Introduction
Colorectal cancer (CRC) is one of the most common
types of cancer globally, and remains associated with a high
mortality rate (1,2). Although surgical resection is effective
for patients with localized disease, ~20% of patients with stage II
and 40% with stage III CRC develop recurrence within 5 years
following surgery (3). This high
probability of postoperative recurrence provides the rationale for
adjuvant chemotherapy following curative resection. 5-fluorouracil
(5-FU)-based chemotherapy is the standard adjuvant treatment for
patients with stage III CRC, and this has been established using
large randomized clinical trials (3,4). Although
the routine use of adjuvant chemotherapy for patients with stage II
CRC is not recommended, a subset of stage II patients with
high-risk characteristics for relapse may benefit from adjuvant
therapy. In addition, there is considerable heterogeneity among
tumors which may lead to differing clinical outcomes and
chemotherapeutic responses. Therefore, it is important to identify
biomarkers associated with differential risk of relapse and
sensitivity to adjuvant chemotherapy.
An aquaporin (AQP) gene was identified in
1992 as a water transport channel (5). Since 1992, the existence of 13
AQP genes has been confirmed in mammals and these AQP genes
are widely expressed in numerous human tissue types (6,7). The
primary function of AQPs is to facilitate passive water transport,
driven by osmotic gradients, across the plasma membrane of the
cell, thus serving an important role in fluid homeostasis and
numerous biological functions (6–9). Previous
studies have demonstrated that certain classes of AQPs were highly
expressed in a variety of cancer types and were associated with
tumor biological functions (8,10–14). Particularly, increased expression
levels of AQP1 have been identified in numerous types of human
cancer, including lung, brain, breast, cervical, renal and CRC
(8,11,15–17).
Certain previous studies have demonstrated that AQP1 is involved in
tumor cell proliferation, invasion, metastasis and angiogenesis,
which may contribute to promoting tumor progression (8,9).
Additionally, previous in vitro studies, which utilized lung
or ovarian cancer cell lines, have also suggested the potential
role of AQP1 expression in modifying the sensitivity to certain
anti-cancer drugs (18,19).
While the clinical and biological relevance of AQP1
has been investigated in numerous types of cancer, including CRC,
only a small number of studies have focused on the prognostic role
of AQP1 expression in CRC, and these results were conflicting
(17,20). Furthermore, it remains to be
determined whether the expression of AQP1 is predictive of response
to chemotherapy. Therefore, the present study aimed to investigate
the association between AQP1 expression and survival outcomes or
chemotherapeutic response in CRC. Specifically, the potential
clinical utility of AQP1 expression as a predictive molecular
marker for disease recurrence was evaluated in patients with CRC
who underwent curative surgery followed by 5-FU-based adjuvant
chemotherapy.
Materials and methods
Tissue samples
A total of 268 surgical specimens of CRC, obtained
at Saitama Medical Center (Kawagoe, Japan) between January 2001 and
March 2010 were used for immunohistochemistry (IHC; Table I). Patients who received preoperative
chemotherapy and/or radiotherapy prior to surgery were not included
in the current study. Tumors were staged at the time of the primary
tumor resection according to the International Union Against Cancer
classification (21). The present
study was performed with the approval of the Ethics Committee of
the Saitama Medical Center, Saitama Medical University (Saitama,
Japan), in accordance with the ethical guidelines for clinical
research (application no. 1143). Written informed consent was
gained from all participants.
 | Table I.Clinicopathological characteristics
of patients with colorectal cancer according to AQP1
expression. |
Table I.
