Introduction
Colorectal cancer (CRC) is the third most common
cancer with ~1.4 million cases (10% of all cancers) diagnosed every
year globally (1). CRC is also the
second leading cause of cancer-associated mortality with ~700,000
mortalities every year (1). At
present, the pathogenesis of CRC remains unclear; however, CRC is
primarily associated with old age and certain lifestyle factors,
including drinking and preserved food, whereas a small proportion
of CRC is associated with underlying genetic disorders. Certain
inherited genetic disorders, including inflammatory bowel disease
(Crohn's disease and ulcerative colitis) and familial adenomatous
polyposis, are considered risk factors for CRC (1). Furthermore, there are other factors that
also increase the risk of CRC, including diet (consumption of
preserved food), obesity, smoking and a lack of physical activity
(2).
As with other types of cancer, the mechanism of
pathogenicity in CRC is fundamentally a disorder of uncontrolled
cellular proliferation. In general, the inactivation of tumor
suppressors, combined with the alteration of DNA repair genes,
results in proto-oncogene mutations to produce oncogenes, which
give rise to abnormal cellular proliferation and invasion (3). Tumor protein p53 (TP53) serves a crucial
role in multicellular organisms in the prevention of tumor
formation through regulating apoptosis, cell cycle and DNA repair
processes (4–6). Different cellular stresses, including
hypoxia, ionizing radiation, DNA damage and chemotherapeutic drugs,
are able to stimulate the activation of TP53. Conversely, TP53
activity may be downregulated through TP53 gene mutations
(loss of function mutations), alterations of upstream factors, or
modifications of the downstream components that mediate TP53
signals (7). It has been reported
that the TP53 mutational frequency is 5–21% in patients with
breast cancer depending upon household income (8). High income patients may acquire fewer
p53 mutations compared with low income patients (8). Approximately 50% of TP53 mutation
result in TP53 losing its (antitumor) activity (9). Previous studies have demonstrated that
the degree of TP53 gene mutation is directly associated with
the Dukes' stage, a staging method prognostically relevant to CRC,
which includes the differentiation grade, extent of local invasion,
liver and lymph node metastasis, and the prognosis of patients with
CRC (9,10).
Apoptosis-stimulating protein of p53 (ASPP) has
recently been identified as a family of proteins with three
members, ASPP1, ASPP2 and iASPP, which selectively regulate the
TP53-mediated apoptotic process. These proteins commonly share an
ankyrin repeat domain, a SRC Homology 3 domain and a
poly-proline-rich domain at the C-terminus. ASPP1 and ASPP2 are
pro-apoptotic factors, whereas iASPP has an anti-apoptotic effect
(4). ASPP2 was revealed to be
downregulated in several types of cancer, including choriocarcinoma
(11), human acute leukemia (12), pancreatic cancer cells (13), pituitary adenoma, gastric cancer
(14), lung cancer (15,16) and
diffuse large B-cell follicular center lymphoma (17). It was reported that patients with
cancer in which ASPP2 is downregulated exhibit metastasis and a
poor prognosis (11–13,17).
Furthermore, iASPP expression is increased in non-small cell lung
cancer, hepatocellular carcinoma and cervical adenocarcinoma, and
is associated with a poor prognosis in these types of cancer
(15,18,19).
Although several reports have studied the role of
ASPP in cancer prognosis (11–13,15,17–19),
studies investigating the association between ASPP and CRC
prognosis are limited. The present study investigated the
expression profiles of ASPP1, ASPP2 and iASPP in 41 samples
collected from CRC patients with different pathological conditions.
The results of the present study will provide valuable pathological
evidence to evaluate the prognosis of CRC in the clinic and to
improve the treatment options for CRC.
Materials and methods
Clinical samples
Samples were collected from 41 patients
pathologically diagnosed with CRC, including 20 males and 21
females, with a median age of 64 years (range, 41–86 years) at the
First Hospital of Jilin University (Changchun, China). CRC tissue
samples and adjacent non-cancerous tissue samples (>5 cm from
the edge of tumor) were obtained by surgical resection between June
2014 and April 2015. One part of the resected tumor and adjacent
non-cancerous tissue was quickly frozen in liquid nitrogen and
stored in a −80°C freezer, while the other part was fixed with
formalin for immunohistochemical analysis. The study research
proposal was approved by the Medical Ethics Committee of the First
Hospital of Jilin University, and written informed consent was
obtained from each patient. The pathological classifications of
these 41 samples are summarized in Table
I.
 | Table I.Pathological classification of human
colorectal cancer. |
Table I.
Pathological classification of human
colorectal cancer.
| Clinical and
pathological profile | Patient number |
|---|
| Early/advanced (TNM
I+II/III+IV)a | 11+7/14+9 |
| Tumor topography
(T1/T2/T3/T4)b | 5/9/16/11 |
| Regional lymph node
metastasis (N0/Nx, x≠0)c | 19/22 |
| Distant metastasis
(M0/M1)d | 10/31 |
| TP53 expression
(Positive/negative)e | 15/26 |
According to the experimental results, the
experimental data were divided into two groups: A TP53-positive
group and a TP53-negtive group. Patients were additionally
classified into early (Stage I+II) and advanced (Stage III+IV)
groups, as well as N0/Nx (x≠0) groups, M0/M1 groups,
good/moderate/poor histological grade groups and colon/rectal
groups based upon the Tumor-Node-Metastasis staging criteria of the
American Joint Cancer Committee (20).
Immunohistochemistry (IHC)
The TP53 and ASPP expression levels of the 41
patient tissue samples were detected by immunohistochemical
staining. Briefly, the CRC and adjacent non-cancerous tissue
samples were fixed with 10% formalin for 24 h at room temperature,
embedded in paraffin and sliced into 4 mm thick sections, which
were used for IHC and immunofluorescence (IF) staining analyses.
