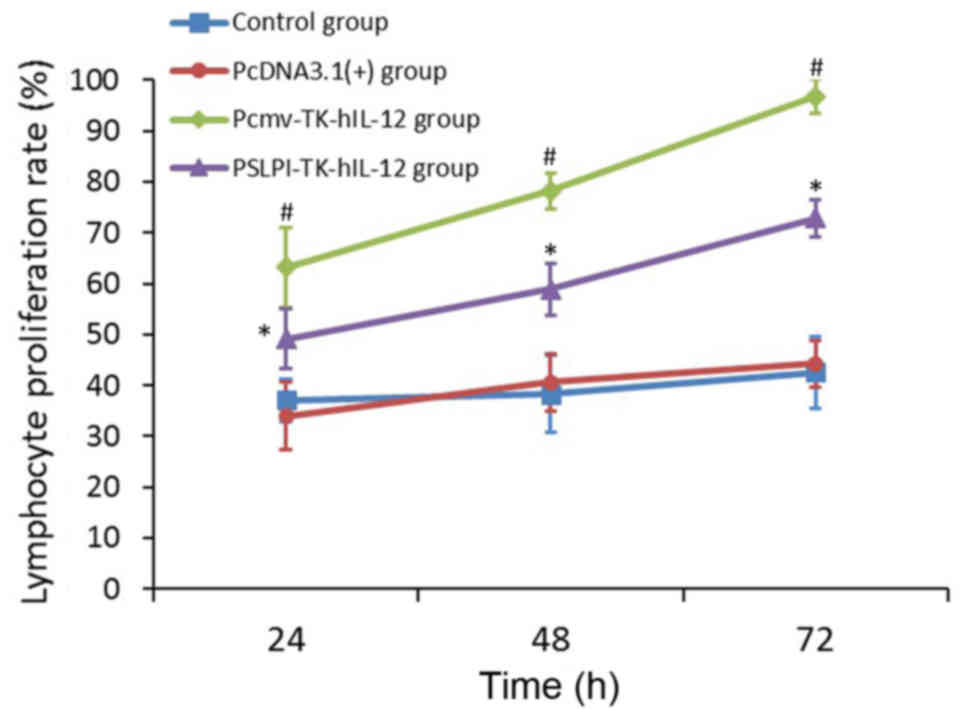
hIL-12 promotes lymphocyte
proliferation
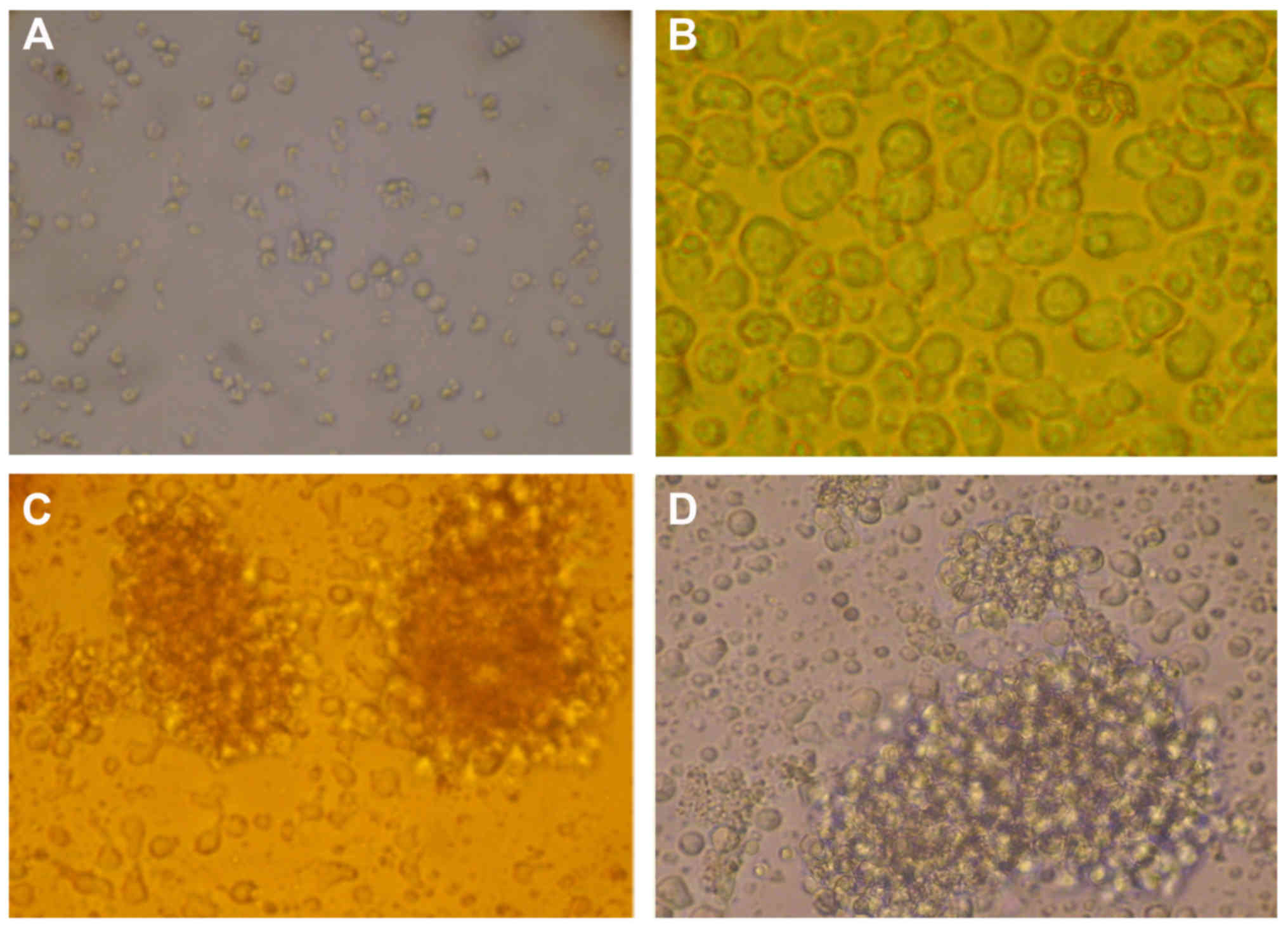
It was observed via optical microscopy that
lymphocytes cultured for 3 days in the control wells of each of the
four groups had a round appearance. Conversely, following
stimulation with PHA, the lymphocytes increased in volume, their
sizes and shapes became irregular and they frequently transformed
into lymphoblasts (Fig. 4A-D). The
results demonstrated lymphocyte proliferation in all experimental
wells, as compared with in the control wells of all of the groups.
