Introduction
Lung cancer is the leading cause of cancer mortality
amongst males globally, and has surpassed breast cancer as the
leading cause of cancer mortality amongst females in developed
countries (1). The 5-year survival
rate has remained poor, at ~17%, over previous decades despite
multiple novel aggressive therapies (2). Furthermore, the majority of patients are
diagnosed whilst in the advanced stages of the disease, due to a
lack of clinical presentation within the early stages. Thus, novel
targets and approaches are required to improve the treatment and
diagnosis of lung cancer.
Recently, the human microbiome has been an active
area of research. A previous study demonstrated that the human
intestine contains ~100 trillion microorganisms, including
~500-1,000 different species (3).
Although the gastrointestinal tract is the major colony site for
these microorganisms, other sites of colonization include the oral
cavity, the respiratory tract, the urogenital tract and the skin.
At present, these complex microbiomes remain incompletely
characterized. However, many studies have focused on the
association between the microbiome and cancer, including
Helicobacter pylori and gastric carcinoma (4), Epstein-Barr virus and nasopharyngeal
carcinoma (5), and Human
papillomavirus and cervical cancer (6). The majority of the earlier studies focus
on a single pathogenic bacteria or virus.
Developments in metagenomics including 16S rRNA gene
pyrosequencing techniques and bioinformatics (7) have assisted in providing an
understanding of the role of the human microbiome within cancer. In
the oral cavity, changes in the level of microorganisms are
considered to be associated with the development of oral cancer
(8). In the pancreatic cancer, the
levels of Neisseria elongata and Streptococcus mitis
in the saliva have been revealed to be increased in patients with
pancreatic cancer, and may become the microbial biomarkers for
pancreatic cancer (9). In the colon,
certain bacterial biofilms were associated with colorectal cancer,
and fecal microbiota may be a potential target for early-stage
detection methods for colorectal cancer (10,11). In
the respiratory tract, microbiota is associated with many lung
diseases, including chronic obstructive pulmonary disease (COPD)
(12,13), asthma (14) and lung cancer (15). These studies demonstrated that the
human microbiome is associated with cancer.
Numerous studies have indicated that inflammation is
an important factor in the promotion of carcinogenesis in pulmonary
adenocarcinoma (16,17). Additionally, recent studies revealed
that the microbiome serves an important role in the regulation of
inflammation through numerous mechanisms, such as influencing the
metabolism of short-chain fatty acids, which were recognized to be
beneficial to the host. Furthermore, the level of inflammation of
the host may be reduced when imbalances within the microbiome are
reversed (18,19). A previous epidemiological study
demonstrated that ~16% of types of human cancer worldwide were
associated with infectious agents or infection-associated chronic
inflammation, with a higher percentage in developing countries, at
22.9%, compared with developed countries, at 7.4% (20).
Understanding the role of the microbiome in lung
cancer is important to identify novel targets and approaches for
the treatment and diagnosis of the disease. In the present study, a
urethane-induced pulmonary adenocarcinoma model was used to explore
the roles of the microbiomes in the lower respiratory and
intestinal tracts, and of inflammation, determined by measuring the
levels of inflammatory cytokines nuclear factor κB (NF-κB), tumor
necrosis factor α (TNF-α), interleukin-1β (IL-1β) and IL-6 present
in lung adenocarcinoma. Additionally, prebiotics were used to alter
the microbiome and levels of inflammation in urethane-induced
pulmonary adenocarcinoma. The result revealed that inflammation and
the microbiome may serve an important role in the carcinogenesis of
lung cancer, and that prebiotics may assist the function of
treatments of lung cancer by modulating the microbiome and the
inflammatory response.
Materials and methods
Animals, groups and sample
collection
A total of 15 6-week-old male BALB/c mice, weighing
18–22 g, were sourced from Vital River Laboratories Co., Ltd.,
(Beijing, China), and were randomly distributed into 3 groups with
access to tap water and an unrestricted diet. Each group included 5
animals in 1 cage. The environment was maintained at 19–22°C, with
40–60% humidity and a standard 12 h day/night rhythm. The animals
were given 2 weeks to adapt to the novel environment.
All experiments (performed once) included: A control
group (C); a urethane-induced adenocarcinoma group (U) and a
prebiotics-gavage intervention group (P). The cases of pulmonary
adenocarcinoma were induced by intraperitoneal (i.p.) injection of
1 g/kg dose of urethane in 100 µl saline once per week for 8 weeks.
The control group was injected i.p. with 100 µl saline, as
described previously (21,22). Group P was treated intragastrically
with 15 mg/kg prebiotics 5 times/week continuously for 16 weeks,
beginning at the 5th week subsequent to the first injection of the
urethane, whilst groups C and U were treated with equivalent
saline. The prebiotics in this experiment were: Dietary fiber
(extracted from endive and konjak), Cordyceps, Ginseng,
Ganoderma lucidum, Seaweed, Hericium and Lycium
barbarum polysaccharides.
All of the mice were sacrificed (via exsanguination
following anesthesia with 5% isoflurane) at the end of the 20th
week after the first injection of urethane. Fresh stool and sera
samples were collected and stored at −80°C prior to sacrifice. The
bronchoalveolar lavage (BAL) procedure was performed, as described
in previous studies (22,23). All BAL samples were stored at −80°C,
and the lung tissue was fixed in 10% paraformaldehyde for
histopathological analyses.
Serum inflammation measurements
NF-κB, TNF-α, IL-1β and IL-6 levels in serum were
detected through a mouse (NF-κB, TNF-α, IL-1β and IL-6) ELISA kit
(Elabscience Biotechnology Co., Ltd., Wuhan, China). The optical
density was read at 450 nm using a microtiter plate reader.
DNA extraction
Extraction of the DNA from 180–220 mg of the fresh
stool samples using the TIANamp Stool DNA kit (DP328; TIANGEN
Biotech Co., Ltd., Beijing, China) was carried out according to
protocol of the manufacturer. However, 400 g of the BAL samples
were first thawed and centrifuged for 5 min at room temperature and
400 × g, and the supernatant was collected. The supernatant samples
were then centrifuged at 13,210 × g for 5 min at room temperature
again. Subsequently, the bacterial DNA from the BAL samples was
extracted using the TIANamp Micro DNA kit (DP316; TIANGEN Biotech
Co., Ltd.) according to protocol outlined previously (23). All DNA quality was assessed using gel
electrophoresis and spectrophotometry, and was stored at −80°C
prior to use.
