Introduction
China has the highest incidence and mortality rate
of nasopharyngeal carcinoma (NPC), a type of malignant epithelial
cell tumor, worldwide (1). NPC
exhibits decreased differentiation and a high metastatic nature
which frequently causes local regional recurrence and distant
metastasis in patients (2), and is
the primary reason for failure of treatment. Therefore, it is
important to investigate the underlying molecular mechanism of
migration and invasion in NPC.
The enhancer of zeste homolog 2 (EZH2) is a core
component of the Polycomb repressive complex 2 which possesses a
highly conserved SET domain with histone methyltransferase
activity. Previous studies have identified that EZH2 was
overexpressed in a variety of carcinomas including NPC, prostate
cancer and breast cancer. The expression levels of EZH2 were
associated with tumor size, depth of invasion, tumor stage, lymph
node metastasis, migration and invasion ability, suggesting that
EZH2 had the potential to be a novel diagnosis biomarker and a
target for antitumor therapy in NPC (3–5).
Rho-associated coiled-coil-containing protein kinase
(ROCK) is a RhoA downstream effector protein. ROCK1 is one of the
isoforms of ROCK and serves a critical role in the regulation of
cell migration and the maintenance of cell migration (6,7).
Previous studies have demonstrated that an ethyl
acetate extract of Celastrus orbiculatus (COE), a
traditional Chinese herb, has a marked antitumor effect in liver
cancer, gastric cancer and other tumors (8–10). In
addition, it has been revealed that COE is able to inhibit tumor
metastasis in gastric cancer (8). The
results of these studies suggested that COE may also have the
ability to inhibit the invasion and metastasis of NPC cells, the
validation of which was an aim of the present study.
In the present study, the effects of COE on
migration and invasion in vitro were investigated, in
addition to the underlying molecular mechanism. The results
indicated that COE inhibited the invasion by suppressing the
EZH2/ROCK1 signaling pathway in NPC cells. Therefore, it is
suggested that COE may have therapeutic effects against NPC via the
EZH2/ROCK1 signaling pathway.
Materials and methods
Reagents and antibodies
RPMI-1640 medium and fetal bovine serum (FBS) were
acquired from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). The Cell Titer 96 Aqueous One solution cell viability assay
(MTS assay) was purchased from Promega Corporation (Madison, WI,
USA). Matrigel was purchased from BD Biosciences (San Jose, CA,
USA). Primary antibodies used were: Rabbit anti-EZH2 (cat. no.
ab186006; 1:1,000; Abcam, Cambridge, MA, USA), rabbit anti-ROCK1
(cat. no. ab45171; 1:2,000; Abcam) and rabbit anti-β-actin (cat.
no. 4970; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA,
USA). The secondary antibody used was: Horseradish peroxidase
(HRP)-conjugated goat anti-rabbit immunoglobulin (Ig)G (cat. no.
HA1001-100; 1:2,000; HuaAn Biotechnology Co., Hangzhou, China).
Chemical reagents used were: 3-Deazaneplanocin A (DZNeP;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and dimethyl
sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA). Other chemicals used
were of analytical grade from commercial sources.
Plant materials
Plant materials were purchased from Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). COE was obtained in the
Department of Chinese Materia Medica Analysis in China
Pharmaceutical University (Nanjing, China), according to a
previously published protocol (11,12). The
resultant COE micropowder was dissolved in DMSO and diluted with
RPMI-1640 medium to various concentrations (6.25, 12.5, 25, 50 and
100 µg/ml).
Cell culture
The human NPC cell line 5–8F was a gift from Fudan
University Cancer Hospital (Shanghai, China). The cell line was
maintained in RPMI-1640 medium and supplemented with 10% FBS, 100
U/ml penicillin and 100 mg/ml streptomycin at 37°C in a humidified
atmosphere containing 5% CO2.
MTS cytotoxicity assay
The Cell Titer 96 Aqueous One solution cell
viability assay (MTS assay) was performed according to the
manufacturer's protocol. Cells were seeded in 96-well plates (100
cells/well) and incubated at 37°C overnight for attachment.
Subsequently, cells were treated with various doses of COE (0,
6.25, 12.5, 25, 50 and 100 µg/ml) for 24 h. A 20 µl volume of MTS
reagent was added to each well and incubated at 37°C for 2 h.
Subsequently, the absorbance was measured at 450 nm using a
microplate reader.