The paraffin-embedded sections were deparaffinized by heating for 1
h at 60°C, then washing with xylene for 15 min twice, and
rehydrated in a descending alcohol series (100, 100, 95, 85 and
75%). Antigen retrieval was performed by boiling, followed by
incubation with citrate buffer (0.01 M, pH 6.0) at room temperature
for 2 min (repeated 5 times). Then, it was cooled to room
temperature and the sections were washed with PBS for 5 mins 3
times. Subsequently, the endogenous peroxidase activity was
inactivated with 3% hydrogen peroxide for 40 min at room
temperature. Following blocking with 5% donkey serum obtained from
healthy animals for 40 min at room temperature, the sections were
incubated with a 1:200 diluted mouse TP53 monoclonal antibody (cat.
no. ZM-0405; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China), a 1:800 diluted mouse ASPP1 monoclonal antibody
(cat. no. A4355; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), a
1:800 diluted mouse ASPP2 monoclonal antibody (cat. no. A4480;
Sigma-Aldrich; Merck KGaA) or a 1:800 diluted rabbit iASPP
polyclonal antibody (cat. no. ab34898; Abcam, Cambridge, MA, USA)
overnight at 4°C. The next day, sections were washed twice with
phosphate-buffered saline (PBS), and then the pre-stained slice was
incubated with the appropriate biotinylated secondary antibody for
1 h at room temperature. Biotin-labeled goat anti-mouse secondary
antibody (cat. no. SP-0024; Beijing Biosynthesis Biotechnology Co.,
Ltd., Beijing, China; ready-to-use dilution) was used for TP53,
ASPP1 and ASPP2 and biotin-labeled goat anti-rabbit secondary
antibody (cat. no. SP-0023; Beijing Biosynthesis Biotechnology Co.,
Ltd.; ready-to-use dilution) was used for iASPP. The sections were
washed three times with PBS. Streptavidin-peroxidase was reacted
for 5 min at room temperature. Subsequently, the target protein was
developed by freshly prepared 3,3′-diaminobenzidine reagent
(Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.). Finally, the
sections were counterstained with hematoxylin for 2 min at room
temperature, dehydrated through an ethanol gradient (50, 70, 80,
90, 95, 100 and 100%) and sealed with neutral gum. The target
protein was subsequently observed at a ×200 magnification under a
BX51 optical microscope (Olympus Corporation, Tokyo, Japan).
Immunofluorescence (IF)
The paraffin-embedded sections were deparaffinized
by heating for 1 h at 60°C, then washing with xylene for 15 min
twice, rehydrated in a descending alcohol series (100, 100, 95, 85
and 75%) and antigen retrieval was performed by boiling, followed
by incubation with citrate buffer (0.01 M, pH 6.0) and at room
temperature for 2 min (repeated 5 times). Following retrieval, the
slides were maintained at room temperature and were treated with
0.1% protease K to expose the antigen. The sections were
subsequently blocked using 5% bovine serum albumin for 60 min at
room temperature and were incubated with the appropriate primary
antibodies (same as those used for IHC) overnight at 4°C. Following
rewarming at 37°C for 1 h, the sections were washed in PBS for 5
min three times and were further incubated with
fluorescence-conjugated secondary antibodies (donkey
anti-mouse-A488, cat. no. ab150105 and donkey anti-rabbit-Tritc,
cat. no. ab6799; both Abcam) at a 1:800 dilution for 30 min at room
temperature. Following washing with PBS three times, the sections
were stained with 0.001% 4′,6-Diamidino-2-phenylindole
dihydrochloride (Sigma-Aldrich; Merck KGaA) for 10 min at room
temperature to stain the nuclei. The fluorescence-stained target
protein was visualized using an Olympus FV1000 fluorescent
microscope (Olympus corporation; magnification, ×200 and ×400). The
omission of the primary antibodies was used as a negative control
in all IF experiments. Quantification of the ASPP1, ASPP2 and iASPP
expression was performed by measuring the total fluorescence
intensity of the positively stained area.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the frozen tissue
specimens using Total RNA Extractor (Sangon Biotech Co., Ltd.,
Shanghai, China). The RNA was reverse transcribed into cDNA using
an AMV First Strand cDNA Synthesis kit (Sangon Biotech Co., Ltd.).
The mixture of total RNA, random Primer p(dN)6 and
rnase-free ddH2O was bathed at 70°C for 5 min, and then
placed in an 0°C ice bath for 10 sec. Then 5X reaction buffer, dNTP
mix (10 mmol/l), Rnase inhibitor (20 U/l) and AMV reverse
transcriptase (10 U/l) were added into the mixture at 37°C for 5
min, 42°C for 60 min and 70°C for 10 min in order to synthesize the
cDNA. RT-qPCR analysis was performed in a Light Cycler 480 (Roche
Diagnostics, Basel, Switzerland) using SG Fast qPCR Master mix (BBI
Solutions, Cardiff, UK). The GAPDH cDNA was employed as an internal
control for each sample. ASPP expression was normalized using the
2−∆∆Cq method (21). The
40 cycles thermocycling conditions were: 95°C for 7 sec, 55°C for
10 sec and 72°C for 15 sec. All the primers used in RT-qPCR are as
follows: GAPDH forward, 5′-TGGGTGTGAACCATGAGAAGT-3′ and reverse,
5′-TGAGTCCTTCCACGATACCAA-3′; ASPP2 forward,
5′-GTGCTGCCTCATGTAACAACG-3′ and reverse,
5′-GTAGCCTTCCTCCATTTCCTC-3′; ASPP1 forward,
5′-CAGTGTATGGTAAGCCCGTTTT-3′ and reverse,
5′-TGGACAGTGACCCGTGAAGA-3′; and iASPP forward,
5′-TGCCTACCACCATCATCACAT-3′ and reverse,
5′-GACCAATGTTTCCCACCCA-3.
Carcinoembryonic antigen (CEA) and
α-fetoprotein (AFP) assay
The concentrations of plasma CEA and AFP in patient
samples were determined using an ADVIA Centaur XP immunoassay
system (Siemens AG, Munich, Germany).