16S sequencing and bioinformatics
16S rRNA (V4) sequencing was carried out to analyze
the changes in the microbiome in accordance with protocol described
in a previous study (24). The
sequences were quality-checked and clustered into de novo
operational taxonomic units (OTUs) at the 97% similarity threshold
for the production of OTUs using UPARSE software (25, http://drive5.com/uparse/). The most abundant sequence
in each OTU was chosen as the representative sequence. UCHIME
software was used to remove chimeric sequences (26). Outliers with a low sequence count and
microbial diversity were removed. Mothur software (version 1.35.1;
https://www.mothur.org/) was then used to
calculate indices of Shannon diversity and richness (27). The Ribosomal Database Project (RDP)
Classifier software (version 16; https://rdp.cme.msu.edu/) was used to classify
sequences.
Statistical analysis
The data was analyzed using a 2-sample t-test and
SPSS 14.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. Two
sample t-tests were performed to identify significant differences
between families and OTUs between each group, and data are
presented as the mean ± standard deviation.
Results
Constructing and identifying the model
of lung cancer
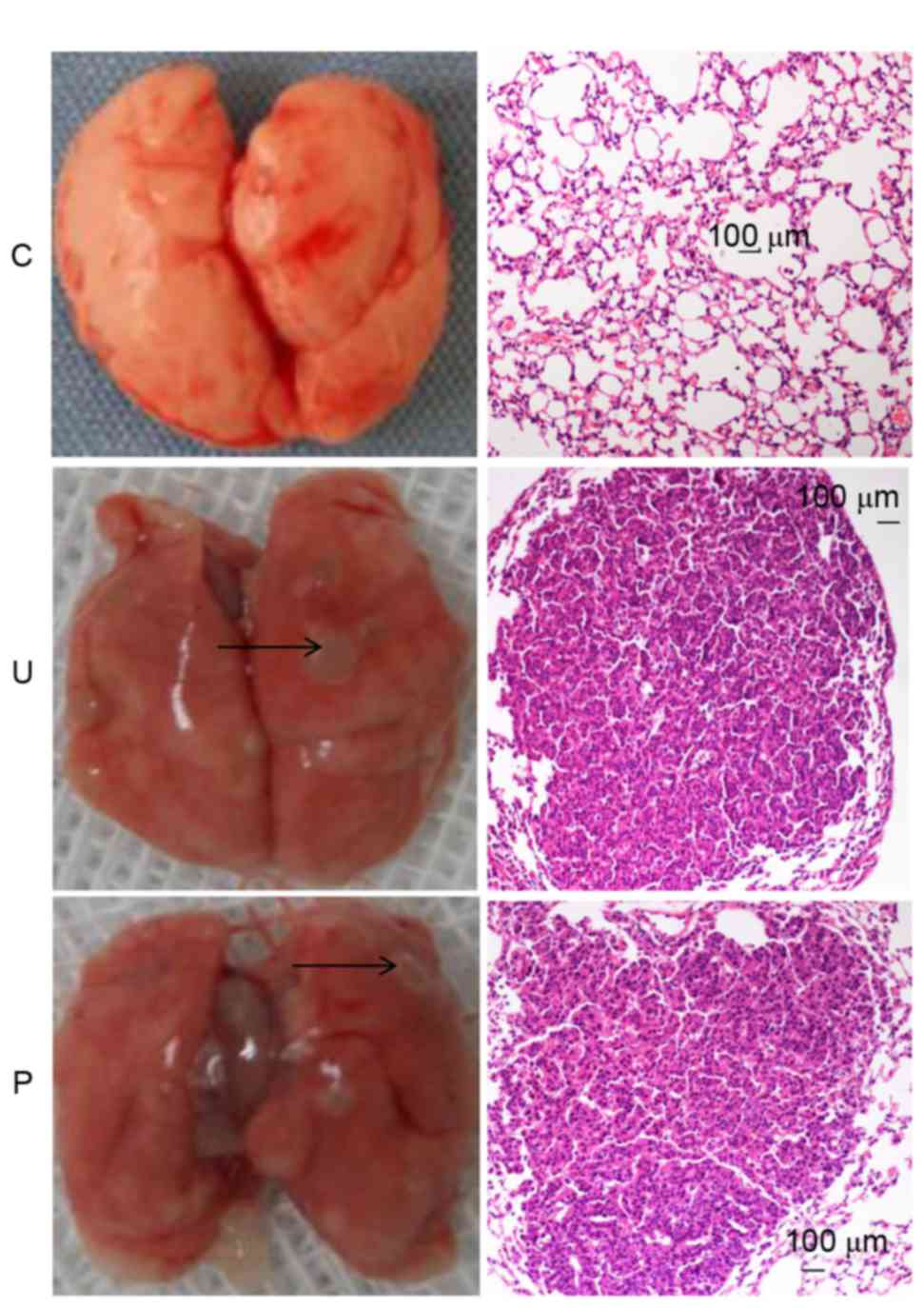
As demonstrated in Fig.
1, at the end of the 20th week, subsequent to the first
injection of urethane, each mouse in groups U and P displayed
altered histopathology and the development of lung adenocarcinoma
tumors, whilst the control group did not.
Prebiotics inhibited urethane-induced
elevation of the level of inflammation (NF-κB, TNF-α, IL-1β, IL-6)
in serum
The levels of the inflammatory markers NF-κB, TNF-α,
IL-1β and IL-6 were markedly higher in groups U and P than in the
control group after urethane-induced treatment, as illustrated in
Fig. 2. When the urethane-induced
adenocarcinoma mice were treated with prebiotics, the levels of
these inflammatory markers markedly decreased to similar levels
observed within the control.