Assessment of apoptosis by flow
cytometry
The Annexin V-fluorescein isothiocyanate (FITC)
apoptosis detection assay was performed according to the
manufacturer's protocol (Bipec Biopharma Corporation, Cambridge,
MA, USA). After 24 h of incubation at 37°C with COE, cells were
harvested using trypsin and washed twice in PBS. Cells were then
re-suspended in 400 µl with 1X binding buffer at a concentration of
1×106 cells/ml prior to the addition of 5 µl Annexin
V-FITC. Cells were then vortex-mixed and incubated for 15 min at
between 4 and 8°C in the dark. A 10 µl volume of propidium iodide
was added to each tube before incubation for another 5 min at
between 4 and 8°C in the dark. The stained cells were analyzed
using flow cytometry by FlowJo version 7.6 software (BD
Biosciences).
Cell invasion and migration
assays
Cell invasion and migration assays were performed
using a Transwell membrane (Corning Incorporated, Corning, NY, USA)
according to the manufacturer's protocol. In the invasion assay,
Matrigel was applied to the upper chamber. Following treatment with
various concentrations (0, 12.5, 25 and 50 µg/ml) of COE, 5–8F
cells (1×105 /well) were seeded into the upper chamber.
RPMI-1640 medium containing 10% FBS was added to the lower chamber.
After 24 h of incubation at 37°C, cells on the upper membrane
surface were removed using a cotton swab and cells on the lower
membrane surface were fixed with 4% paraformaldehyde and stained
with crystal violet. The controls of these experiments were cells
treated with 0 µg/ml COE. The number of invading cells was counted
for each chamber in five random fields (magnification, ×200) by an
Olympus IX73 fluorescence microscope (Olympus Corporation, Tokyo,
Japan) and were captured by digital camera (Olympus Corporation).
For the migration assays, the procedures were carried out in the
same way, with the exception that the upper membrane was not coated
with Matrigel. Each experiment was performed three times.
Quantification of ROCK1 mRNA
expression by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
Total RNA was extracted from the COE-treated cells
using the TRIzol® reagent (Life Technologies; Thermo
Fisher Scientific, Inc.), and RT was performed in a 20 µl reaction
with 200 ng total RNA using the Two Step RT PCR kit (Takara
Biotechnology Co., Ltd., Dalian, China). The RNA concentration was
assessed by measuring the absorption (A260/A280) on the NanoDrop
Spectrophotometer ND-1000 (NanoDrop, Wilmington, DE, USA). RT was
performed under the following conditions: 37°C for 15 min, 85°C for
5 sec, and then held at 4°C until use. RT-qPCR was performed in
triplicate using SYBR Premix Ex Taq™ kit (Takara
Biotechnology Co., Ltd., Dalian, China) on a
LightCycler® 480 Real-Time PCR system. The primers used
are presented in Table I. qPCR was
performed under the following conditions: Initiation step at 95°C
for 30 sec for one cycle, denaturation step at 95°C for 5 sec and
60°C for 30 sec for 40 cycles, annealing at 95°C for 5 sec and 60°C
for 1 min for one cycle, and an elongation step at 50°C for 30 sec
for 1 cycle. mRNA levels of EZH2 and ROCK1 were measured by the
relative fluorescent intensity to the internal control β-actin
using the 2−ΔΔCq method (13).
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction analysis.
| Gene | Forward primer
sequence | Reverse primer
sequence |
|---|
| EZH2 |
5′-TCAAAGAACTACCTGGATGCTGT-3′ |
5′-CTTGAGCTGTCTCAGTCGCA-3′ |
| ROCK1 |
5′-CCAACAGTCCTTGGGTTGTTCA-3′ |
5′-TTTCAGGCACATCATAGTTGCTC-3′ |
| β-actin |
5′-TTCCTTCCTGGGCATGGAGT-3′ |
5′-TCTTCATTGTGCTGGGTGCC-3′ |
Western blot analysis
Protein expression levels were analyzed by western
blot analysis. Experiments were performed at least three times. The
cells were scraped into 0.3 ml lysis buffer on ice. Cell debris was
removed by centrifugation (12,000 × g, 4°C, 10 min). The protein
concentrations were quantified using the Bradford method. A total
of 30 µg protein/lane was separated by SDS-PAGE (5–10% gel) and
transferred onto polyvinylidine difluoride membranes (Immobilon;
EMD Millipore, Billerica, MA, USA). The membranes were blocked with
5% bovine serum albumin and incubated overnight at 4°C with primary
antibodies. Following three washes with TBST, the membranes were
incubated for 2 h at room temperature with HRP-conjugated goat
anti-rabbit IgG. Immunoreactive protein bands were detected using
an enhanced chemiluminescence system (GE Healthcare Life Sciences,
Chalfont, UK). To quantify protein levels of the western blots,
densitometric analysis was performed using ImageJ v1.46 software
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SPSS software (version 16.0; SPSS, Inc., Chicago,
IL, USA) was used to analyze the results of the present study.