Compared with the control group, the cell proliferation rate did
not change significantly in the pcDNA3.1(+) group; though it
increased significantly in the Pcmv-TK-hIL-12 and PSLPI-TK-hIL-12
groups (P<0.01). The increased expression level in the
PSLPI-TK-hIL-12 group was still lower than that in the
Pcmv-TK-hIL-12 group (P<0.01; Fig.
5). The results indicated that the hIL-12 expressed by
the fusion gene eukaryotic expression vectors had notable
proliferation activity to the lymphocytes that were stimulated with
PHA. The activity increased with the amount of hIL-12
expression and the duration of exposure (P<0.01).
Specific and cooperative killing effect
of the HSV-TK/hIL-12 fusion gene regulated by the hSLPI promoter in
hNSCLC in vitro
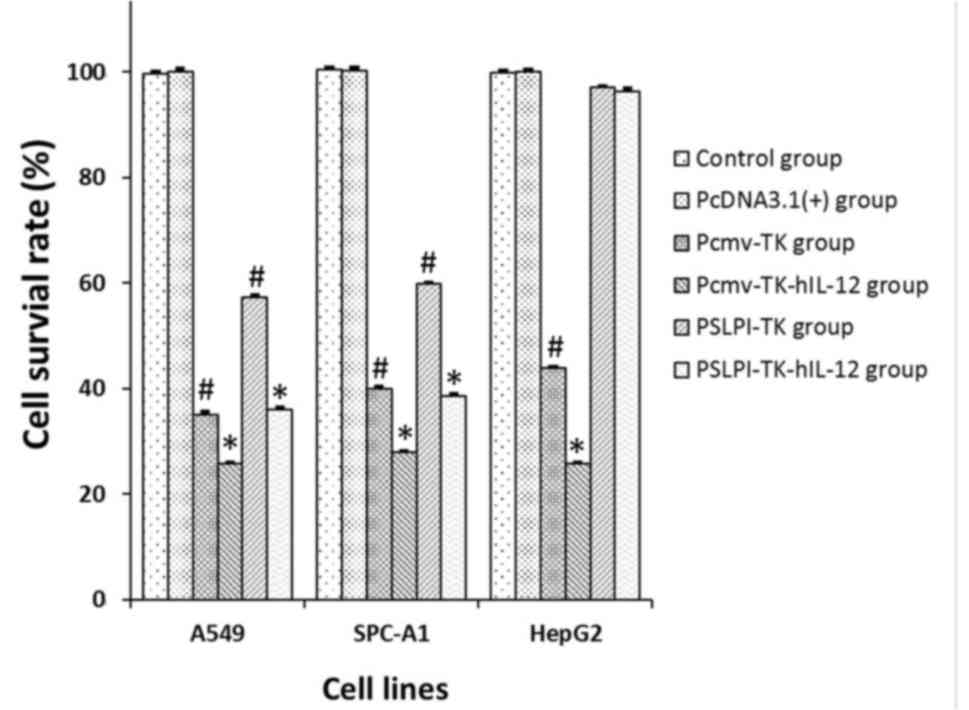
Direct antitumor effect of the
HSV-TK/hIL-12 fusion gene
The killing effect of lymphocytes at a 20:1
effector-target ratio combined with GCV at a final concentration of
10 µg/ml was evaluated using an MTT assay after 72 h of culture.
For all three cell lines, the cell survival rate in the pcDNA3.1
groups did not change significantly, as compared with in the
control groups (P>0.05). In the PSLPI-TK and PSLP-TK-hIL-12
groups of the two lung cancer cell lines, cell survival rate
declined significantly, compared with that in the control groups
(P<0.01), while no significant difference was noted with the
HepG2 cell line (P>0.05). In the Pcmv-TK and Pcmv-TK-hIL-12
groups of the three cell lines, cell survival rate declined
significantly, compared with that in the control groups (P<0.01;
Fig. 6). These results indicated that
TK/GCV gene regulated by the hSLPI promoter
had a notable killing effect on the lung cancer cells specifically
(P<0.01). Conversely, the CMV promoter had no tissue
specificity.