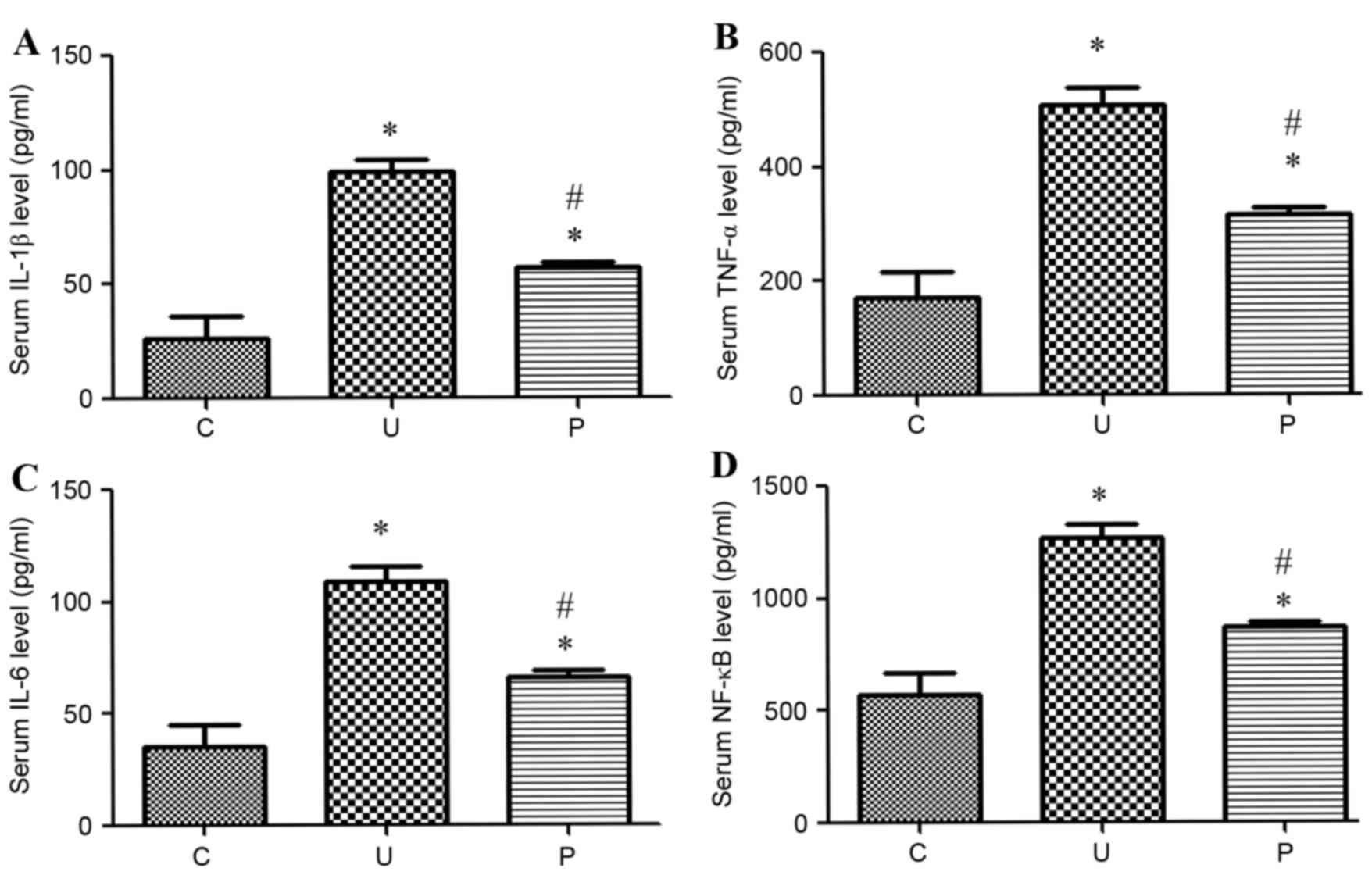 | Figure 2.Levels of inflammation (pg/ml) in
sera in each group (n=5 for each group). The levels of inflammatory
markers (A) IL-1β, (B) TNF-α, (C) IL-6 and (D) NF-κB were
significantly higher in both groups U (P<0.0001, <0.0001,
<0.0001 and <0.0001 respectively) and P (P<0.0001,
<0.0002, <0.0001 and 0.0002 respectively) than in the control
group C. The level of inflammatory markers was significantly
decreased in group P compared with group U (P<0.0001,
<0.0001, <0.0001 and <0.0001 respectively). Data are
presented as the mean ± standard deviation. *P<0.05 vs. group C.
#P<0.05 vs. group U. IL-1β, Interleukin-1β; TNF-α,
tumor necrosis factor α; NF-κB, nuclear factor κB; C, control
group; U, urethane-induced adenocarcinoma group; P,
prebiotics-gavage intervention group. |
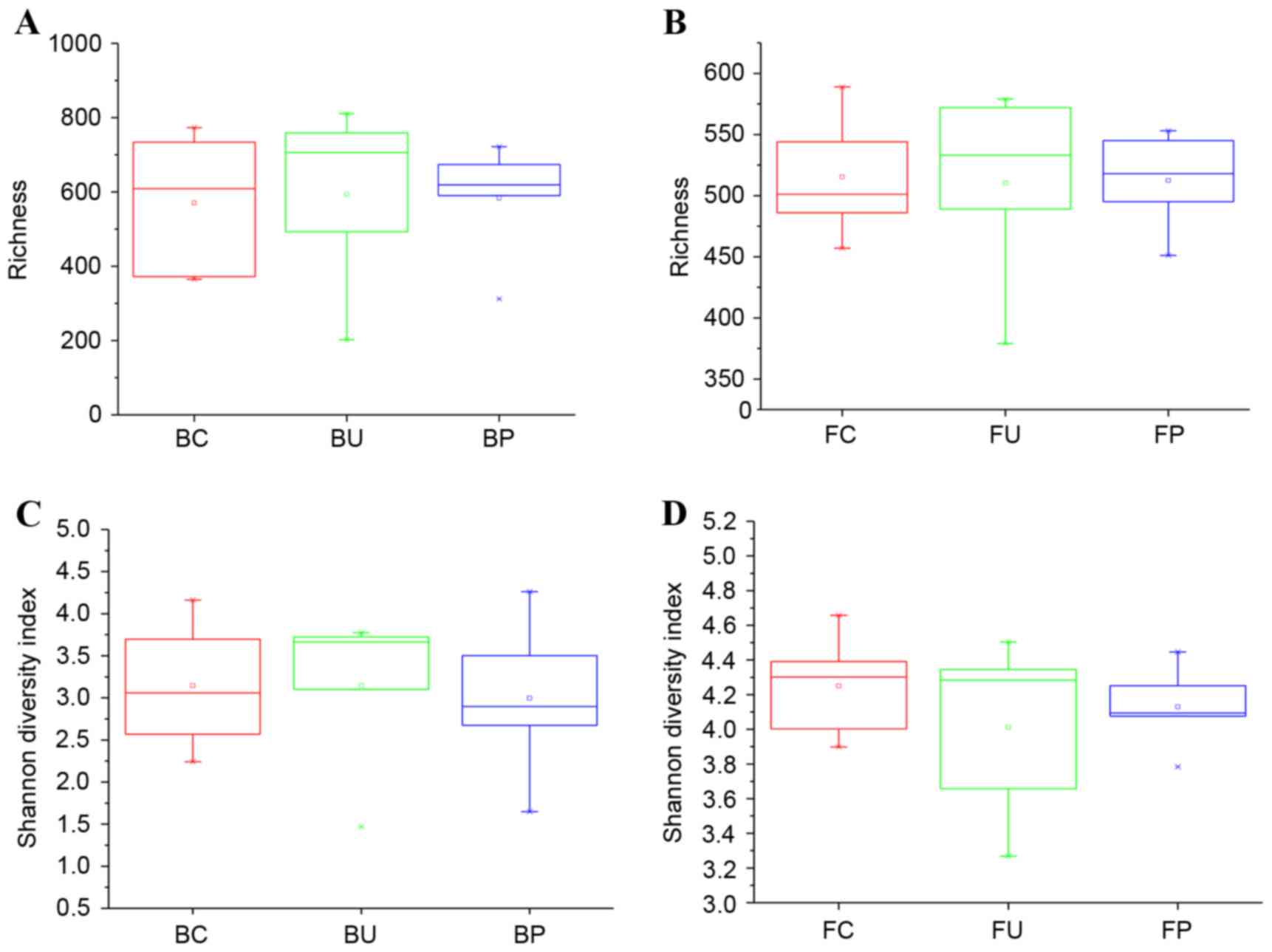
Richness and diversity of microbiome
in lung and intestine of mice
To investigate the change in the composition of the
microbiome, the bacterial richness and Shannon diversity indices of
each group were calculated and compared for the lung tissues, with
samples obtained from BAL, and for the intestinal tissues, with
samples obtained from feces. The results are demonstrated in
Fig. 3A-D.