Results are presented as the mean ± standard deviation and assessed
by the two-tailed Student's t-test. To quantify protein levels of
western blots, densitometric analysis was performed using ImageJ
software (version 1.46; National Institutes of Health, Bethesda,
MD, USA). P<0.05 was considered to indicate a statistically
significant difference. Experiments were performed at least three
times.
Results
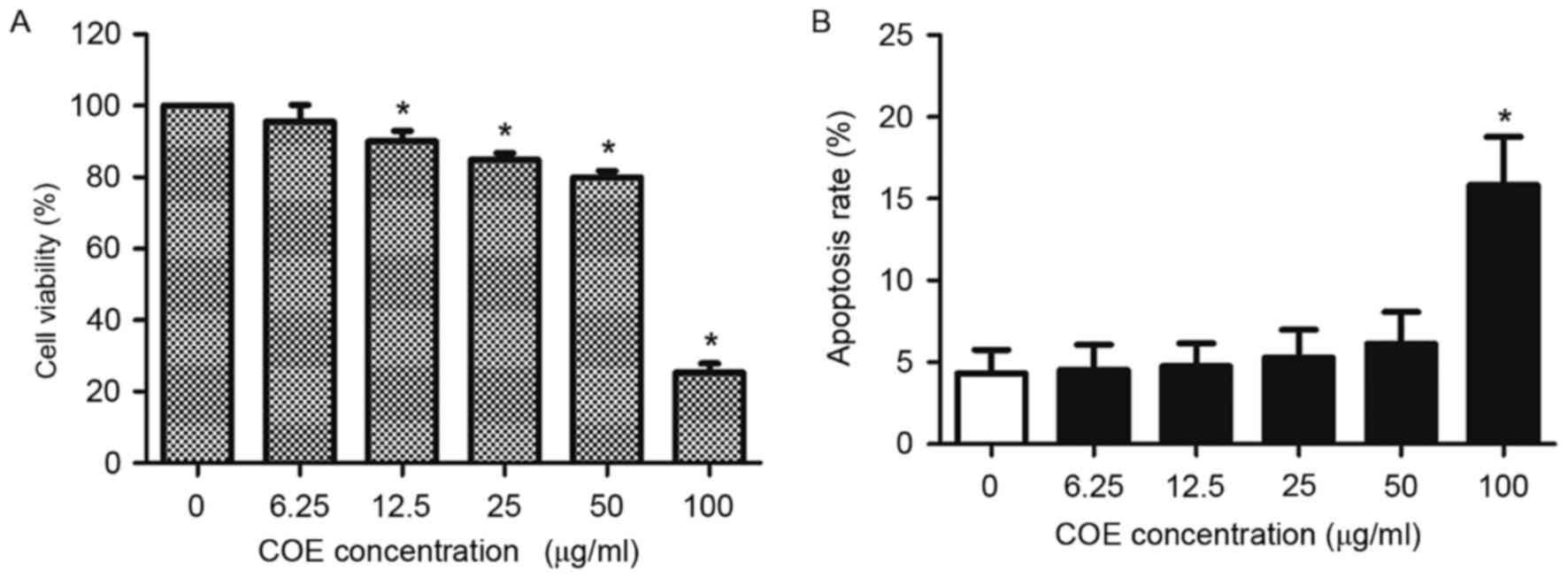
Effect of COE on viability of 5–8F
cells
COE decreased viability of 5–8F cells in a
concentration-dependent manner (Fig.
1A). Additionally, COE increased the apoptotic rate of 5–8F
cells in a concentration-dependent manner (Fig. 1B). However, no significant difference
in the apoptotic rates of 5–8F cells was identified for doses
<100 µg/ml COE for 24 h. Therefore, concentrations <100 µg/ml
COE were selected for the subsequent experiments, to ensure that
the effect of COE on NPC cells was not caused by direct
cytotoxicity of COE.