Clinicopathological characteristics
of patients with colorectal cancer according to AQP1
expression.
|
|
| AQP1
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n (%) | Positive, n
(%) | Negative, n
(%) | P-value |
|---|
| All patients | 268 | 112 | 156 | – |
| Age, years |
|
|
| 0.09 |
|
<65 | 99 (36.9) | 47 (42.0) | 52 (33.3) |
|
|
≥65 | 169 (63.1) | 65 (58.0) | 104 (66.7) |
|
| Gender |
|
|
| 0.34 |
|
Male | 172 (64.2) | 74 (66.1) | 98 (62.8) |
|
|
Female | 96 (35.8) | 38 (33.9) | 58 (37.2) |
|
| Location |
|
|
| <0.01 |
|
Right | 90 (33.6) | 27 (24.1) | 63 (40.4) |
|
|
Left | 178 (66.4) | 85 (75.9) | 93 (59.6) |
|
| Histology |
|
|
| 0.02 |
|
Well | 124 (46.3) | 41 (36.6) | 83 (53.2) |
|
|
Moderately | 130 (48.5) | 66 (58.9) | 64 (41.0) |
|
|
Poorly/others | 14 (5.2) | 5 (4.5) | 9 (5.8) |
|
| Depth of
invasion |
|
|
| 0.03 |
|
Tis | 25 (9.3) | 4 (3.6) | 21 (13.5) |
|
| T1 | 16 (6.0) | 5 (4.5) | 11 (7.1) |
|
| T2 | 25 (9.3) | 10 (8.9) | 15 (9.6) |
|
| T3 | 126 (47.0) | 58 (51.8) | 68 (43.6) |
|
| T4 | 76 (28.4) | 35 (31.3) | 41 (26.3) |
|
| Lymph node
metastasis |
|
|
| 0.03 |
|
Absent | 136 (50.7) | 49 (43.8) | 87 (55.8) |
|
|
Present | 132 (49.3) | 63 (56.3) | 69 (44.2) |
|
| Lymphatic
invasion |
|
|
| <0.01 |
|
Negative | 77 (28.7) | 19 (17.0) | 58 (37.2) |
|
|
Positive | 191 (71.3) | 93 (83.0) | 98 (62.8) |
|
| Venous
invasion |
|
|
| <0.01 |
|
Negative | 85 (31.7) | 26 (23.2) | 59 (37.8) |
|
|
Positive | 183 (68.3) | 86 (76.8) | 97 (62.2) |
|
| Stage |
|
|
| 0.12 |
| 0 | 25 (9.3) | 4 (3.6) | 21 (13.5) |
|
| I | 28 (10.4) | 8 (7.1) | 20 (12.8) |
|
| II | 65 (24.3) | 32 (28.6) | 33 (21.2) |
|
|
III | 87 (32.5) | 43 (38.4) | 44 (28.2) |
|
| IV | 63 (23.5) | 25 (22.3) | 38 (24.4) |
|
| Liver
metastasis |
|
|
| 0.53 |
|
Absent | 217 (81.0) | 91 (81.3) | 126 (80.8) |
|
|
Present | 51 (19.0) | 21 (18.8) | 30 (19.2) |
|
IHC and evaluation of AQP1
expression
The expression of AQP1 was examined using IHC.
Briefly, 4-µm-thick, fixed in 20% formalin for ~24 h at room
temperature, paraffin-embedded sections were deparaffinized in
xylene and rehydrated in ethanol. Endogenous peroxidases were
blocked with 0.3% hydrogen peroxide. Antigens were retrieved by 10
min autoclaving in 10 mM citrate buffer solution [(pH 6.0) 105°C].
Primary rabbit polyclonal anti-human AQP1 antibody (cat. no.
HPA019206; Atlas Antibodies AB, Bromma, Sweden) was incubated in a
1:1,000 dilution of 10 mM PBS at 4°C overnight. The sections were
the incubated with horseradish peroxidase (HRP)-coupled anti-rabbit
secondary antibody polymer for 30 min at room temperature according
to the manufacturer's protocol of the EnVision™ HRP system (Dako;
Agilent Technologies GmbH, Waldbronn, Germany; dilution,
ready-to-use). Following this, sections were incubated with
3,3′-diaminobenzene for 3–5 min at room temperature. Blocking was
performed using 0.3% hydrogen peroxide for 30 min at room
temperature and then washed in PBS. Sections were counterstained
with Carrazzi's hematoxylin for 1 min at room temperature.