The present study compared the killing effect
between single gene and fusion gene treatments. In the CMV control
group, the fusion gene therapy had a lower cell survival rate in
A549, SPC-A1 and HepG2 cells (25.12, 25.77 and 25.82%,
respectively), compared with single gene therapy (35.01, 40.29 and
43.91%, respectively; P<0.01). In the hSLPI control
group, the cell survival rate in the fusion gene groups of A549 and
SPC-A1 cells (35.99 and 38.54%, respectively) was also lower than
that in the single gene groups (57.29 and 59.90%, respectively;
P<0.01). These results indicated that the
HSV-TK/hIL-12 fusion gene induced cooperative
killings.
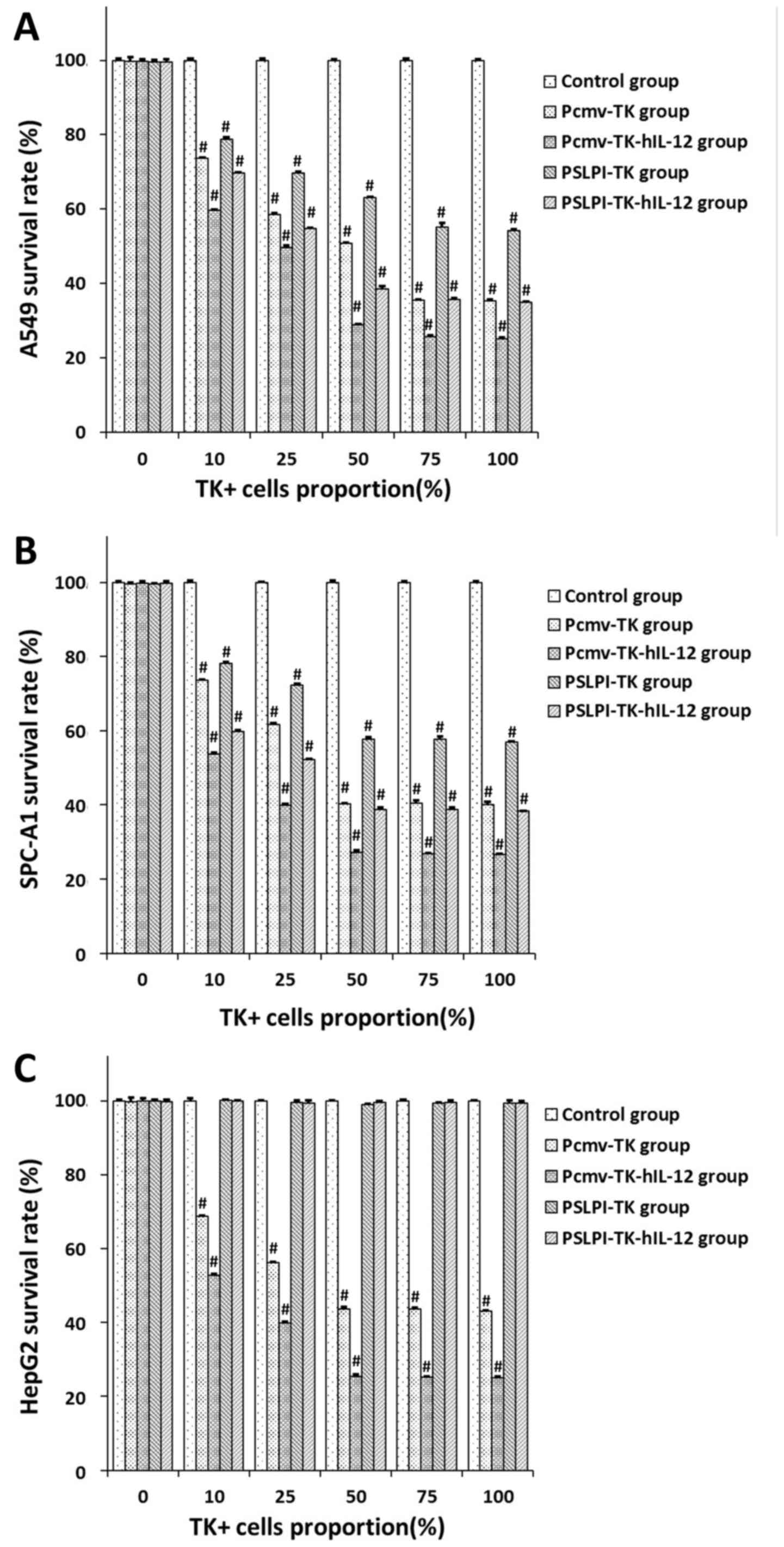
BSE of the HSV-TK/hIL-12 fusion
gene
GCV at a final concentration of 10 µg/ml and
lymphocytes at 20:1 effector-target ratio were added into tumor
cells, of which TK+ cells accounted for 0, 10, 25, 50,
75 and 100%. An MTT assay was performed 72 h later, and the cell
survival rate was calculated. For A549 and SPC-A1 cell lines, the
cell survival rate in the Pcmv-TK, Pcmv-TK-hIL-12, PSLPI-TK and
PSLP-TK-hIL-12 groups declined significantly from 10%
TK+, compared with that in the control groups
(P<0.01; Fig. 7A and B). For HepG2
cells, the cell survival rate in the Pcmv-TK and Pcmv-TK-hIL-12
groups declined significantly from 10% TK+, compared
with that in the control groups (P<0.01), while no significant
difference was noted with the PSLPI-TK and PSLP-TK-hIL-12 groups
(P>0.05; Fig. 7C). For the A549
cells at 75% TK+ the survival rate in the control,
Pcmv-TK, Pcmv-TK-hIL-12, PSLPI-TK and PSLPI-TK-hIL-12 groups did
not change significantly, compared with 100% TK+
(P>0.05; Fig. 8A). For the SPC-A1
cells and HepG2 cells at 50% TK+ the survival rate in
the control, Pcmv-TK, Pcmv-TK-hIL-12, PSLPI-TK and PSLPI-TK-hIL-12
groups did not change significantly, compared with 75%
TK+ and 100% TK+ (P>0.05; Fig. 8B and C). These results indicated that
the HSV-TK/GCV system had a BSE on tumor cells, and that the
phSLPI-TK/GCV system had a targeted killing effect on lung cancer
cells.