Statistical analysis
Two independent variables were analyzed using the
Mann-Whitney U test, comparisons among multiple groups were
performed using the Kruskal-Wallis test, and Pearson's correlation
analysis was used to compare the associations. Statistical analyses
were performed using the SPSS Version 16.0 statistical software
package (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Altered ASPP expression in human CRC
tissues
The protein expression of ASPP1, ASPP2 and iASPP was
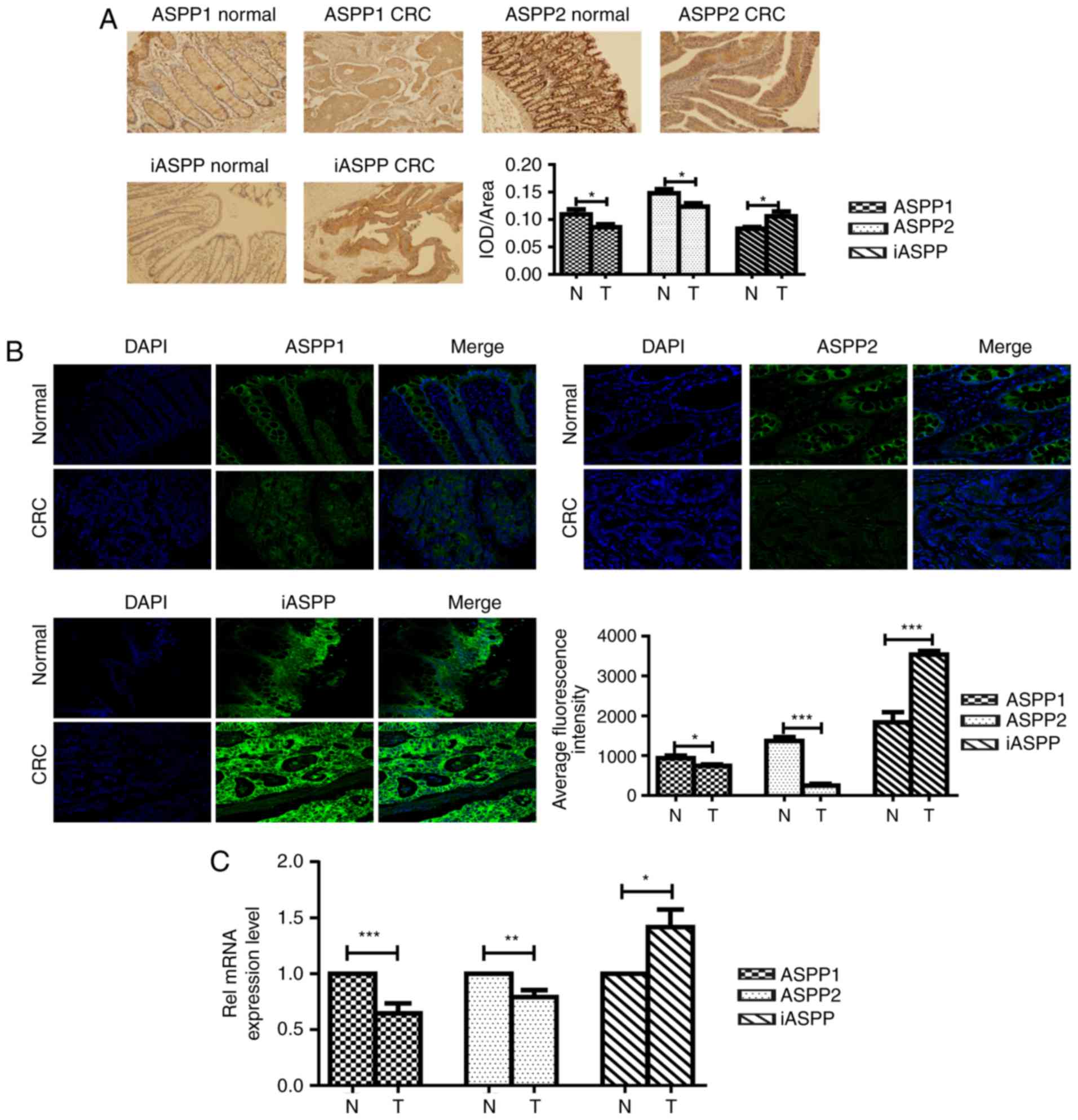
detected in human CRC specimens using IHC (Fig. 1A). The corresponding adjacent
non-cancerous tissues were used as controls (Fig. 1A). The expression of ASPP proteins was
detectable in normal epithelium, lamina propria and glands tissues
in the CRC tissues, as well as the corresponding adjacent
non-cancerous tissues. The results of the present study
demonstrated that ASPPs are distributed in the nucleus and
cytoplasm; however, they were more abundant in the cytoplasm.
Quantification analysis of ASPPs in the IHC samples revealed that
ASPP1 and ASPP2 proteins were expressed at significantly low levels
(P<0.05), while iASPP protein expression was significantly
upregulated (P<0.05) in the CRC samples compared with the
adjacent non-cancerous tissues (Fig.
1A). In order to confirm these observations, the human CRC
samples and the adjacent non-cancerous tissues were further
analyzed using IF staining (Fig. 1B).
IF staining results were consistent with the IHC data, whereby the
expression of ASPP1 and ASPP2 was considerably downregulated and
the expression of iASPP was significantly upregulated in CRC
samples, compared with the adjacent non-cancerous controls
(P<0.05). In order to further evaluate whether alteration in the
protein levels of ASPPs in CRC is associated with RNA deregulation,
the mRNA levels of ASPPs in human CRC samples were examined using
RT-qPCR. As demonstrated in Fig. 1C,
mRNA levels of ASPP1 and ASPP2 were decreased in CRC samples
compared with expression adjacent non-cancerous tissues, whereas
iASPP RNA expression was elevated in CRC tissues (P<0.05).
ASPP1 expression was significantly
decreased in the TP53-positive CRC group
ASPPs are a protein family that regulates the
apoptotic process; ASPP1 and ASPP2 activate apoptosis, while iASPP
inactivates apoptosis. In order to investigate the association