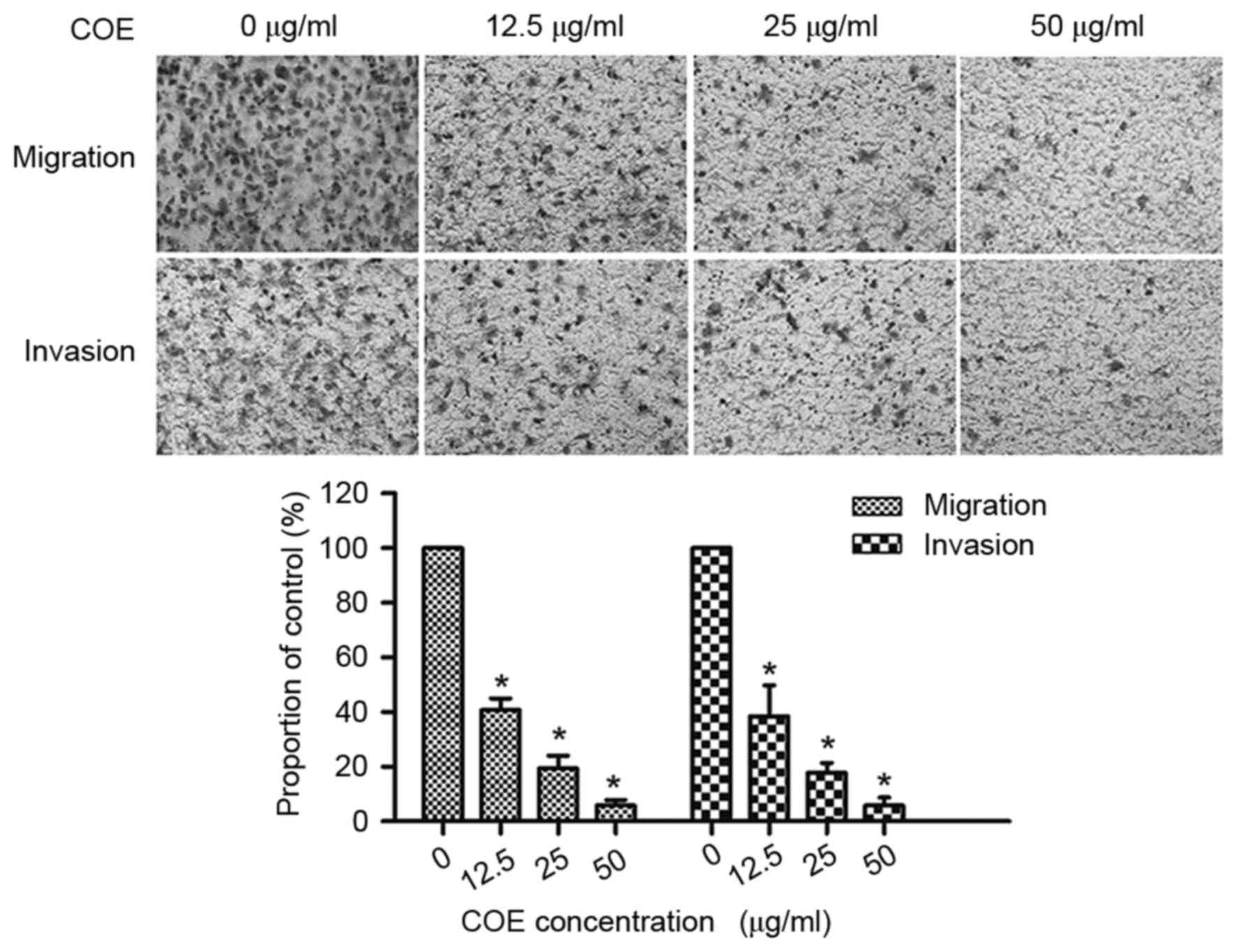
COE inhibits migration and invasion in
5–8F cells
COE markedly decreased the number of cells which
migrated and invaded the lower chamber, in a dose-dependent manner.
As presented in Fig. 2, treatment
with COE (12.5, 25 and 50 µg/ml) inhibited 59.3, 80.6 and 94.21% of
cell migration and inhibited 61.54, 82.2 and 94.23% of cell
invasion in 5–8F cells, respectively. The results of the present
study identified that COE inhibited migration and invasion of 5–8F
cells in a dose-dependent manner.