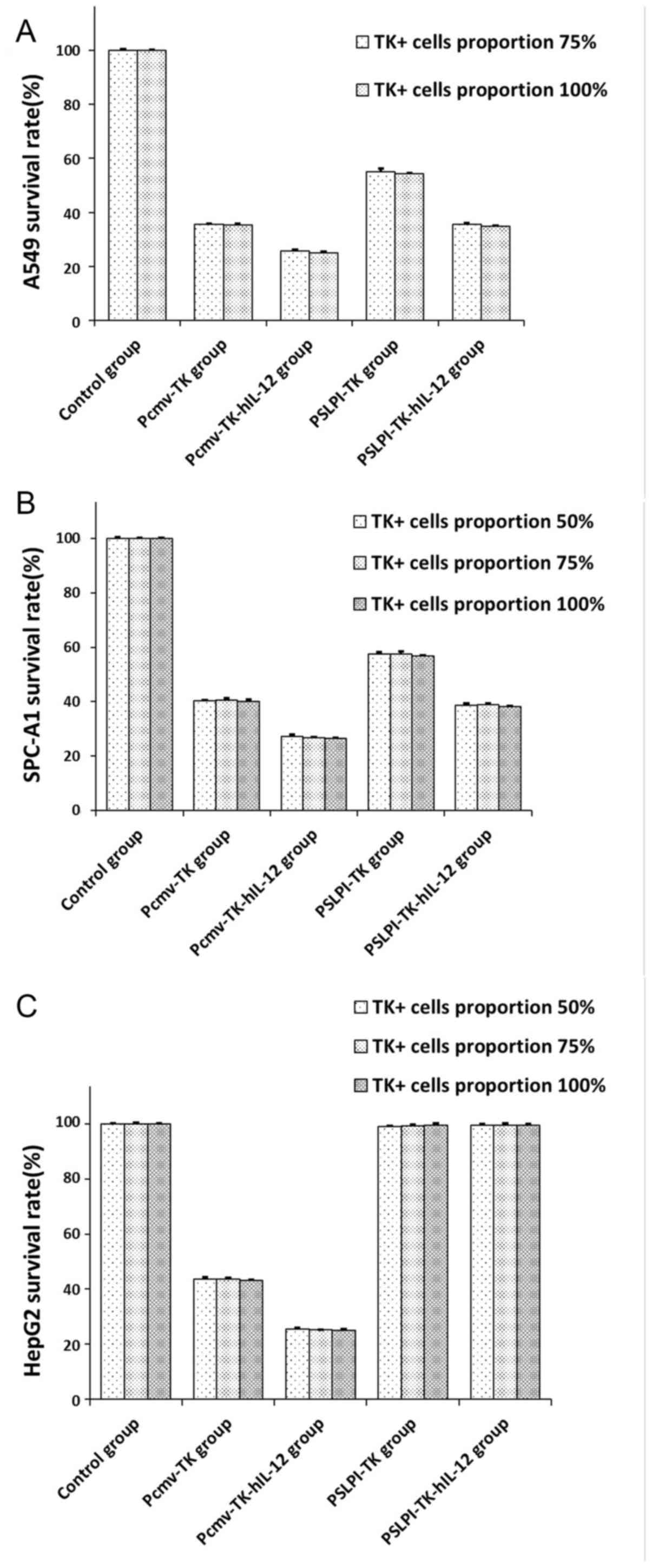 | Figure 8.Bystander effects on three cell
lines. (A) A549 cells: at 75% TK+, the survival rate in
the control, Pcmv-TK, Pcmv-TK-hIL-12, PSLPI-TK and PSLPI-TK-hIL-12
groups did not change significantly, compared with 100%
TK+ (P>0.05). (B) SPC-A1 cells and (C) HepG2 cells:
at 50% TK+, the survival rate in the control, Pcmv-TK,
Pcmv-TK-hIL-12, PSLPI-TK and PSLPI-TK-hIL-12 groups did not change
significantly, compared with 75 and 100% TK+
(P>0.05). TK-hIL-12, thymidine kinase-human interleukin-12. |
Discussion
In recent years, gene therapy has emerged as a
promising strategy for the treatment of cancer (24); however, a number of limitations are
associated with its clinical application, particularly the reduced
specificity in delivering functional therapeutic genes into tumor
cells (25). Therefore, prior studies
have been focused on developing targeting strategies (26,27).