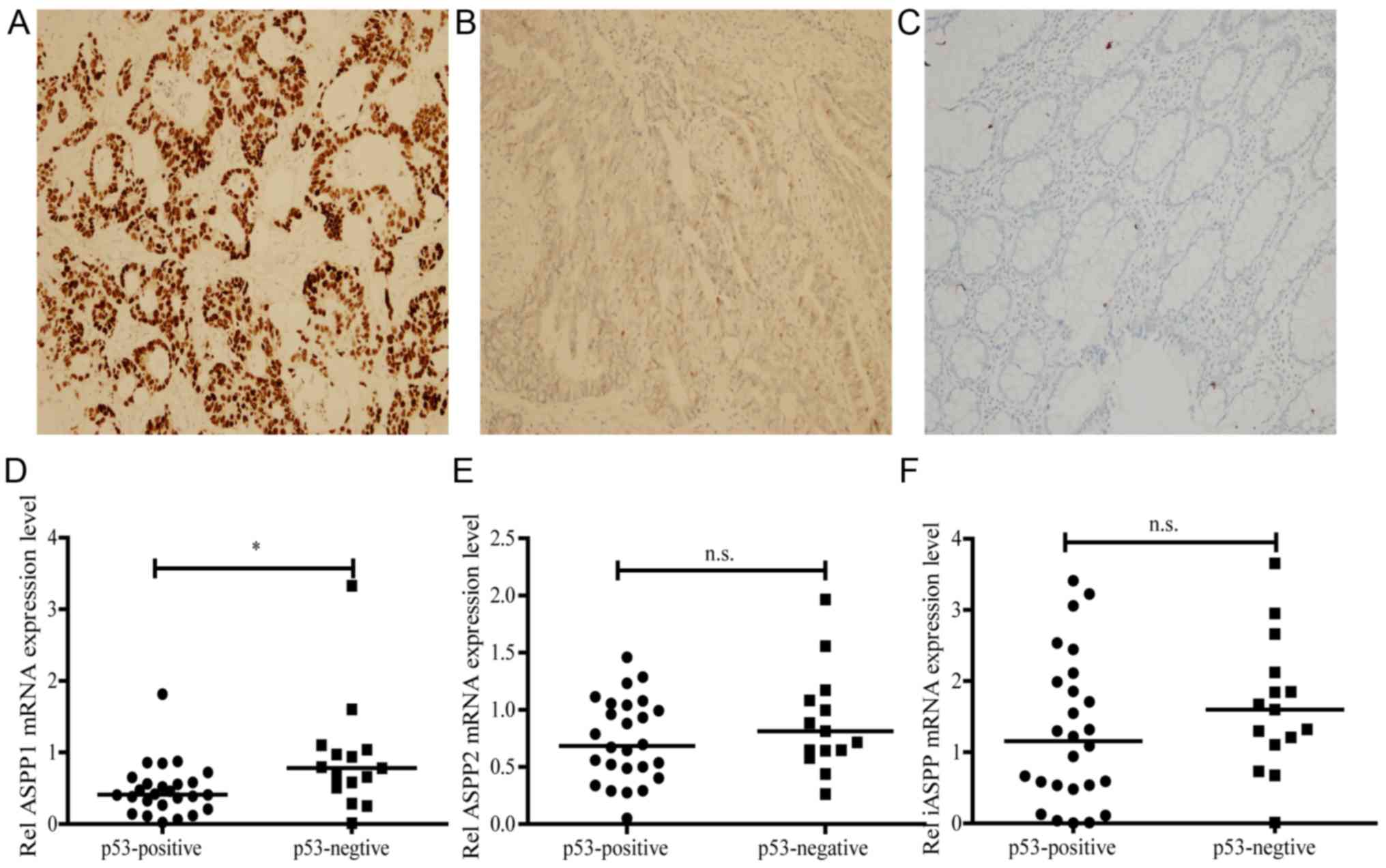
between ASPP and TP53 in human CRC tissues, the human CRC specimens
were divided into TP53-positive (n=15; Fig. 2A) and TP53-negtive (n=26; Fig. 2B) groups by IHC staining. The TP53
level of the corresponding adjacent non-cancerous tissues in IHC
was used as a negative control (Fig.
2C). The ASPP levels were subsequently determined using RT-qPCR
in both the TP53-positive and -negative groups. The results of the
present study demonstrated that, compared with the TP53-negative
group, only the levels of ASPP1 were declined in the TP53-positive
group (P<0.05; Fig. 2D). However,
no significant difference was observed in the expression levels of
ASPP2 and iASPP between the two groups (Fig. 2E and F).
iASPP expression is correlated with
the clinical course, and the size and extent of the primary
tumor
In order to investigate the association between ASPP
expression and disease pathological properties, the human CRC
samples were classified into early (Stage I + II)/advanced (Stage
III + IV) groups, as well as N0/Nx (x≠0) groups, M0/M1 groups,
good/moderate/poor histological grade groups, and colon/rectal
group based upon the Tumor-Node-Metastasis staging criteria of the
American Joint Cancer Committee (22). T1, T2, T3 and T4 were based on the
size and extent of the primary tumor (Table I). The ASPP mRNA expression was
determined by RT-qPCR in these groups (Fig. 1C). ASPP1 and ASPP2 expression did not
exhibit any difference in the advanced stage group and the early
stage group, however the iASPP level was markedly elevated in the
advanced stage group compared with the early stage group (Fig. 3A-C). Furthermore, ASPP1 and ASPP2
expression also had no significant difference but the iASPP
expression exhibited a gradient increase along with the enhancement
of the tumor size and extent, where iASPP expression levels in the
T3 and T4 group were markedly higher than those in the T1 or T2
group (Fig. 3D-F). However, no
considerable changes in ASPP1, ASPP2, and iASPP expression were
observed when CRC patients were classified based on regional lymph
nodes and distant metastasis (Fig.
3G-L).
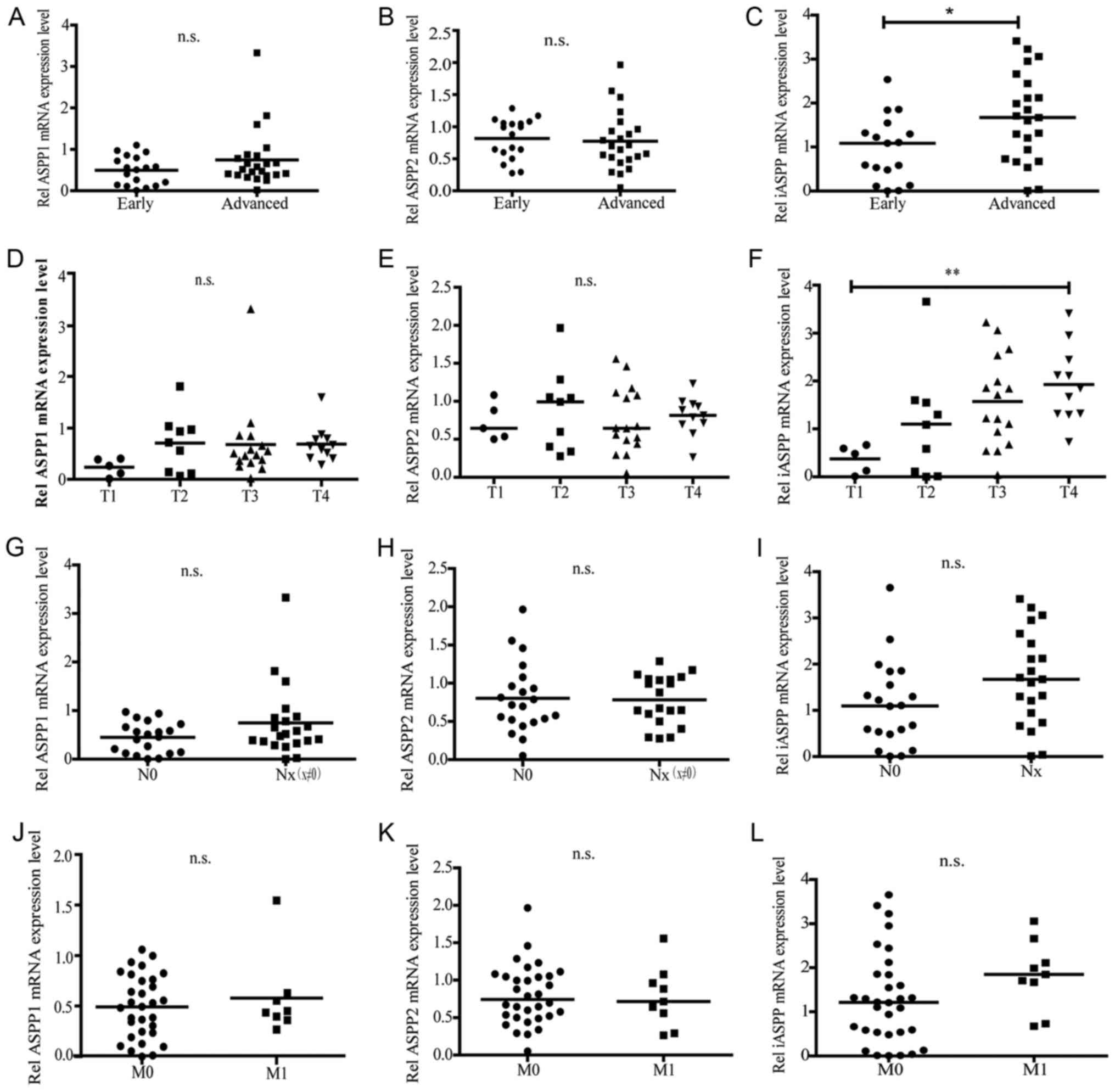 | Figure 3.Associations between ASPP expression
levels and the pathological grade of CRC malignancy, tumor
topography, regional lymph node metastasis or distant metastases.