Immunohistochemical evaluations were performed independently by two
histopathologists who were blinded to the clinicopathological
characteristics of the patients. In total >200 cancer cells were
investigated under high power (×200) magnification using a light
microscope for AQP1 expression, following screening for areas with
the highest staining intensity under lower power (×40)
magnification. AQP1 staining of membranous or cytoplasmic staining
within the cancer cells was semi-quantitatively scored as 0 (0–5%),
1 (5–20%), 2 (20–50%) or 3 (>50%). Subsequently, tumors
determined to have scores of 2 and 3 were considered to have
AQP1-positive expression, whilst tumors with scores of 0 and 1 were
considered to be negative for AQP1 expression.
Statistical analysis
Statistical analysis of the associations between
AQP1 expression and various clinicopathological characteristics was
performed using a Fisher's exact test, χ2 test or
Mann-Whitney U test. Cumulative survival was assessed using the
Kaplan-Meier method and the differences between the two groups were
analyzed using a log-rank test. All statistical analyses were
two-sided. Statistical analyses were conducted using GraphPad Prism
v6.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS
Statistics version 22 (IBM SPSS Corporation, Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association between AQP1 expression
and clinicopathological characteristics
Clinical characteristics of patients included in the
current study are presented in Table
I and representative images of AQP1 staining in non-tumor and
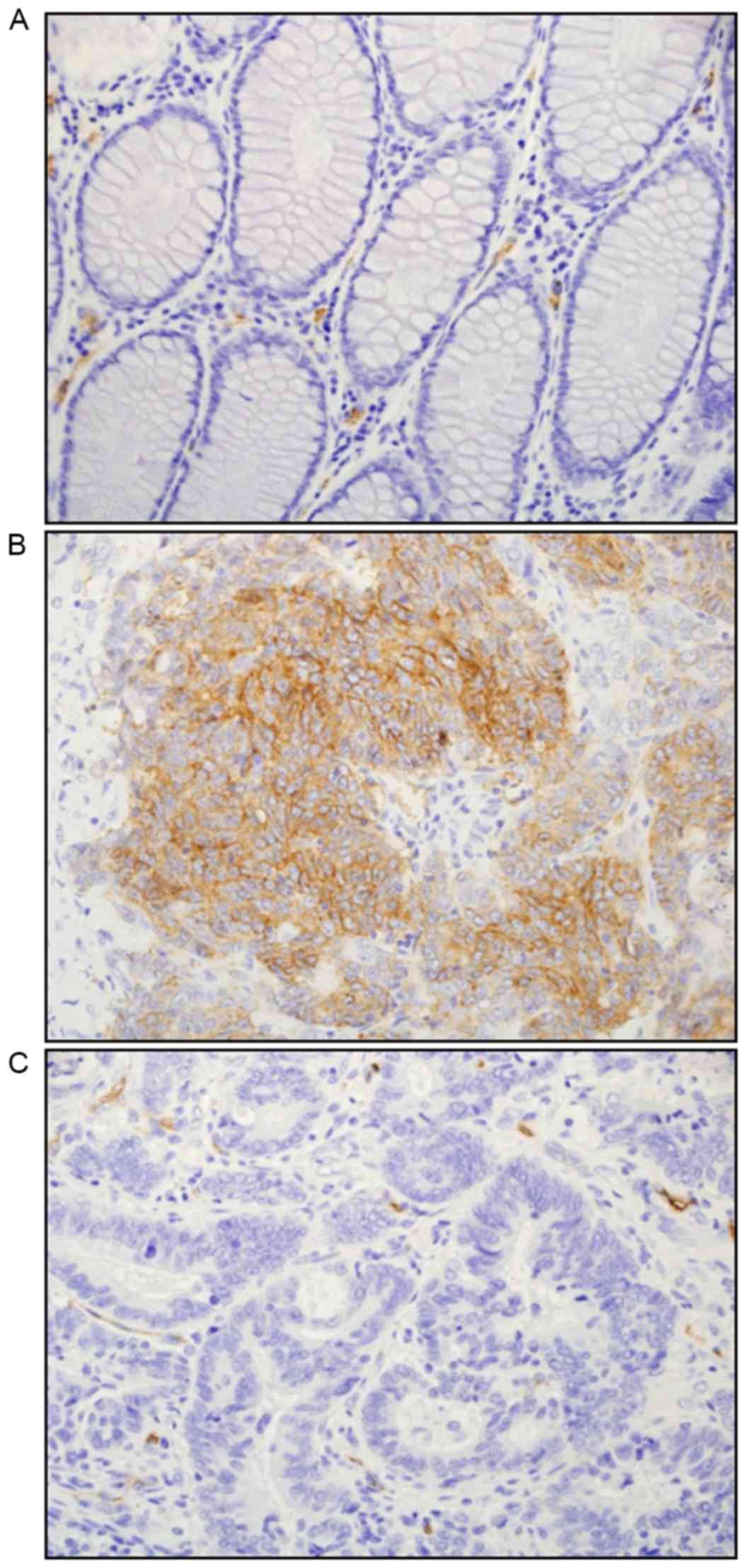
tumor tissues are shown in Fig. 1A-C.