Tissue-specific promoters represent one of the primary methods of
gene therapy (28,29).
hSLPI is produced by cells in the respiratory
tract and genital mucosa epithelium, and is highly expressed in
hNSCLC (19); by contrast, it has low
expression in other normal tissues, particularly in the liver
(22). This indicated potential
therapeutic inhibitory effects mediated by the expression of tumor
necrosis factor (TNF)-associated apoptosis-inducing ligand, TNF-α,
death receptor (DR)-4, DR-5 and TNF receptor-I, which lead to the
activation of the apoptosis pathway via caspase-2, −8 and −9
(30). Previous studies indicated
that SLPI was significantly overexpressed in ovarian cancer
samples, compared with in matched normal samples (31). Additionally, studies used the
hSLPI promoter to regulate the suicide gene therapy of
cervical (32) and ovarian cancer
(33) had achieved an antitumor
effect. In 2004, Maemondo et al (34) used this promoter in the therapy of
hNSCLC (squamous cell carcinoma HS-24 and adenocarcinoma A549 and
H358 cells) and achieved a potent antitumor effect in vivo
and in vitro. In the present study, the eukaryotic
expression vector of HSV-TK/hIL-12 fusion gene regulated by
the hSLPI promoter was constructed, specifically expressed
in lung cancer cells so as to improve the safety and effectiveness
of lung cancer gene therapy. To further investigate the specificity
of the hSLPI gene promoter in lung cancer, the HepG2 cell
line was selected as the negative control, which had already been
constructed in the lab to prove the effectiveness of gene targeting
therapy. The HSV-TK/hIL-12 gene expression at the
mRNA level was investigated using RT-PCR. The results indicated
that genes regulated by the CMV promoter were expressed in three
types of tumor cell lines at the same level (P>0.05), without
cell specificity. The genes regulated by the hSLPI promoter
were expressed in the two types of lung cancer cell lines, and not
in HepG2 cells. Coincidentally, an ELISA was preformed to detect
the hIL-12 expression, and the results were similar to the
RT-PCR findings. The MTT assay also revealed that the
HSV-TK/hIL-12 gene regulated by the hSLPI
promoter had a notable and specific killing effect on the lung
cancer cells (P<0.01). These results demonstrated that the genes
regulated by the hSLPI promoter had an effect on lung cancer
cells in a targeted manner.
Currently, two commonly used vector systems exist
for tumor gene therapy: Viral and non-viral vectors (35). Nanni et al (36) transferred the mIL-12 gene into
tumor cells using retroviral vectors. The administration of this
product as a vaccine led to tumor regression in 80–90% of the
tumor-burdened mice. The output of mIL-12 reached 400-2, 500
pg/l06/24 h. Loskog et al (37) used the adenovirus vector with the
mIL-12 gene in bladder cancer therapy and obtained an
antineoplastic effect. The production of mIL-12 reached 6–11 ng/ml
after the cells were cultured for 48 h in vitro; however,
viral vectors have a number of disadvantages (38–42). The
major problem is the generation of neutralizing antibodies, which
are formed due to immunogenicity and inflammatory toxicity
(41). Ring et al (42) determined that when the suicide gene,
regulated by the ERBB2 promoter, was transferred into cells using
viral vectors, the promoter lost its transcriptional activity and
specificity, and its specific antitumor effect was weakened. In
comparison, cationic lipids as a non-viral vehicle have several
advantages, including low toxicity, no immunogenicity, simple
operation, good reproducibility and applicability to mitotic and
non-mitotic cells in vivo and in vitro (26). Through continual improvement, the
transfection efficiency of liposomes has been increased up to ~90%
(43,44); therefore, in the present study,
Lipofectamine® 2000 was used, which could provide high
transfection efficiency and high levels of transgene expression in
a range of mammalian cell types in vitro (45). The present study indicated that the
production of the hIL-12 gene, regulated by the CMV
promoter, was 16.52±0.11 ng/106/72 h, 16.45±0.22
ng/106/72 h and 16.57±0.14 ng/106/72 h, in
A549, SPC-A1 and HepG2 cells, respectively. The data were
consistent with those of previous studies.
Since the first application of the TK gene
in tumor treatment studies by Moolten in 1986 (46), it had been extensively studied in
various human tumor types (9,10,47,48);
however, a number of basic and clinical studies indicated that the
combination therapy of the HSV-TK gene along with
immune-associated genes had a greater success than single TK
gene therapy (9–14). Ramesh et al (49) demonstrated that the
TK/GCV gene did not inhibit tumor growth effectively
in nude mice without immunity, with the transfection rate being
100%. On the contrary, when the transfection rate was 50%, the
tumor inhibition rate could reach 100% in mice with normal
immunity. The results indicated that the integrity of the host
immune system is essential for TK gene therapy. In the
present study, by comparing the killing effect of the single gene
with the effect of the fusion gene, it was demonstrated that in CMV
control groups (A549, SPC-A1 and HepG2) and hSLPI control
groups (A549 and SPC-A1), the fusion gene had a lower cell survival
rate, compared with the single gene. The results indicated that the
HSV-TK/hIL-12 fusion gene induced the cooperative
antitumor effect on hNSCLC cell lines in vitro.
Previous studies demonstrated that in the
HSV-TK/GCV gene antitumor system not only are the
cells transfected with the TK gene killed, but the adjacent
non-transfected tumor cells are also (46,50,51). This
phenomenon is known as a BSE (52).