The CRC samples were classified into multiple groups based on the
different Tumor-Node-Metastasis staging system. The groups were
classified as early or advanced groups based on the clinical
course; T1, T2, T3 or T4 groups based on the on the size and/or
extent of the primary tumor; N0 (negative) and Nx (positive) groups
based on whether the cancer had migrated to the regional lymph
nodes; and M0 (negative) or M1 (positive) based on whether there
were distant metastases. The mRNA expression level of ASPP1, ASPP2
and iASPP were examined by reverse transcription-quantitative
polymerase chain reaction. (A-C) The expression of ASPP1, ASPP2,
iASPP in the early stage group and the advanced stage group. (D-F)
The expression of ASPP1, ASPP2, iASPP in the T1~T4 groups. (G-I)
The expression of ASPP1, ASPP2, iASPP in the N0 and Nx groups.
(J-L) The expression of ASPP1, ASPP2, iASPP in the M0 and M1
groups. *P<0.05; **P<0.01. ASPP, apoptosis-stimulating
protein of p53; CRC, colorectal cancer; n.s., no significant
difference. |
Significant correlation was observed
between plasma CEA levels and ASPP2 mRNA expression in CRC
samples
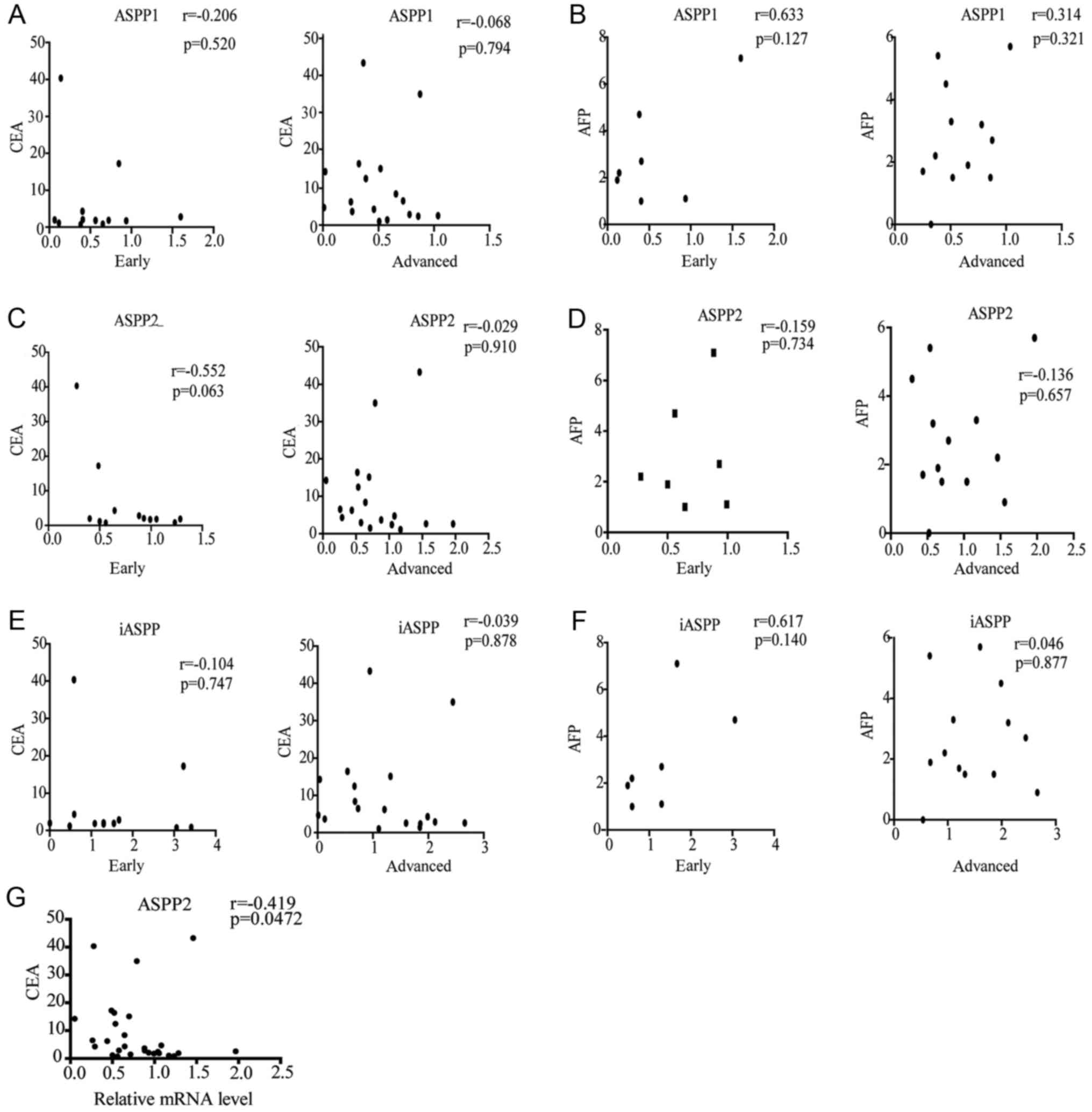
Since the levels of CEA and AFP in the plasma remain
valuable biomarkers for evaluating CRC progression (23–25), the
potential association between ASPP mRNA expression and CEA or AFP
concentrations in the samples was examined in the early and
advanced groups (Fig. 4). No
correlation was identified between the ASPP expression and AFP/CEA
concentrations in the early or advanced groups (Fig. 4A-F). However, the ASPP2 level was
negatively correlated with CEA expression in all the samples
(r=−0.0419; P=0.0472; Fig. 4G).