In the lower airways, the mean richness values were
570.6±194.2, 594.2±250.4, 583.4±160.0 for groups C, U and P,
respectively, and were not statistically significant as illustrated
in (Fig. 3A) (U vs. C; P=0.8719; P
vs. C, P=0.9122; P vs. U, P=0.9372). In the intestinal tract, the
mean richness values which were 515.4±51.8, 510.4±81.8, 512.4±41.3
for groups C, U and P, respectively, and were also not
significantly different, as demonstrated in Fig. 3B (U vs. C; P=0.9109; P vs. C,
P=0.9218; P vs. U, P=0.9623). In the aforementioned 3 groups,
differences in the Shannon diversity indices were not statistically
significant in the lower respiratory or the intestinal tracts, as
demonstrated in Fig. 3C (U vs. C;
P=0.9978; P vs. C, P=0.7978; P vs. U; P=0.8837) and Fig. 3D (U vs. C; P=0.4056; P vs. C,
P=0.5106; P vs. U, P=0.6586).
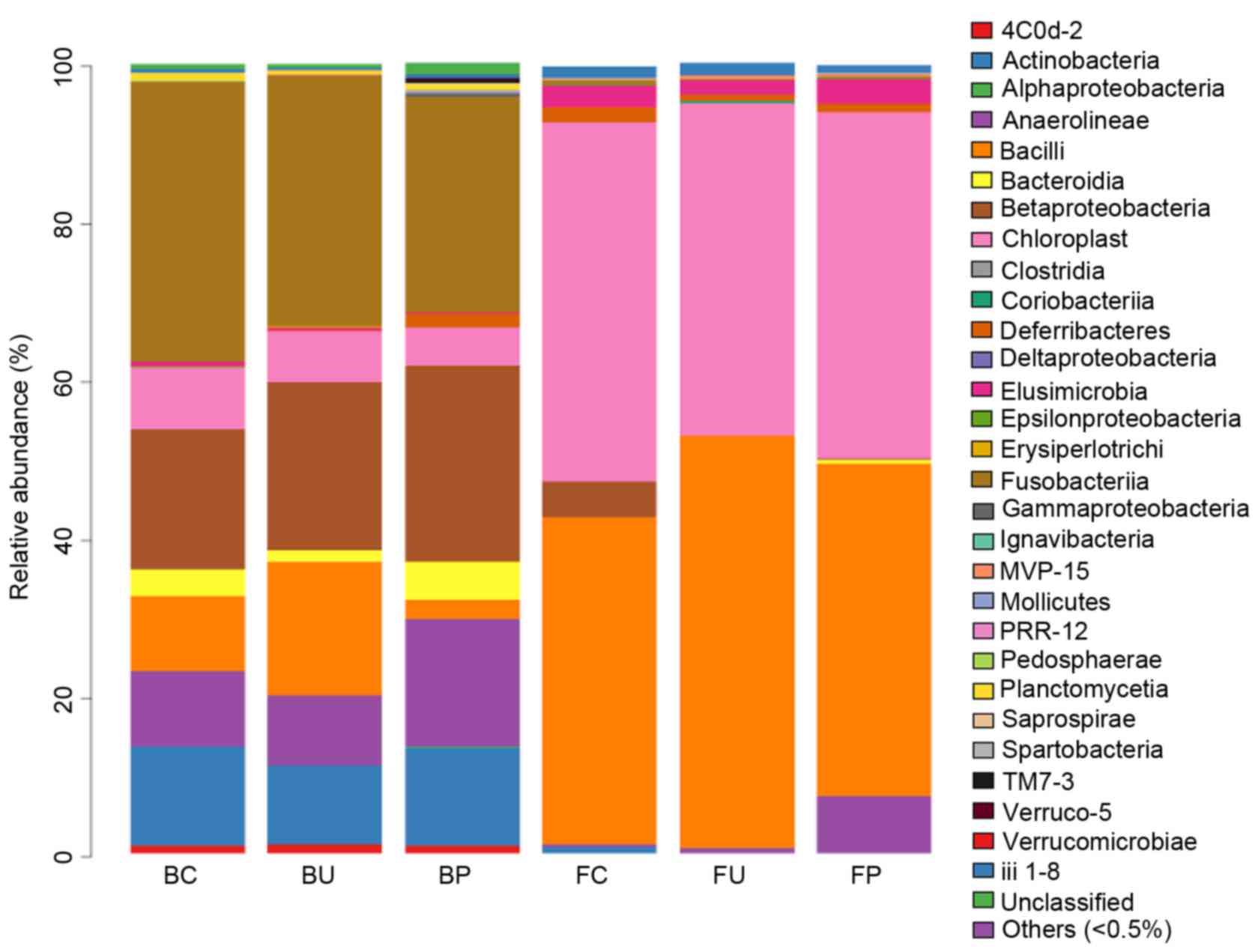
Composition of microbial communities
at the class level
A total of 30 classes of bacteria were identified
using the RDP classifier. Gammaproteobacteria, Chloroplasts,
Alphaproteobacteria, Bacteroidia, Bacilli, Clostridia and
Betaproteobacteria were the most abundant classes in BAL, and
occupied 94.9% of the microbiota in the 3 BC, BU and BP groups, as
illustrated in Fig. 4. No significant
difference was observed in the types of classes of bacteria between
the groups C, P and U in the lower respiratory tract microbiome. In
the intestinal tract, Clostridia and Bacteroidia were the most
abundant types of class, and occupied 88.99% of the microbiota in
the 3 (FC, FU and FP) groups. No significant variation in the types
of classes of bacteria was observed between the 3 groups in these
samples.