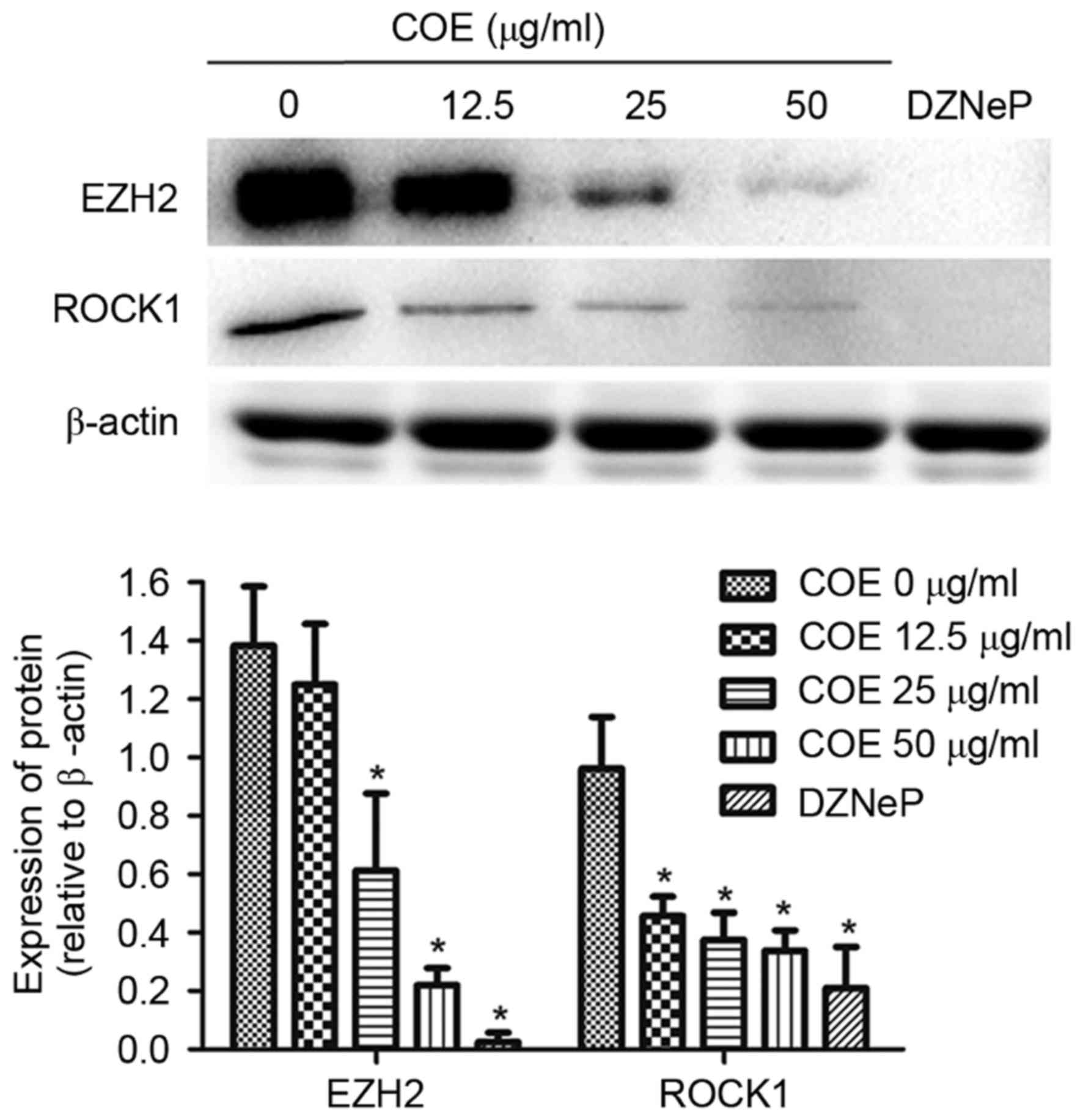
COE inhibits protein expression of
EZH2 and ROCK1 in 5–8F cells
To determine the underlying molecular mechanism of
COE in NPC cells, the expression levels of EZH2 and ROCK1 were
analyzed. Treating cells with COE decreased EZH2 and ROCK1 protein
expression levels in a dose-dependent manner. EZH2 and ROCK1
protein expression levels were significantly decreased following
treatment with COE at 50 µg/ml and 2 µM DZNeP, an inhibitor of
EZH2, for 24 h (Fig. 3).