In normal and cancer stromal areas, AQP1 expression was detected in
the endothelial cells of microvessels and erythrocytes, which is
consistent with previous studies (9,17,22). AQP1 expression was not detectable in
normal epithelial cells (Fig. 1A). In
CRC tissues, the expression of AQP1 was identified in the cell
membrane and cytoplasm of the cancer cells (Fig. 1B). Among the 268 patients with stage
0–IV CRC, positive AQP1 expression was observed in 112 (41.8%)
patients, and the remaining 156 (58.2%) patients were identified to
be AQP1-negative (Fig. 1B and C,
respectively). The associations between AQP1 expression and the
clinicopathological features of the 268 patients with CRC are
presented in Table I. Positive AQP1
expression was significantly associated with left-sided tumors vs.
right-sided tumors (P<0.01). AQP1 expression was also
significantly associated with numerous aggressive characteristics,
including greater depth of invasion (P=0.03), lymph node metastasis
(P=0.03), lymphatic invasion (P<0.01) and venous invasion
(P<0.01). Degree of differentiation was also associated with
AQP1 expression (P=0.02), with well-differentiated adenocarcinomas
more commonly negative for AQP1 expression. However, there was no
significant difference in the proportion of patients with liver
metastasis between the AQP1-positive and -negative groups.
Prognostic outcomes of AQP1 expression
levels in stage II–III CRC
To determine the prognostic performance of AQP1
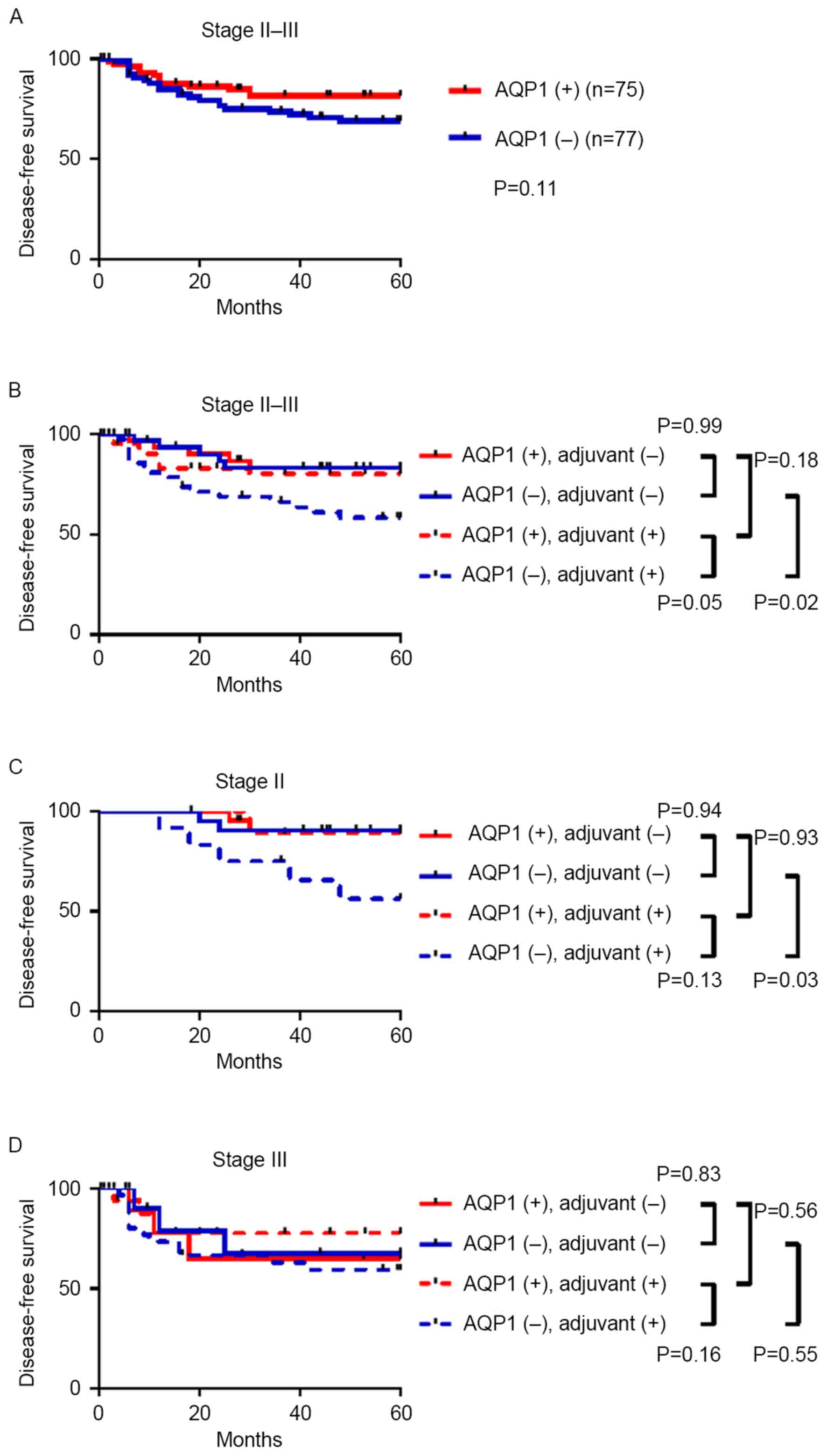
expression in patients with stage II–III CRC, 5-year disease-free
survival (DFS) data was analyzed. Although this did not reach
statistical significance, patients with AQP1-positive CRC had an