Although certain changes in ASPP expression were detected in other
observations, these differences were not statistically significant
(data not shown). The mean value of CEA and AFP concentrations from
all CRC tissues were 3.64 (range, 0.74–43.28) and 2.2 (range,
0–7.1) ng/ml, respectively.
Discussion
Previously, the alteration of ASPP expression during
cancer development was reported in a number of tumor types
(17,26,27).
However, few studies have focused on the role of ASPP in CRC
development in either basic or clinical studies. Therefore, the
present study aimed to investigate the role of ASPP in CRC
progression and whether there is an association between ASPP and
the pathology of the disease.
The preliminary data revealed that the protein and
mRNA expression levels of ASPP1 and ASPP2 in human CRC specimens
were significantly decreased compared with the corresponding
adjacent non-cancerous controls. These observations are consistent
with the results of other studies that reported low levels of ASPP
in several tumor types, including breast, lung, non-small cell lung
carcinoma, mesothelioma and leukemia (16,28–31).
Therefore, these results indicated that the decreased expression of
ASPP1 and ASPP2 weakened their anti-oncogene ability, causing the
CRC cells to proliferate abnormally. The results of the present
study demonstrated that the iASPP level in CRC samples was markedly
augmented compared with that in the control samples (Fig. 1). This result is similar to those of
other studies, in that iASPP is upregulated in numerous different
tumors, including human breast carcinomas (32) and acute leukemia (AL) (33). iASPP, as a pro-tumorigenic factor, has
been demonstrated to inhibit TP53 (34). Therefore, the abundant iASPP competed
with the weakened ASPP1/ASPP2 to bind to TP53, resulting in the
inhibition of the pro-apoptotic activity of TP53 and the promotion
of CRC development (35). The
detailed mechanism of the oncogenic cellular proliferation
controlled by ASPPs is well established to be mediated through the
TP53-mediated apoptotic regulation, but not through the
TP53-involved cell cycle arrest (4,35).
TP53 has been reported to exist in wild-type and
mutated forms in healthy mammals, which directly interferes with
its tumor suppressing activity (36).
Mutation of the amino acids, namely 178His, 181Arg, 243Met, 247Arg,
248Arg and 273Arg, in the ASPP2 binding area of TP53 abolished its
anti-oncogenic activity in a crystal structure analysis (36). In fact, these mutation sites are all
frequently reported in human cancer (36). Notably, the mutated residues of 248Arg
and 273Arg are involved in DNA and ASPP2 binding. A total of 5–21%
of cancer patients harbor the mutated TP53 protein (8). Therefore, the presence and type of TP53
mutation is considered in patients with cancer, and whether it
affects TP53 expression. It may be useful to determine whether or
not ASPP expression is associated with the activity status of TP53
in human CRC. A study undertaken by Mori et al (17) was unable to identify any association
between ASPP expression and TP53 status in lung cancer cell lines.
Li et al (16) demonstrated
that lower expression levels of ASPP1 and ASPP2 in non-small cell
lung cancer (NSCLC) tumor tissues were more significantly
associated with wild-type TP53 than with mutant TP53.
In the present study, ASPP expression was analyzed in the
TP53-positive group (a sample was considered TP53-positive if TP53
expression rate was >30% compared with the healthy control using
IHC analysis) and the TP53-negative group (TP53 expression rate
≤30% compared with the healthy control). The results demonstrated
that the ASPP1 expression level was significantly lower in the
TP53-positive group than in the TP53-negative group (Fig. 2D). This result was identical to the
observations made by Li et al (16) in NSCLC tumor tissues. No notable
changes were observed in the expression of either ASPP2 or iASPP
(Fig. 2E and F). These data suggested
that the anti-oncogenic capability of the patients with CRC was
adjusted through the homeostatic system following the aberrant
proliferation of the CRC cells. Therefore, the higher expression of
ASPP1 in patients with negative TP53 may be triggered in order to
resist the pro-oncogenic forces. Since the antibody that was
utilized in the IHC staining in the present study was unable to
distinguish the wild-type from the mutated form of TP53, it is
difficult to differentiate between the two and to analyze each one
independently. However, the pro-oncogenic activity of TP53
is controlled by ASPP1 or ASPP2; therefore, the declined ASPP1
expression will primarily affect the pro-oncogenic ability of
TP53 in this type of CRC.
A study undertaken by Zhang et al (34) revealed that iASPP expression was
markedly higher in the blood cells of patients with acute leukemia
(AL) than in those of healthy donors or patients with AL who had
achieved complete remission. This observation suggested that iASPP
may be employed as a marker of disease progression in AL (33). In addition, increased iASPP expression
was also associated with a poor prognosis in FIGO IA2-IIA cervical
adenocarcinoma (19). Low ASPP2
expression was reported to correlate with a poor prognosis in
patients with diffuse large B-cell lymphoma (17) or breast cancer (26). Furthermore, metastatic breast cancer
was revealed to be associated with a decreased ASPP2 expression
compared with non-metastatic breast cancer (37). In order to observe whether or not the
alteration of ASPP expression was associated with prognosis in
patients with CRC, human CRC samples were divided into early and
advanced groups based on the clinical course, N0/Nx (x≠0) groups,
M0/M1 groups, good/moderate/poor histological grade groups, and
colon/rectal groups based on TNM grading system, and the
histological grade and tumor location. Notably, upregulated iASPP
expression was revealed to be associated with the grade of
malignancy in patients with CRC (Fig. 3C
and F), but was not associated with metastasis to the regional
lymph nodes (Fig. 3I), distant
metastasis (Fig. 3L) or tumor
location (data not shown). No significant association was observed
between the expression of ASPP1 or that of ASPP2 in any of the
groups.