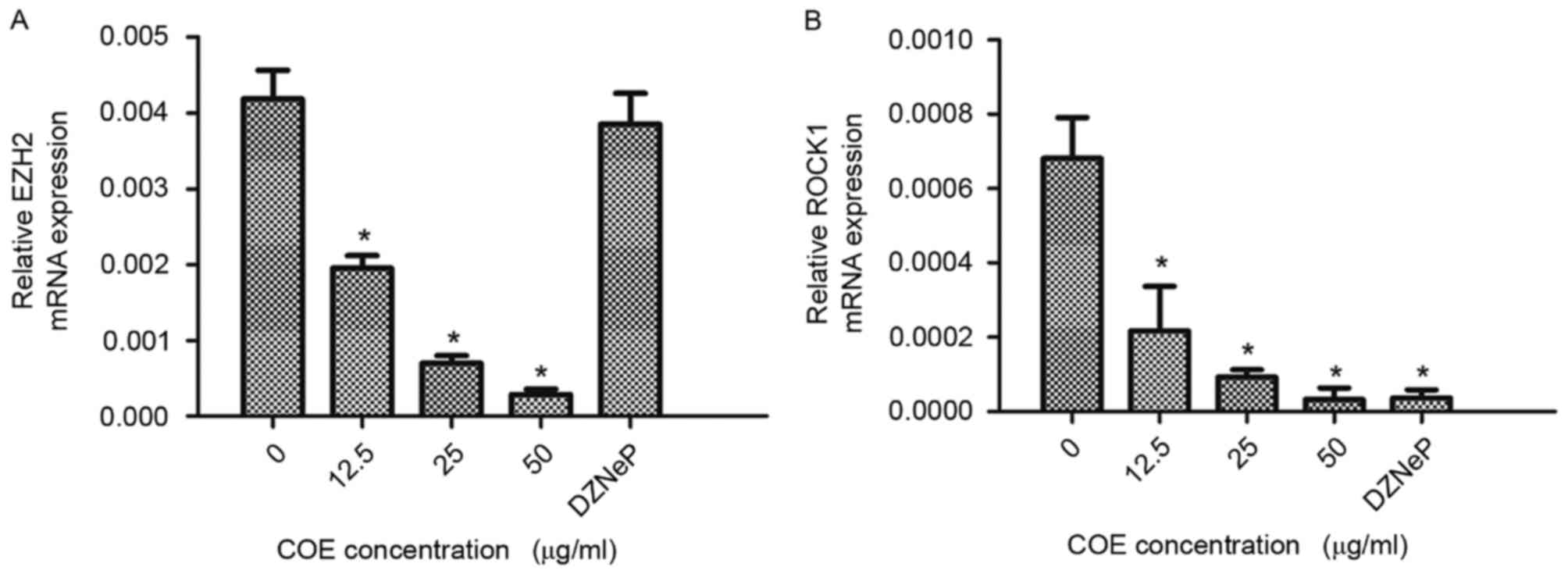
COE inhibits mRNA expression of EZH2
and ROCK1 in 5–8F cells
RT-qPCR was used to determine mRNA expression levels
of EZH2 and ROCK1 in 5–8F cells. Cells treated with COE exhibited
decreased EZH2 and ROCK1 mRNA expression levels in a dose-dependent
manner. RT-qPCR revealed that EZH2 mRNA expression levels were
significantly decreased following treatment with COE at 50 µg/ml
for 24 h; however, a similar effect was not observed with DZNeP
treatment. ROCK1 mRNA expression levels were significantly
decreased following treatment with various doses of COE and 2 µM
DZNeP for 24 h. Results are presented in Fig. 4. The results of the present study
suggest that expression levels of EZH2 and ROCK1 mRNA were
significantly decreased by COE in a dose-dependent manner in 5–8F
cells.
Discussion
COE serves diverse roles in antitumor activity
through modulation of cell viability, apoptosis, angiogenesis,
invasion and metastasis. Although a number of studies have
suggested that the COE is associated with tumor metastasis
(8,14), its function in the EZH2/ROCK1
signaling pathway remains unclear. To identify potential
therapeutic targets, elucidating the underlying molecular
tumorigenic mechanisms of COE is required.
EZH2 overexpression has been identified in NPC,
non-small cell lung cancer, chronic lymphocytic leukemia and breast
cancer (15–17). Collectively, these studies suggest
that EZH2 is a novel target oncogene and pharmacological inhibition
of EZH2 may be therapeutic for certain types of cancer.
The ROCK signaling pathway is one of the most
important cellular invasion mechanisms. Activated Rho GTP binds to
ROCK, changing the conformation of ROCK and exposing the catalytic
domain so that downstream effector molecules may be phosphorylated.
Subsequently, ROCK reorganizes the cytoskeleton and promote
formation of focal adhesion (18).
Previous studies have demonstrated that the regulation of human
lung cancer cell movement by placental growth factor is primarily
due to ROCK (19,20). Therefore, ROCK1 is associated with
cancer growth, invasion and metastasis, and may be a target of
tumor metastasis.