The BSE can expand the killing effect of the TK/GCV
gene significantly (52); therefore,
the present study explored the BSE of the TK/GCV system on A549,
SPC-A1 and HepG2 cells. The results of the MTT assay indicated
that, at 10% TK+, the cell survival rate in the
Pcmv-TK/Pcmv-TK-hIL-12 groups (A549, SPC-A1 and HepG2) and the
PSLPI-TK/PSLPI-TK-hIL-12 groups (A549 and SPC-A1) declined
significantly, compared with the control group (P<0.01). In A549
cells, at 75% TK+, it could achieve the efficiency of
the 100% TK+ group. For the SPC-A1 and HepG2 cells, at
50% TK+, it could achieve the efficiency of the 100%
TK+ group. These results indicated that the
HSV-TK/GCV system had a BSE on tumor cells.
In conclusion, the present study selected the
hSLPI promoter as a target for hNSCLC gene therapy.
The data indicated that the fusion gene regulated by the
hSLPI promoter had targeted expression in hNSCLC, and
combined suicide gene therapy with immune gene therapy generated
significantly stronger therapeutic antitumor effects, compared with
single gene therapy. The present study provided evidence to warrant
preclinical studies of this lung cancer treatment, and may present
the theoretical basis for a novel therapeutic strategy.
Acknowledgements
This study was supported by the Projects of Health
Management Department of Jilin, China (grant no. 20132003), the
Department of Science and Technology of Jilin, China (grant nos.
20140311006YY, 20150312022ZG and 20150204028YY), and the
Development and Reform Commission of Jilin, China (grant nos.
2013C014-4 and 2014G073).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Itaya T, Yamaoto N, Ando M, Ebisawa M,
Nakamura Y, Murakami H, Asai G, Endo M and Takahashi T: Influence
of histological type, smoking history and chemotherapy on survival
after first-line therapy in patients with advanced non-small cell
lung cancer. Cancer Sci. 98:226–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang M, Shen H, Qiu C, Ni Y, Wang L, Dong
W, Liao Y and Du J: High expression of miR-21 and miR-155 predicts
recurrence and unfavourable survival in non-small cell lung cancer.
Eur J Cancer. 49:604–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun S, Schiller JH, Spinola M and Minna
JD: New molecularly targeted therapies for lung cancer. J Clin
Invest. 117:2740–2750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zarogoulidis P, Darwiche K, Sakkas A,
Yarmus L, Huang H, Li Q, Freitag L, Zarogoulidis K and Malecki M:
Suicide gene therapy for cancer-current strategies. J Genet Syndr
Gene Ther. 4:168492013.PubMed/NCBI
|
|
6
|
Lv SQ, Zhang KB, Zhang EE, Gao FY, Yin CL,
Huang CJ, He JQ and Yang H: Antitumor efficiency of the cytosine
deaminase/5-fluorocytosine suicide gene therapy system on malignant
gliomas: an in vivo study. Med Sci Monit. 15:BR13–BR20.
2009.PubMed/NCBI
|
|
7
|
Finocchiaro LM, Riveros MD and Glikin GC:
Cytokine-enhanced vaccine and suicide gene therapy as adjuvant
treatments of metastatic melanoma in a horse. Vet Rec. 164:278–279.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ju DW, Yang Y, Tao Q, Song WG, He L, Chen
G, Gu S, Ting CC and Cao X: Interleukin-18 gene transfer increases
antitumor effects of suicide gene therapy through efficient
induction of antitumor immunity. Gene Ther. 7:1672–1679. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drozdzik M, Qian C, Xie X, Peng D, Bilbao
R, Mazzolini G and Prieto J: Combined gene therapy with suicide
gene and interleukin-12 is more efficient than therapy with one
gene alone in a murine model of hepatocellular carcinoma. J
Hepatol. 32:279–286. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Warren P, Song W, Holle E, Holmes L, Wei
Y, Li J, Wagner T and Yu X: Combined HSV-TK/GCV and secondary
lymphoid tissue chemokine gene therapy inhibits tumor growth and
elicits potent antitumor CTL response in tumor-bearing mice.
Anticancer Res. 22:599–604. 2002.PubMed/NCBI
|
|
11
|
Kerkar SP, Muranski P, Kaiser A, Boni A,
Sanchez-Perez L, Yu Z, Palmer DC, Reger RN, Borman ZA, Zhang L, et
al: Tumor-specific CD8+ T cells expressing
interleukin-12 eradicate established cancers in lymphodepleted
hosts. Cancer Res. 70:6725–6234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terao S, Shirakawa T, Goda K, Kamidono S,
Fujisawa M and Gotoh A: Recombinant interleukin-2 enhanced the
antitumor effect of ADV/RSV-HSV-tk/ACV therapy in a murine bladder
cancer model. Anticancer Res. 25:2757–2760. 2005.PubMed/NCBI
|
|
13
|
Hall SJ, Canfield SE, Yan Y, Hassen W,
Selleck WA and Chen SH: A novel bystander effect involving tumor
cell-derived Fas and FasL interactions following Ad.HSV-tk and
Ad.mIL12 gene therapies in experimental prostate cancer. Gene Ther.