In healthy patients, CEA is expressed at very low
levels; however, certain types of cancer have been reported to have
elevated levels of CEA in the serum. CEA, as a cell
surface-anchored glycoprotein, is a functional ligand in colon
carcinoma L-selectin and E-selectin, which may be involved in the
metastatic dissemination of colon carcinoma cells (38–40). In
the present study, no correlation was identified between plasma CEA
concentration and APSS expression in early or advanced human CRC
groups (Fig. 4). However, the
alteration in the expression of ASPP2 in the entire CRC sample
population exhibited a negative correlation with CEA concentration
(Fig. 4G). This observation indicated
that ASPP2 may be a valuable biomarker for CRC progression and a
novel biomarker for serological cancer. It may be possible to make
a preliminary diagnosis by detecting the level of ASPP2 expression
in the serum. Additionally, in FIGO IA2-IIA cervical
adenocarcinoma, the increased expression of iASPP was associated
with a poor prognosis (26) and, in
gestational trophoblastic disease, the downregulation of ASPP1 was
correlated with clinical outcome (41). However, it is unclear if ASPP2 is
correlated with clinical outcome and further investigation is
required.
AFP is a major plasma protein during fetal
development. Since it was revealed to be abnormally increased in
certain types of tumor, AFP is frequently used as a diagnostic
marker for several cancer types, including hepatocellular
carcinomas (42), hepatoblastoma
(43) and yolk sac tumors (44). The majority of tumors with increased
AFP originate from the stomach, bile duct or pancreas (45), and upregulation of AFP is extremely
rare in CRC due to the fact that the colorectum is initiated from
the hindgut endoderm. As of yet, only 11 cases of CRC with
increased-AFP have been reported in the early stages without
distant metastases (45). The results
of the present study demonstrated no correlation between AFP
concentration and ASPP expression in early or advanced cases of CRC
(Fig. 4). This may be attributed to
the small sample size but, in order to validate these observations,
a larger sample size is required.
In conclusion, the results of the present study
confirmed that ASPP1 and ASPP2 expression were reduced, while iASPP
expression was elevated in human CRC specimens, compared to the
corresponding adjacent non-cancerous tissues. In addition, the
upregulated iASPP may be used as a valuable biomarker for the grade
of malignancy in human CRC. Finally, it was revealed that the
upregulation of AFP is accompanied with an increase in iASPP
expression in the early CRC group, compared with the advanced CRC
group. These observations not only enrich the epidemic and clinical
data of human CRC, but also provide valuable information for the
development of novel drugs for the treatment of CRC, and for the
improvement of CRC diagnosis, prognosis and therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 30972610 and 81273240),
Jilin Province Science and Technology Agency (grant nos.
20160101037JC, 20170622009JC, 2017C021 and 2017J039), Norman
Bethune Program of Jilin University (2012206), The fund of the
State Key Laboratory of Kidney Diseases in PLA General
Hospital.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
LW designed the experiment. LY, YL and XW performed
the experiments and LY wrote the paper. YS, HH and CL processed the
data. YJ participated in all of these processes, and revised the
paper and approved the final version to be published.
Ethics and consent to participate
The study research proposal was approved by the
Medical Ethics Committee of the First Hospital of Jilin University,
and written informed consent was obtained from each patient.
Consent for publication
All participants provided written informed consent
for the publication of the data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shah TA: Colonic cancer; presentation and
management in a surgical unit at allied hospital faisalabad. Prof
Med J. 23:251–256. 2016.
|
|
3
|
Croce CM: Oncogenes and cancer. N Engl J
Med. 358:502–511. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trigiante G and Lu X: ASPP [corrected] and
cancer. Nat Rev Cancer. 6:217–226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chao C, Saito S, Kang J, Anderson CW,
Appella E and Xu Y: p53 transcriptional activity is essential for
p53-dependent apoptosis following DNA damage. EMBO J. 19:4967–4975.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vousden KH and Lu X: Live or let die: The
cell's response to p53. Nat Rev Cancer. 2:594–604. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Starks AM, Martin DN, Dorsey TH, Boersma
BJ, Wallace TA and Ambs S: Household income is associated with the
p53 mutation frequency in human breast tumors. PLoS One.
8:e573612013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soussi T, Kato S, Levy PP and Ishioka C:
Reassessment of the TP53 mutation database in human disease by data
mining with a library of TP53 missense mutations. Hum Mutat.
25:6–17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vazquez A, Bond EE, Levine AJ and Bond GL:
The genetics of the p53 pathway, apoptosis and cancer therapy. Nat
Rev Drug Discov. 7:979–987. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi Y, Han Y, Xie F, Wang A, Feng X, Li N,
Guo H and Chen D: ASPP2 enhances oxaliplatin (L-OHP)-induced
colorectal cancer cell apoptosis in a p53-independent manner by
inhibiting cell autophagy. J Cell Mol Med. 19:535–543. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mak VC, Lee L, Siu MK, Wong OG, Lu X, Ngan
HY, Wong ES and Cheung AN: Downregulation of ASPP2 in
choriocarcinoma contributes to increased migratory potential
through Src signaling pathway activation. Carcinogenesis.
34:2170–2177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schittenhelm MM, Illing B, Ahmut F, Rasp
KH, Blumenstock G, Döhner K, Lopez CD and Kampa-Schittenhelm KM:
Attenuated expression of apoptosis stimulating protein of p53-2
(ASPP2) in human acute leukemia is associated with therapy failure.
PLoS One. 8:e801932013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song B, Bian Q, Zhang YJ, Shao CH, Li G,
Liu AA, Jing W, Liu R, Zhou YQ, Jin G and Hu XG: Downregulation of
ASPP2 in pancreatic cancer cells contributes to increased
resistance to gemcitabine through autophagy activation. Mol Cancer.
14:1772015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng WD, Chu RX, Wang BZ, Wang LP, Ma LL
and Wang LX: Infection à Helicobacter pylori et les expressions de
l'apoptose liée à p53, PAES et PPEEE dans le cancer gastrique et
des lésions précancéreuses. Pathol Biol. 61:199–202. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li S, Shi G, Yuan H, Zhou T, Zhang Q, Zhu
H and Wang X: Abnormal expression pattern of the ASPP family of
proteins in human non-small cell lung cancer and regulatory
functions on apoptosis through p53 by iASPP. Oncol Rep. 28:133–140.