In order to determine the effect of COE on the
EZH2/ROCK1 signaling pathway, a series of experiments were
conducted in NPC cells. The function of COE was determined using an
MTS cytotoxicity assay and flow cytometry. The increased dose of
COE may be attributed to the decreased viability capacity and
increased apoptotic rates.
The results of the present study demonstrated that
COE serves a crucial role in decreasing tumor migration and
invasion by regulation of the EZH2/ROCK1 signaling pathway. COE
inhibited invasion and migration of 5–8F cells at low-toxic doses
(12.5, 25 and 50 µg/ml), suggesting that inhibition of invasion and
migration of 5–8F cells by COE was not due to its cytotoxic
effect.
EZH2 and ROCK1 protein and mRNA expression levels
were significantly decreased following COE treatment. The
abrogation of EZH2 function by DZNeP decreased the protein and mRNA
expression levels of ROCK1; however, DZNeP decreased the protein
expression levels of EZH2 only. The results of the study of Girard
et al (21) were consistent
with those of the present study; DZNeP decreased protein expression
levels, but not mRNA expression levels, of EZH2 in chondrosarcoma
cells. The results of the present study, together with those of
Girard et al (21), indicated
that DZNeP may regulate EZH2 expression levels at the
post-transcriptional level.
Previous studies have demonstrated that EZH2 is
upregulated in NPC tissues. Overexpression of EZH2 was associated
with an advanced clinical stage and increased risk of relapse, and
EZH2 served as an independent poor prognostic factor for patients
with NPC (3,22). Therefore, ROCK1 may be partially
involved in EZH2-induced progression of NPC.
EZH2 was overexpressed in NPC cell lines compared
with normal nasopharyngeal cells. EZH2 knockdown by short hairpin
RNA increased the expression of epithelial cadherin and markedly
decreased the invasiveness and metastasis in NPC cells (23). ROCK1 serves a critical role in
regulating cell migration and invasion (7,19). The
results of the present study demonstrated that the expression of
EZH2 and ROCK1 was suppressed by COE at the level of transcription
initiation. However, further studies are required to determine
whether COE regulates EZH2 and ROCK1 directly and the underlying
molecular mechanism for the association between EZH2 and ROCK1 in
NPC.
COE was identified to be an inhibitor of EZH2 in NPC
and inhibited the invasion and migration by downregulating the
EZH2/ROCK1 signaling pathway. To the best of our knowledge, the
present study is the first to identify the association between COE
and the EZH2/ROCK1 signaling pathway. COE may therefore be used as
a novel anticancer drug, particularly for types of cancer
exhibiting increased expression of EZH2. The results of the present
study identified the value of COE in treating metastatic NPC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81573656, 81274141,
81450051 and 81403232), the Plans of Colleges and Universities in
Jiangsu Province to Postgraduate Research and Innovation (grant no.
KYZZ15-0368), the Foundation of SuBei People's Hospital (grant no.
yzucms201409), the Natural Science Foundation of Jiangsu Province
(grant no. BK20141280) and the second batch of scientific research
projects of the National Traditional Chinese medicine clinical
research base construction (grant no. JDZX2015254).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and YH designed the research, performed the
experiments, analyzed data and wrote the paper. YC, YM, FY, XD, LT
and HW performed the experiments. YQ and RG assisted with data
acquisition and statistical analysis. YL performed the Celastrus
orbiculatus extraction and provided all the reagents. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee AW, Ng WT, Chan YH, Sze H, Chan C and
Lam TH: The battle against nasopharyngeal cancer. Radiother Oncol.
104:272–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hwang CF, Huang HY, Chen CH, Chien CY, Hsu
YC, Li CF and Fang FM: Enhancer of zeste homolog 2 overexpression
in nasopharyngeal carcinoma: An independent poor prognosticator
that enhances cell growth. Int J Radiat Oncol Biol Phys.