9:511–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nasu Y, Bangma CH, Hull GW, Yang G, Wang
J, Shimura S, McCurdy MA, Ebara S, Lee HM, Timme TL and Thompson
TC: Combination gene therapy with adenoviral vector-mediated
HSV-tk+GCV and IL-12 in an orthotopic mouse model for prostate
cancer. Prostate Cancer Prostatic Dis. 4:44–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fogar P, Greco E, Basso D, Habeler W,
Navaglia F, Zambon CF, Tormen D, Gallo N, Cecchetto A, Plebani M
and Pedrazzoli S: Suicide gene therapy with HSV-TK in pancreatic
cancer has no effect in vivo in a mouse model. Eur J Surg Oncol.
29:721–730. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada Y, Okada N, Mizuguchi H, Hayakawa T,
Nakagawa S and Mayumi T: Transcriptional targeting of RGD
fiber-mutant adenovirus vectors can improve the safety of suicide
gene therapy for murine melanoma. Cancer Gene Ther. 12:608–616.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schoensiegel F, Paschen A, Sieger S,
Eskerski H, Mier W, Rothfels H, Kleinschmidt J, Schadendorf D and
Haberkorn U: MIA (melanoma inhibitory activity) promoter mediated
tissue-specific suicide gene therapy of malignant melanoma. Cancer
Gene Ther. 11:408–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curvelo JA, Barreto AL, Portela MB,
Alviano DS, Holandino C, Souto-Padrón T and Soares RM: Effect of
the secretory leucocyte proteinase inhibitor (SLPI) on Candida
albicans biologicalprocesses: A therapeutic alternative? Arch
Oral Biol. 59:928–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ameshima S, Ishizaki T, Demura Y, Imamura
Y, Miyamori I and Mitsuhashi H: Increased secretory leukoprotease
inhibitor in patients with non-small cell lung carcinoma. Cancer.
89:1448–1456. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zuo J, Zhang C, Ren C, Pang D, Li Y, Xie
X, Tang Z and Jiang X: Secretory leukocyte protease inhibitor is a
proliferation and survival factor for pancreatic cancer cells. Clin
Transl Oncol. 17:314–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hough CD, Cho KR, Zonderman AB, Schwartz
DR and Morin PJ: Coordinately up-regulated genes in ovarian cancer.
Cancer Res. 61:3869–3876. 2001.PubMed/NCBI
|
|
22
|
Franken C, Meijer CJ and Dijkman JH:
Tissue distribution of antileukoprotease and lysozyme in humans. J
Histochem Cytochem. 37:493–498. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen PJ, Kuo MY, Chen ML, Tu SJ, Chiu MN,
Wu HL, Hsu HC and Chen DS: Continuous expression and replication of
the hepatitis delta virus genome in Hep G2 hepatoblastoma cells
transfected with cloned viral DNA. Proc Natl Acad Sci USA.
87:5253–5257. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alexandrova R: Experimental strategies in
gene therapy of cancer. J Buon. 14:S23–S32. 2009.PubMed/NCBI
|
|
25
|
Fukazawa T, Matsuoka J, Yamatsuji T, Maeda
Y, Durbin ML and Naomoto Y: Adenovirus-mediated cancer gene therapy
and virotherapy (Review). Int J Mol Med. 25:3–10. 2010.PubMed/NCBI
|
|
26
|
Das SK, Menezes ME, Bhatia S, Wang XY,
Emdad L, Sarkar D and Fisher PB: Gene therapies for cancer:
Strategies, challenges and successes. J Cell Physiol. 230:259–271.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nettelbeck DM, Jérôme V and Müller R: Gene
therapy: Designer promoters for tumour targeting. Trends Genet.
16:174–181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo XR, Li JS, Niu Y and Miao L: Targeted
killing effects of double CD and TK suicide genes controlled by
survivin promoter on gastric cancer cell. Mol Biol Rep.
38:1201–1207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Teimoori-Toolabi L, Azadmanesh K,
Amanzadeh A and Zeinali S: Selective suicide gene therapy of colon
cancer exploiting the urokinase plasminogen activator receptor
promoter. BioDrugs. 24:131–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakamura K, Takamoto N, Hongo A, Kodama J,
Abrzua F, Nasu Y, Kumon H and Hiramatsu Y: Secretory leukoprotease
inhibitor inhibits cell growth through apoptotic pathway on ovarian
cancer. Oncol Rep. 19:1085–1091. 2008.PubMed/NCBI
|
|
31
|
Tsukishiro S, Suzumori N, Nishikawa H,
Arakawa A and Suzumori K: Use of serum secretory leukocyte protease
inhibitor levels in patients to improve specificity of ovarian
cancer diagnosis. Gynecol Oncol. 96:516–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Robertson MW III, Wang M, Siegal GP,
Rosenfeld M, Ashford RS II, Alvarez RD, Garver RI and Curiel DT:
Use of a tissue-specific promoter for targeted expression of the
herpes simplex virus thymidine kinase gene in cervical carcinoma
cells. Cancer Gene Ther. 5:331–336. 1998.PubMed/NCBI
|
|
33
|
Barker SD, Coolidge CJ, Kanerva A,
Hakkarainen T, Yamamoto M, Liu B, Rivera AA, Bhoola SM, Barnes MN,
Alvarez RD, et al: The secretory leukoprotease inhibitor (SLPI)
promoter for ovarian cancer gene therapy. J Gene Med. 5:300–310.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Maemondo M, Saijo Y, Narumi K, Kikuchi T,
Usui K, Tazawa R, Matsumoto K, Nakamura T, Sasaki K, Takahashi M,
et al: Gene therapy with secretory leukoprotease inhibitor
promoter-controlled replication-competent adenovirus for non-small
cell lung cancer. Cancer Res. 64:4611–4620. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Uchibori R, Okada T, Ito T, Urabe M,
Mizukami H, Kume A and Ozawa K: Retroviral vector-producting
mesenchymal stem cells for targeted suicide cancer gene therapy. J
Gene Med. 11:373–381. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nanni P, Rossil L, De Giovanni C, Landuzzi
L, Nicoletti G, Stoppacciaro A, Parenza M, Colombo MP and Lollini
PL: Interleukin 12 gene therapy of MHC-negative murine melanoma
metastases. Cancer Res. 58:1225–1230. 1998.PubMed/NCBI
|
|
37
|
Loskog A, Björkland A, Brown MP, Korsgren
O, Malmström PU and Tötterman TH: Potent antitumor effects of CD154
transduced tumor cells in experimental bladder cancer. J Urol.