2012.PubMed/NCBI
|
|
17
|
Mori T, Okamoto H, Takahashi N, Ueda R and
Okamoto T: Aberrant overexpression of 53BP2 mRNA in lung cancer
cell lines. FEBS Lett. 465:124–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lossos IS, Natkunam Y, Levy R and Lopez
CD: Apoptosis stimulating protein of p53 (ASPP2) expression differs
in diffuse large B-cell and follicular center lymphoma: Correlation
with clinical outcome. Leuk Lymphoma. 43:2309–2317. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu B, Guo H, Zhao J, Wang C, Wu G, Pang M,
Tong X, Bu F, Liang A, Hou S, et al: Increased expression of iASPP,
regulated by hepatitis B virus X protein-mediated NF-κB activation,
in hepatocellular carcinoma. Gastroenterology. 139:2183–2194.e5.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim KH, Yang SS, Yoon YS, Lim SB, Yu CS
and Kim JC: Validation of the seventh edition of the American joint
committee on cancer tumor-node-metastasis (AJCC TNM) staging in
patients with stage II and stage III colorectal carcinoma: Analysis
of 2511 cases from a medical centre in Korea. Colorectal Dis.
13:e220–e226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sgroi DC, Teng S, Robinson G, LeVangie R,
Hudson JR Jr and Elkahloun AG: In vivo gene expression profile
analysis of human breast cancer progression. Cancer Res.
59:5656–5661. 1999.PubMed/NCBI
|
|
23
|
Anzai H, Kazama S, Kiyomatsu T, Nishikawa
T, Tanaka T, Tanaka J, Hata K, Kawai K, Yamaguchi H, Nozawa H, et
al: Alpha-fetoprotein-producing early rectal carcinoma: A rare case
report and review. World J Surg Oncol. 13:1802015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michl M, Stintzing S, von Weikersthal
Fischer L, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE,
Heintges T, Lerchenmueller C, Kahl C, et al: CEA response is
associated with tumor response and survival in patients with KRAS
exon 2 wild-type and extended RAS wild-type metastatic colorectal
cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab
(FIRE-3 trial). Ann Oncol. 27:1565–1572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huo YR, Glenn D, Liauw W, Power M, Zhao J
and Morris DL: Evaluation of carcinoembryonic antigen (CEA) density
as a prognostic factor for percutaneous ablation of pulmonary
colorectal metastases. Eur Radiol. 27:128–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kong F, Shi X, Li H, Li P, Yu J, Li X,
Chen J, Sun Y and Jia Y: Increased expression of iASPP correlates
with poor prognosis in FIGO IA2-IIA cervical adenocarcinoma
following a curative resection. Am J Cancer Res.
5:12172015.PubMed/NCBI
|
|
27
|
Cobleigh MA, Tabesh B, Bitterman P, Baker
J, Cronin M, Liu ML, Borchik R, Mosquera JM, Walker MG and Shak S:
Tumor gene expression and prognosis in breast cancer patients with
10 or more positive lymph nodes. Clin Cancer Res. 11:8623–8631.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vives V, Su J, Zhong S, Ratnayaka I, Slee
E, Goldin R and Lu X: ASPP2 is a haploinsufficient tumor suppressor
that cooperates with p53 to suppress tumor growth. Genes Dev.
20:1262–1267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Samuels-Lev Y, O'Connor DJ, Bergamaschi D,
Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T
and Lu X: ASPP proteins specifically stimulate the apoptotic
function of p53. Mol Cell. 8:781–794. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZJ, Lu X, Zhang Y, Zhong S, Gu SZ,
Zhang XB, Yang X and Xin HM: Downregulated mRNA expression of ASPP
and the hypermethylation of the 5′-untranslated region in cancer
cell lines retaining wild-type p53. FEBS Lett. 579:1587–1590. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mori S, Ito G, Usami N, Yoshioka H, Ueda
Y, Kodama Y, Takahashi M, Fong KM, Shimokata K and Sekido Y: p53
apoptotic pathway molecules are frequently and simultaneously
altered in nonsmall cell lung carcinoma. Cancer. 100:1673–1682.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sullivan A and Lu X: ASPP: A new family of
oncogenes and tumour suppressor genes. Br J Cancer. 96:196–200.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bergamaschi D, Samuels Y, O'Neil NJ,
Trigiante G, Crook T, Hsieh JK, O'Connor DJ, Zhong S, Campargue I,
Tomlinson ML, et al: iASPP oncoprotein is a key inhibitor of p53
conserved from worm to human. Nat Genet. 33:162–167. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Wang M, Zhou C, Chen S and Wang
J: The expression of iASPP in acute leukemias. Leuk Res.
29:179–183. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu ZJ, Zhang Y, Zhang XB and Yang X:
Abnormal mRNA expression of ASPP members in leukemia cell lines.
Leukemia. 18:8802004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lettre G, Kritikou EA, Jaeggi M, Calixto
A, Fraser AG, Kamath RS, Ahringer J and Hengartner MO: Genome-wide
RNAi identifies p53-dependent and -independent regulators of germ
cell apoptosis in C. elegans. Cell Death Differ. 11:1198–1203.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gorina S and Pavletich NP: Structure of
the p53 tumor suppressor bound to the ankyrin and SH3 domains of
53BP2. Science. 274:1001–1005. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Denoix PF: Enquete permanent dans les
centres anticancereaux. Bull Inst Natl Hyg. 1:701946.
|
|
39
|
Thomas SN, Zhu F, Schnaar RL, Alves CS and
Konstantopoulos K: Carcinoembryonic antigen and CD44 variant
isoforms cooperate to mediate colon carcinoma cell adhesion to E-
and L-selectin in shear flow. J Biol Chem. 283:15647–15655. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Konstantopoulos K and Thomas SN: Cancer
cells in transit: The vascular interactions of tumor cells. Annu
Rev Biomed Eng. 11:177–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mak VC, Lee L, Siu MK, Wong OG, Lu X, Ngan
HY, Wong ES and Cheung AN: Downregulation of ASPP1 in gestational
trophpblastic disease: Correlation with hypermethylation, apoptopic
activity and clinical outcome. Mod Pathol. 24:522–532. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thomas SN, Tong Z, Stebe KJ and
Konstantopoulos K: Identification, characterization and utilization
of tumor cell selectin ligands in the design of colon cancer
diagnostics. Biorheology. 46:207–225. 2009.PubMed/NCBI
|
|
43
|
O'Conor GT, Tatarinov YS, Abelev GI and
Uriel J: A collaborative study for the evaluation of a serologic
test for primary liver cancer. Cancer. 25:1091–1098. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Smith CJ, Ajdukiewicz A and Kelleher PC:
Concanavalin-A-affinity molecular heterogeneity of human hepatoma
AFP and cord-serum AFP. Ann N Y Acad Sci. 417:69–74. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Norgaard-Pedersen R, Albrechtsen R and
Teilum G: Serum alpha-foetoprotein as a marker for endodermal sinus
tumour (yolk sac tumour) or a vitelline component of
‘teratocarcinoma’. Acta Pathol Microbiol Scand A. 83:573–589.
1975.PubMed/NCBI
|