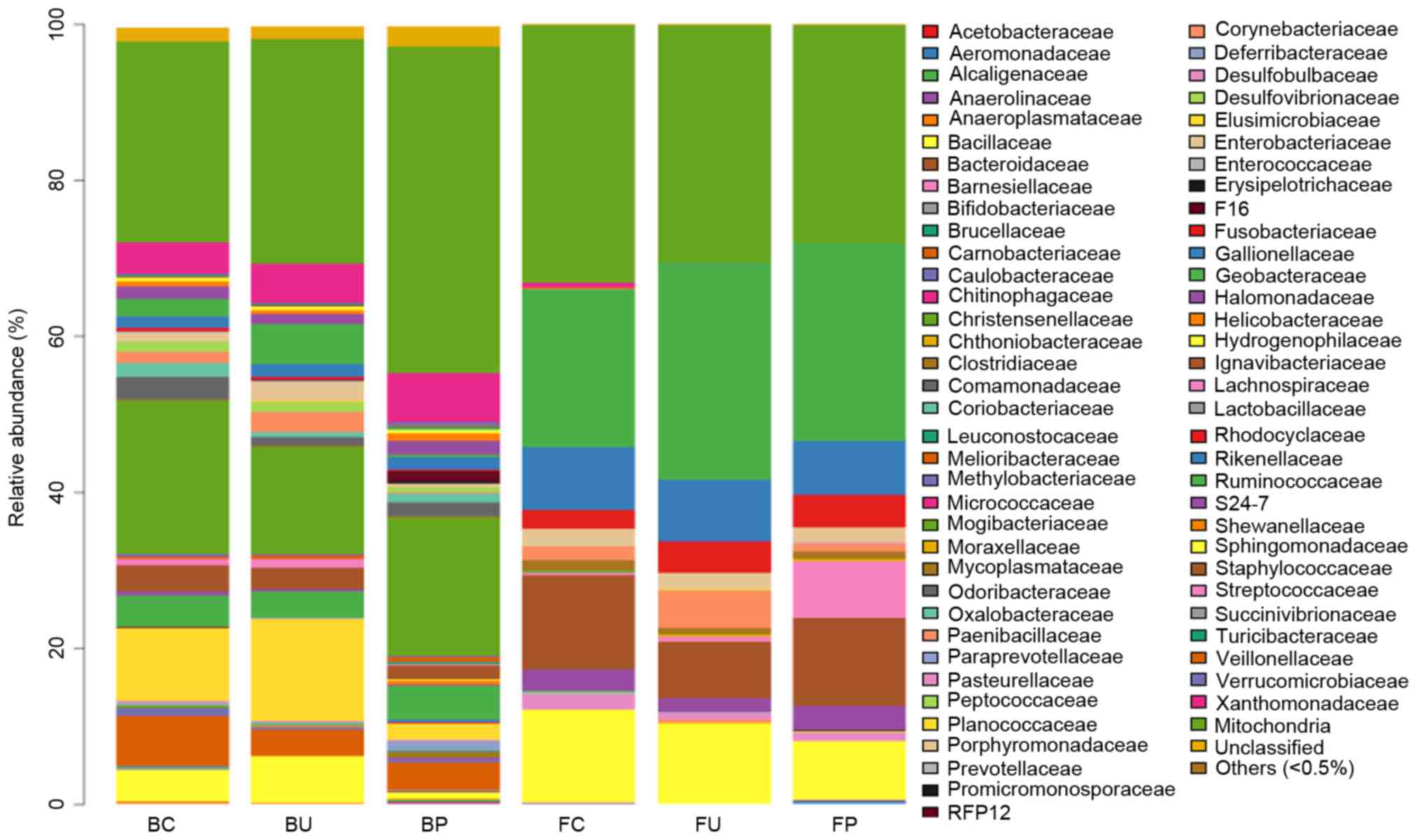
Composition of microbial communities
at the family level
To analyze the systematic differences in microbial
communities among groups C, U and P, 68 families except the
‘Others’ group, comprising other types of microbes whose total
ratio was <0.5%, were clustered based on their respective
relative abundance, as demonstrated in Fig. 5. In the lower respiratory tract
microbiome, no significant difference was identified between groups
C, U and P. In the intestinal tract, the levels of abundance of the
Oxalobacteraceae and Paenibacillaceae families were lower in group
U compared with group C. The levels of abundance of these families
were higher in group P compared with group U, although the
difference was not statistically significant. The levels of
abundance of the Enterobacteriaceae, Moraxellaceae, and
Shewanellaceae families significantly increased in group P compared
with group U. Additionally, all abundance levels of these families
were lower in group U compared with group C, although the
difference was not statistically significant (the data that
exhibited significant variation is listed in Table I, and others are not shown).
 | Table I.Relative abundances of bacterial
families in the lower respiratory and intestinal tracts. |
Table I.
Relative abundances of bacterial
families in the lower respiratory and intestinal tracts.
|
| Relative mean
abundance (%) |
|---|
|
|
|
|---|
| Bacterial
families | n= (5) | n=(5) | n=(5) |
|---|
|
Enterobacteriaceae | 9.375 (BC) | 13.042 (BU) | 2.084
(BP)c |
|
Sphingomonadaceae | 0.564 (BC) | 0.551 (BU) | 1.059
(BP)a,c |
|
Oxalobacteraceae | 0.006 (FC) | 0.000
(FU)a | 0.006 (FP) |
|
Paenibacillaceae | 0.002 (FC) | 0.000
(FU)b | 0.005 (FP) |
|
Enterobacteriaceae | 0.074 (FC) | 0.016 (FU) | 0.048
(FP)c |
| Moraxellaceae | 0.188 (FC) | 0.001 (FU) | 0.052
(FP)c |
| Shewanellaceae | 0.038 (FC) | 0.001 (FU) | 0.006
(FP)c |
Effect of prebiotics to the OTU of
microbiome
Finally, the OTU was used to analyse the difference
between microbiota. In the lower respiratory tract, the levels of
abundance of Clostridiales, Lachnospiraceae, Pedobacter and iii1-15
families decreased in group U compared with group C, and the
abundance of the Clostridiales and Lachnosipraceae families became
equivalent to the level in the control group in group P when the
prebiotics were administered. The levels of abundance of S24-7 and
Erythroba families increased in group U compared with group C, and
the level in group P became equivalent to the levels of the control
group when prebiotics were administered. These data are
demonstrated in Table II; other
families without significant differences among groups were not
included due to the large number within this group. In the
intestinal tract, significant differences between the microbiomes
of each group were observed, as illustrated in Table III. For example, the level of
abundance of the S24-7, Bacteroidales and Firmicutes families
increased in group U compared with group C, becoming equivalent to
those in the control group subsequent to the administration of
prebiotics, as demonstrated in group P. The abundance levels of
Clostridiales, Ruminococcus and Flexispira families exhibited a
decrease in group U compared with group C, becoming equivalent to
those in the control group subsequent to the administration of
prebiotics, as demonstrated in group P. Furthermore, the
Lachnospiraceae family demonstrated a similar result within the
lower respiratory tract, as summarized in Table III. Other families without
significant differences between the groups were not included, due
to the large number within this group.
 | Table II.OTU of the respiratory
microbiome. |
Table II.