82:597–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang CJ and Hung MC: The role of EZH2 in
tumour progression. Br J Cancer. 106:243–247. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crea F, Paolicchi E, Marquez VE and Danesi
R: Polycomb genes and cancer: Time for clinical application. Crit
Rev Oncol Hematol. 83:184–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shi Y, Pontrello CG, DeFea KA, Reichardt
LF and Ethell IM: Focal adhesion kinase acts downstream of EphB
receptors to maintain mature dendritic spines by regulating cofilin
activity. J Neurosci. 29:8129–8142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Newell-Litwa KA, Badoual M, Asmussen H,
Patel H, Whitmore L and Horwitz AR: ROCK1 and 2 differentially
regulate actomyosin organization to drive cell and synaptic
polarity. J Cell Biol. 210:225–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Antimetastatic effects of Celastrus
orbiculatus on human gastric adenocarcinoma by inhibiting
epithelial-mesenchymal transition and NF-κB/snail signaling
pathway. Integr Cancer Ther. 14:271–281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang H, Qian Y, Liu Y, Li G, Cui P, Zhu
Y, Ma H, Ji X, Guo S and Tadashi H: Celastrus orbiculatus
extract induces mitochondrial-mediated apoptosis in human
hepatocellular carcinoma cells. J Tradit Chin Med. 32:621–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qian YY, Zhang H, Hou Y, Yuan L, Li GQ,
Guo SY, Hisamits T and Liu YQ: Celastrus orbiculatus extract
inhibits tumor angiogenesis by targeting vascular endothelial
growth factor signaling pathway and shows potent antitumor activity
in hepatocarcinomas in vitro and in vivo. Chin J Integr Med.
18:752–760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li JJ, Yang J, LÜ F, Qi YT, Liu YQ, Sun Y
and Wang Q: Chemical constituents from the stems of Celastrus
orbiculatus. Chin J Nat Med. 10:pp279–283. 2012. View Article : Google Scholar
|
|
12
|
Zan K, Chen XQ, Wang Q and Cao L: Chemical
constituents in stem of Celastrus orbiculatus. Chin Trad
Herbal Drugs. 38:1455–1457. 2007.
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Y, Liu Y, Qian Y, Dai X, Yang L, Chen
J, Guo S and Hisamitsu T: Research on the efficacy of Celastrus
Orbiculatus in suppressing TGF-β1-induced
epithelial-mesenchymal transition by inhibiting HSP27 and
TNF-α-induced NF-κ B/Snail signaling pathway in human gastric
adenocarcinoma. BMC Complement Altern Med. 14:4332014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Zhao H, Lv L, Bao L, Wang X and
Han S: Prognostic significance of EZH2 expression in non-small cell
lung cancer: A meta-analysis. Sci Rep. 6:192392016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rabello Ddo A, Lucena-Araujo AR,
Alves-Silva JC, da Eira VB, de Vasconcellos MC, de Oliveira FM,
Rego EM, Saldanha-Araujo F and Silva Pittella F: Overexpression of
EZH2 associates with a poor prognosis in chronic lymphocytic
leukemia. Blood Cells Mol Dis. 54:97–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song X, Gao T, Wang N, Feng Q, You X, Ye
T, Lei Q, Zhu Y, Xiong M, Xia Y, et al: Selective inhibition of
EZH2 by ZLD1039 blocks H3K27 methylation and leads to potent
anti-tumor activity in breast cancer. Sci Rep. 6:208642016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Croft DR, Crighton D, Samuel MS, Lourenco
FC, Munro J, Wood J, Bensaad K, Vousden KH, Sansom OJ, Ryan KM and
Olson MF: p53-mediated transcriptional regulation and activation of
the actin cytoskeleton regulatory RhoC to LIMK2 signaling pathway
promotes cell survival. Cell Res. 21:666–682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vigil D, Kim TY, Plachco A, Garton AJ,
Castaldo L, Pachter JA, Dong H, Chen X, Tokar B, Campbell SL and
Der CJ: ROCK1 and ROCK2 are required for non-small cell lung cancer
anchorage-independent growth and invasion. Cancer Res.
72:5338–5347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Ye L, Zhang L and Jiang WG:
Placenta growth factor, PLGF, influences the motility of lung
cancer cells, the role of Rho associated kinase, Rock1. J Cell
Biochem. 105:313–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Girard N, Bazille C, Lhuissier E, Benateau
H, Llombart-Bosch A, Boumediene K and Bauge C: 3-Deazaneplanocin A
(DZNep), an inhibitor of the histone methyltransferase EZH2,
induces apoptosis and reduces cell migration in chondrosarcoma
cells. PLoS One. 9:e981762014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Zhao FP, Peng Z, Zhang MW, Lin SX,
Liang BJ, Zhang B, Liu X, Wang L, Li G, et al: EZH2 promotes
angiogenesis through inhibition of miR-1/Endothelin-1 axis in
nasopharyngeal carcinoma. Oncotarget. 5:11319–11332. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ,
Liu YH, Zhang HB, Liao YJ, Zheng F, Zhu W, et al: EZH2 supports
nasopharyngeal carcinoma cell aggressiveness by forming a
co-repressor complex with HDAC1/HDAC2 and snail to inhibit
E-cadherin. Oncogene. 31:583–594. 2012. View Article : Google Scholar : PubMed/NCBI
|