166:1093–1097. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brand K, Arnold W, Bartels T, Lieber A,
Kay MA, Strauss M and Dörken B: Liver-associated toxicity of the
HSV-tk/GCV approach and adenoviral vectors. Cancer Gene Ther.
4:9–16. 1997.PubMed/NCBI
|
|
39
|
van der Eb MM, Cramer SJ, Vergouwe Y,
Schagen FH, van Krieken JH, van der Eb AJ, Rinkes Borel IH, van de
Velde CJ and Hoeben RC: Severe hepatic dysfunction after
adenovirus-mediated transfer of the herpes simplex virusthymidine
kinase gene and ganciclovir administration. Gene Ther. 5:451–458.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Larocca C and Schlom J: Viral vector-based
therapeutic cancer vaccines. Cancer J. 17:359–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sterman DH, Recio A, Haas AR, Vachani A,
Katz SI, Gillespie CT, Cheng G, Sun J, Moon E, Pereira L, et al: A
phase I trial of repeated intrapleural adenoviral-mediated
interferon-beta gene transfer for mesothelioma and metastatic
pleural effusions. Mol Ther. 18:852–860. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ring C, Harris JD, Hurst HC and Lemoine
NR: Suicide gene expression induced in tumor cells transduced with
recombinant adenoviral, retroviral and plasmid vectors containing
the promoter. Gene Ther. 3:1094–1103. 1996.PubMed/NCBI
|
|
43
|
Ejeskär K, Fransson S, Zaibak F and
Ioannou PA: Method for efficient transfection of in
vitro-transcribed mRNA into SK-N-AS and HEK293 cells: Difference in
the toxicity of nuclear EGFP compared to cytoplasmic EGFP. Int J
Mol Med. 17:1011–1016. 2006.PubMed/NCBI
|
|
44
|
Kamaci N, Emnacar T, Karakas N, Arikan G,
Tsutsui K and Isik S: Selective silencing of DNA topoisomerase IIβ
in human mesenchymal stem cells by siRNAs (small interfering RNAs).
Cell Biol Int Rep (2010). 18:e000102011.PubMed/NCBI
|
|
45
|
Dalby B, Cates S, Harris A, Ohki EC,
Tilkins ML, Price PJ and Ciccarone VC: Advanced transfection with
Lipofectamine 2000 reagent: Primary neurons, siRNA, and
high-throughput applications. Methods. 33:95–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moolten FL: Tumor chemosensitivity
conferred by inserted herpes thymidine kinase genes: Paradigm for a
prospective cancer control strategy. Cancer Res. 46:5276–2581.
1986.PubMed/NCBI
|
|
47
|
Ambade AV, Joshi GV and Mulherkar R:
Effect of suicide gene therapy in combination with immunotherapy on
antitumour immuneresponse & tumour regression in a xenograft
mouse model for head & neck squamous cellcarcinoma. Indian J
Med Res. 132:415–422. 2010.PubMed/NCBI
|
|
48
|
Rodriguez SS, Castro MG, Brown OA, Goya RG
and Console GM: Gene therapy for the treatment of pituitary tumors.
Expert Rev Endocrinol Metab. 4:359–370. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ramesh R, Marrogi AJ, Munshi A, Abboud CN
and Freeman SM: In vivo analysis of the ‘bystander effect’: A
cytokine cascade. Exp Hamatol. 24:829–838. 1996.
|
|
50
|
Wei MX, Bougnoux P, Sacré-Salem B, Peyrat
MB, Lhuillery C, Salzmann JL and Klatzmann D: Suicide gene therapy
of chemically induced mammary tumor in rat: Efficacy and distant
bystander effect. Cancer Res. 58:3529–3532. 1998.PubMed/NCBI
|
|
51
|
Kianmanesh AR, Perrin H, Panis Y, Fabre M,
Nagy HJ, Houssin D and Klatzmann D: A ‘distant’ bystander effect of
suicide gene therapy: Regression of nontransduced tumors together
with a distant transduced tumor. Hum Gene Ther. 8:1807–1814. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van Dillen IJ, Mulder NH, Vaalburg W, de
Vries EF and Hospers GA: Influence of the bystander effect on
HSV-tk/GCV gene therapy. A review. Curr Gene Ther. 2:307–322. 2002.
View Article : Google Scholar : PubMed/NCBI
|