OTU of the respiratory
microbiome.
|
| Average of relative
abundance in different group |
|---|
|
|
|
|---|
| OTU | BC (n=5) | BU (n=5) | BP (n=5) |
|---|
| Clostridiales | 2.8 | 0.8a | 1.2 |
|
Lachnospiraceae | 2.6 | 0.0b | 0.8 |
| Pedobacter | 2.4 | 0.2b | 0.0b |
| iii1-15 | 1.2 | 0.0b | 0.0b |
| Rikenellaceae | 0.0 | 7.0b | 1.0 |
| S24-7 | 0.2 | 4.2b | 2.4 |
| Erythroba | 0.4 | 2.2 | 0c |
 | Table III.OTU of the intestinal microbiome. |
Table III.
OTU of the intestinal microbiome.
|
| Average of relative
abundance in different group |
|---|
|
|
|
|---|
| Out | FC (n=5) | FU (n=5) | FP (n=5) |
|---|
| S24-7 | 1,187.5 |
4,050.6a | 863.2d |
| Clostridiales |
41.8 |
10.4a | 57.0d |
| Bacteroidales |
0.0 |
0.6c | 0.0e |
| Firmicutes |
1.0 |
7.2c | 0.0e |
| Ruminococcus | 85 |
27.4a | 40.6 |
| Ruminococcus
gn |
24.4 |
5b | 17.2 |
| Bacteroides |
0.2 |
1.8c | 0.8 |
| Flexispira |
132.6 |
0.0c | 84.0 |
| Adlercreutzia |
0.0 |
1.6c | 0.6 |
|
Oxalobacteraceae |
1.2 |
0.0c | 1.8 |
|
Lachnospiraceae |
18.4 |
2.2c | 10.6 |
|
Desulfovibrionaceae | 35 |
0.2c | 0.0c |
| Paenibacillus |
0.8 |
0.0c | 2.0 |
| AF12 |
0.4 |
141.4c | 48.0c |
| Bacteria | 0 | 0 | 2.0e |
| Lactobacillus |
0.4 | 0 | 3.2e |
| Lactobacillus
ruminis |
0.2 | 0 | 0.6e |
| Lactococcus
garvieae |
1.8 |
2.8 | 0.0e |
| Rikenellaceae | 0 | 0 | 1.4e |
| Shewanella
algae |
15.4 |
0.4 | 2.4e |
|
Ruminococcaceae |
1.2 |
2.2 | 0.4e |
| Escherichia
coli | 2,850.4 | 4,058.6 | 510.0d |
|
Helicobacteraceae |
0.8 |
0.2 | 3.6e |
Discussion
At present, intestinal microbiota are considered to
be the most important type of microbiome in humans, with known
associations with numerous diseases including obesity (28), diabetes (29), inflammatory bowel disease (30) and cancer (9,18). In
experimental animals and humans, there is evidence to support the
hypothesis that the commensal microbiome serves an important role
in carcinogenesis, tumor progression and therapy (18).
The association between the intestinal microbiome,
inflammation and cancer has also been widely studied. For example,
a recent study concluded that the intestinal microbiome affected
the intestine of the host and other organs such as the lungs, due
to the circulation of metabolites produced in the intestines
throughout the body (31). Multiple
studies have demonstrated that the microbiome existing in the
respiratory tract is associated with numerous diseases of the
lungs, including COPD (12,13), asthma (14) and lung cancer (15). Additionally, the microbiome of the
respiratory tract serves an important role in the exacerbation of
chronic lung diseases (32).
Otherwise, the microbiomes of the airway and intestinal tracts may
affect each other through the gut-lung axle (23,33). For
example, a study conducted by Madan et al (34) indicated that changes in diet resulted
in an alteration of the intestinal tract microflora, and in an
alteration in the respiratory tract microflora. Previous studies
have demonstrated that respiratory and intestinal microbiomes are
associated with inflammation, which was demonstrated to be one of
the most important factors in the carcinogenesis of lung cancer
(16,17). Investigation into the association
between microbiomes in the lower airways and the intestinal tract,
inflammation and lung cancer is therefore required.
Furthermore, the development of metagenomics,
including 16S rRNA gene pyrosequencing technique and bioinformatics
(7) have provided an increased
understanding of the role of the human microbiome in the
development of cancer. The microbiome has become an important area
of research. However, data associating the changes in the airway
and intestinal microbiome to lung cancer remain scarce. Thus, the
aim of the present study was to investigate and explain the
association between microbiota, inflammation and lung cancer
through a urethane-induced pulmonary adenocarcinoma mouse model,
which is widely used in research in lung cancer (21) and the microbiome (35).
The present study identified no significant
variation in the richness and diversity of microbiome among the 3
groups, as demonstrated in Fig. 1,
which may be associated with the individual variation and the
sample number of each group. However, there were some significant
variations between the intestinal and the respiratory tract
microbiomes at a familial level, which is demonstrated in Tables I, II
and III. The levels of abundance of
the S24-7, Bacteroidales and Firmicutes families were increased in
the intestinal tract of urethane-induced pulmonary adenocarcinoma
mice (U) compared with the control group (C), but were reduced when
prebiotics were administered to the urethane-induced pulmonary
adenocarcinoma mice (group P). The abundance levels of the
Clostridiales family were decreased in the intestinal tract of
urethane-induced pulmonary adenocarcinoma mice (U) compared with
the control group (C), but were increased when prebiotics were
administered to the urethane-induced pulmonary adenocarcinoma mice
(group P). Additionally, the S24-7, Clostridiales and
Lachnospiraceae families exhibited similar variation in the
respiratory and intestinal tracts, and the variation between each
group and different organ sites- the lungs and the
intestine-indicated that an interaction existed between the
intestinal and respiratory tract microbiomes, and prebiotics may
affect these microbiomes, which was similar to data revealed in a
previous study (34).
Multiple previous studies identified similar changes
in the levels of abundance of the Clostridiales, Bacteroidales and
S24-7 families observed in the present study. For example, Baxter
et al (36) demonstrated that
the number of Bacteroidales families present was associated with a
higher rate of tumorigenesis, whilst the numbers of Clostridiales
families present was associated with a lower rate of tumorigenesis.
In the present study, the levels of abundance of the Bacteroidales
and S24-7 families increased in group U compared with group C in
the lower respiratory and intestinal tracts, whilst the level of
abundance of the Clostridiales families decreased. These variations
were also found in a study by Schwab et al (37) concerning murine microbiota activity
and interactions with the host during acute inflammation and
recovery. Furthermore, a reduction in the level of abundance of
intestinal Clostridiales families was reported to precede the
development of nosocomial Clostridium difficile infection
(38), and decreased abundance in
Clostridiales families was strongly correlated with inflammatory
bowel disease (IBD) status (39).
Whilst an increase in the relative proportion of Bacteroidales
families was identified in high-fat-fed mice with high levels of
inflammation (40), Bacteroidales
families were also found in oral mucosae, and exhibited higher
levels of abundance in recurrent aphthous stomatitis patients
compared with healthy controls (41).
Thus, the variations in Clostridials, Bacteroidales and S24-7
families in the lower respiratory and intestinal tracts may exert
significant effects on the levels of inflammation and the
carcinogenesis of lung cancer.
In the present study, the results from the analysis
of the inflammatory markers revealed that the inflammation level in
the urethane-induced pulmonary adenocarcinoma mice was higher than
the level of the control group, which was similar to the data
observed in previous studies (21,42).
Additionally, the level of inflammation decreased when prebiotics
were administered to the group of pulmonary adenocarcinoma mice.
The prebiotics used consisted of a mixture plant polysaccharides,
which are utilized by the microbiome to promote the growth of
certain microorganisms, particularly probiotic microorganisms such
as Lactobacillus, the level of which increased in group P in the
intestinal tract, as demonstrated in Table III.
In conclusion, the carcinogen urethane affected the
levels of inflammation and the composition of the microbiome, and
prebiotics inhibited the urethane-induced elevation of the level of
inflammation in mice, possibly through the regulation of the
microbiome in the intestinal and respiratory tracts, but did not
inhibit the development of pulmonary adenocarcinoma that was
possibly associated with the interference time and the
concentration of the prebiotics. Additional studies are required to
investigate these issues. In the present study, it was demonstrated
that prebiotics may increase levels of inflammation and the
composition of the microbiome in the intestinal and respiratory
tracts, and improve the treatment of lung cancer. Understanding the
role of the intestinal and respiratory microbiomes is important, in
order to identify novel targets and approaches for improving the
treatment and diagnosis of lung cancer.
Acknowledgements
The authors would like to thank Professor Yiming Lu
(Beijing Institute of Radiation Medicine, State Key Laboratory of
Proteomics, Cognitive and Mental Health Research Center of The PLA,
Beijing, China) for technical assistance with 16S rRNA sequence raw
data processing.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 82171206 and
31401141) and the Foundation of the Center of Translational
Medicine between Beijing Proteome Research Center and Baodi
Clinical Institute of Tianjin medical university (grant no.
TMRC201301).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ and GW conceived and designed the study. ZD, ZL,
CS and HX performed the animal experiments, sera preparation and
DNA extraction. ZD and CZ performed 16S data generation and
analysis. ZC, JL and HW conducted pathological analysis. ZD, ZL, CZ
and GW participated in the design of the study and drafted the
manuscript.
Ethics approval and consent to
participate
All mouse experiments were performed in accordance
with the approved guidelines of the Academy of Military Medical
Sciences. The experimental protocol was approved by the Ethics
Committee for Animal Experimentation of the Academy of Military
Medical Sciences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamada N, Seo SU, Chen GY and Nunez G:
Role of the gut microbiota in immunity and inflammatory disease.
Nat Rev Immunol. 13:321–335. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seoane A, Bessa X, Balleste B, O'Callaghan
E, Panadès A, Alameda F, Navarro S, Gallén M, Andreu M and Bory F:
Helicobacter pylori and gastric cancer: Relationship with
histological subtype and tumor location. Gastroenterol Hepatol.
28:60–64. 2005.(In Spanish). View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith C: EBV and nasopharyngeal carcinoma:
A target for cellular therapies. Immunotherapy. 5:821–824. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynge E and Rebolj M: Primary HPV
screening for cervical cancer prevention: Results from European
trials. Nat Rev Clin Oncol. 6:699–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin R, Miquel S, Langella P and
Bermudez-Humaran LG: The role of metagenomics in understanding the
human microbiome in health and disease. Virulence. 5:413–423. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt BL, Kuczynski J, Bhattacharya A,
Huey B, Corby PM, Queiroz EL, Nightingale K, Kerr AR, DeLacure MD,
Veeramachaneni R, et al: Changes in abundance of oral microbiota
associated with oral cancer. PLoS One. 9:e987412014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farrell JJ, Zhang L, Zhou H, Chia D,
Elashoff D, Akin D, Paster BJ, Joshipura K and Wong DT: Variations
of oral microbiota are associated with pancreatic diseases
including pancreatic cancer. Gut. 61:582–588. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dejea CM, Wick EC, Hechenbleikner EM,
White JR, Welch Mark JL, Rossetti BJ, Peterson SN, Snesrud EC,
Borisy GG, Lazarev M, et al: Microbiota organization is a distinct
feature of proximal colorectal cancers. Proc Natl Acad Sci USA.
111:18321–18326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeller G, Tap J, Voigt AY, Sunagawa S,
Kultima JR, Costea PI, Amiot A, Böhm J, Brunetti F, Habermann N, et
al: Potential of fecal microbiota for early-stage detection of
colorectal cancer. Mol Syst Biol. 10:7662014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han MK, Huang YJ, Lipuma JJ, Boushey HA,
Boucher RC, Cookson WO, Curtis JL, Erb-Downward J, Lynch SV, Sethi
S, et al: Significance of the microbiome in obstructive lung
disease. Thorax. 67:456–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia-Nuñez M, Millares L, Pomares X,
Ferrari R, Pérez-Brocal V, Gallego M, Espasa M, Moya A and Monsó E:
Severity-related changes of bronchial microbiome in chronic
obstructive pulmonary disease. J Clin Microbiol. 52:4217–4223.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gurwitz D and Lunshof JE: Farm microbiome
and childhood asthma. N Engl J Med. 364:19722011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hosgood HD III, Sapkota AR, Rothman N,
Rohan T, Hu W, Xu J, Vermeulen R, He X, White JR, Wu G, et al: The
potential role of lung microbiota in lung cancer attributed to
household coal burning exposures. Environ Mol Mutagen. 55:643–651.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stathopoulos GT, Sherrill TP, Cheng DS,
Scoggins RM, Han W, Polosukhin VV, Connelly L, Yull FE, Fingleton B
and Blackwell TS: Epithelial NF-kappa B activation promotes
urethane-induced lung carcinogenesis. Proc Natl Acad Sci USA.
104:18514–18519. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malkinson AM: Role of inflammation in
mouse lung tumorigenesis: A review. Exp Lung Res. 31:57–82. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dzutsev A, Goldszmid RS, Viaud S, Zitvogel
L and Trinchieri G: The role of the microbiota in inflammation,
carcinogenesis, and cancer therapy. Eur J Immunol. 45:17–31. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Belkaid Y and Hand TW: Role of the
microbiota in immunity and inflammation. Cell. 157:121–141. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Narayan C and Kumar A: Constitutive over
expression of IL-1β, IL-6, NF-κB, and Stat3 is a potential cause of
lung tumorgenesis in urethane (ethyl carbamate) induced Balb/c
mice. J Carcinog. 11:92012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ihara S, Kida H, Arase H, Tripathi LP,
Chen YA, Kimura T, Yoshida M, Kashiwa Y, Hirata H, Fukamizu R, et
al: Inhibitory roles of signal transducer and activator of
transcription 3 in antitumor immunity during carcinogen-induced
lung tumorigenesis. Cancer Res. 72:2990–2999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barfod KK, Roggenbuck M, Hansen LH,
Schjorring S, Larsen ST, Sorensen SJ and Krogfelt KA: The murine
lung microbiome in relation to the intestinal and vaginal bacterial
communities. BMC Microbiol. 13:3032013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou D, Zhang H, Bai Z, Zhang A, Bai F,
Luo X, Hou Y, Ding X, Sun B, Sun X, et al: Exposure to soil, house
dust and decaying plants increases gut microbial diversity and
decreases serum immunoglobulin E levels in BALB/c mice. Environ
Microbiol. 18:1326–1337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edgar RC: UPARSE: Highly accurate OTU
sequences from microbial amplicon reads. Nat Methods. 10:996–998.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edgar RC, Haas BJ, Clemente JC, Quince C
and Knight R: UCHIME improves sensitivity and speed of chimera
detection. Bioinformatics. 27:2194–2200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rodrigues NF, Kästle J, Coutinho TJ,
Amorim AT, Campos GB, Santos VM, Marques LM, Timenetsky J and de
Farias ST: Qualitative analysis of the vaginal microbiota of
healthy cattle and cattle with genital-tract disease. Genet Mol
Res. 14:6518–6528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferolla SM, Armiliato GN, Couto CA and
Ferrari TC: The role of intestinal bacteria overgrowth in
obesity-related nonalcoholic fatty liver disease. Nutrients.
6:5583–5599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karlsson F, Tremaroli V, Nielsen J and
Backhed F: Assessing the Human Gut Microbiota in Metabolic
Diseases. Diabetes. 62:3341–3349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tong M, Li X, Parfrey Wegener L, Roth B,
Ippoliti A, Wei B, Borneman J, McGovern DP, Frank DN, Li E, et al:
A modular organization of the human intestinal mucosal microbiota
and its association with inflammatory bowel disease. PLoS One.
8:e807022013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohtani N: Microbiome and cancer. Semin
Immunopathol. 37:65–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dickson RP, Martinez FJ and Huffnagle GB:
The role of the microbiome in exacerbations of chronic lung
diseases. Lancet. 384:691–702. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sze MA, Tsuruta M, Yang SW, Oh Y, Man SF,
Hogg JC and Sin DD: Changes in the bacterial microbiota in gut,
blood, and lungs following acute LPS instillation into mice lungs.
PLoS One. 9:e1112282014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Madan JC, Koestler DC, Stanton BA,
Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison
HG, Sogin ML, et al: Serial analysis of the gut and respiratory
microbiome in cystic fibrosis in infancy: Interaction between
intestinal and respiratory tracts and impact of nutritional
exposures. MBio. 3:e00251–12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garzoni C, Brugger SD, Qi W, Wasmer S,
Cusini A, Dumont P, Gorgievski-Hrisoho M, Mühlemann K, von Garnier
C and Hilty M: Microbial communities in the respiratory tract of
patients with interstitial lung disease. Thorax. 68:1150–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baxter NT, Zackular JP, Chen GY and
Schloss PD: Structure of the gut microbiome following colonization
with human feces determines colonic tumor burden. Microbiome.
2:202014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schwab C, Berry D, Rauch I, Rennisch I,
Ramesmayer J, Hainzl E, Heider S, Decker T, Kenner L, Müller M, et
al: Longitudinal study of murine microbiota activity and
interactions with the host during acute inflammation and recovery.
ISME J. 8:1101–1114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vincent C, Stephens DA, Loo VG, Edens TJ,
Behr MA, Dewar K and Manges AR: Reductions in intestinal
Clostridiales precede the development of nosocomial Clostridium
difficile infection. Microbiome. 1:182013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gevers D, Kugathasan S, Denson LA,
Vázquez-Baeza Y, Van Treuren W, Ren B, Schwager E, Knights D, Song
SJ, Yassour M, et al: The treatment-naive microbiome in new-onset
Crohn's disease. Cell Host Microbe. 15:382–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de La Serre CB, Ellis CL, Lee J, Hartman
AL, Rutledge JC and Raybould HE: Propensity to high-fat
diet-induced obesity in rats is associated with changes in the gut
microbiota and gut inflammation. Am J Physiol Gastrointest Liver
Physiol. 299:G440–G448. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hijazi K, Lowe T, Meharg C, Berry SH,
Foley J and Hold GL: Mucosal Microbiome in Patients with Recurrent
Aphthous Stomatitis. J Dent Res. 94:872015S-94S. View Article : Google Scholar
|
|
42
|
Treda Jan C, Fukuhara T, Suzuki T,
Nakamura A, Zaini J, Kikuchi T, Ebina M and Nukiwa T: Secretory
leukocyte protease inhibitor modulates urethane-induced lung
carcinogenesis. Carcinogenesis. 35:8962014. View Article : Google Scholar : PubMed/NCBI
|