improved DFS compared with that of AQP1-negative patients [hazard
ratio (HR), 0.58; 95% confidence interval (CI) 0.30–1.12; P=0.11;
Fig. 2A]. Therefore, the prognostic
efficacy of AQP1 in stage II–III CRC was not validated in the
present study.
Association between AQP1 expression
and therapeutic outcomes of patients treated with and without
5-FU-based adjuvant chemotherapy
Of the 152 patients with stage II–III CRC, 84
patients received adjuvant chemotherapy following curative surgery,
whilst 67 patients were treated with surgery alone. For the 84
patients with adjuvant therapy, regimens primarily comprised
intravenous or oral administration of 5-FU-based drugs; however, 9
(10.7%) were treated with 5-FU-based regimens in combination with
oxaliplatin (mFOLFOX6 regimen). Among patients that received
adjuvant chemotherapy, positive expression of AQP1 was
significantly associated with improved DFS (HR, 0.45; 95% CI,
0.21–1.00; P=0.05; Fig. 2B). By
contrast, for patients who were not administered adjuvant
chemotherapy, AQP1 expression had no effect on DFS (HR, 1.00; 95%
CI, 0.29–3.45; P=0.99; Fig. 2B). As
shown in Fig. 2C and D, a similar
trend was identified in the stratified analyses of stage II and III
patients who received adjuvant chemotherapy, although this did not
reach statistical significance due to the small number of patients
in each group (HR, 0.22; 95% CI, 0.06–1.42; P=0.13; and HR, 0.53;
95% CI, 0.21–1.29; P=0.16, respectively). In addition, among the 77
patients with stage II and III AQP1-negative CRC, patients who were
administered adjuvant chemotherapy had significantly reduced DFS
compared with patients treated with surgery alone (P=0.02).
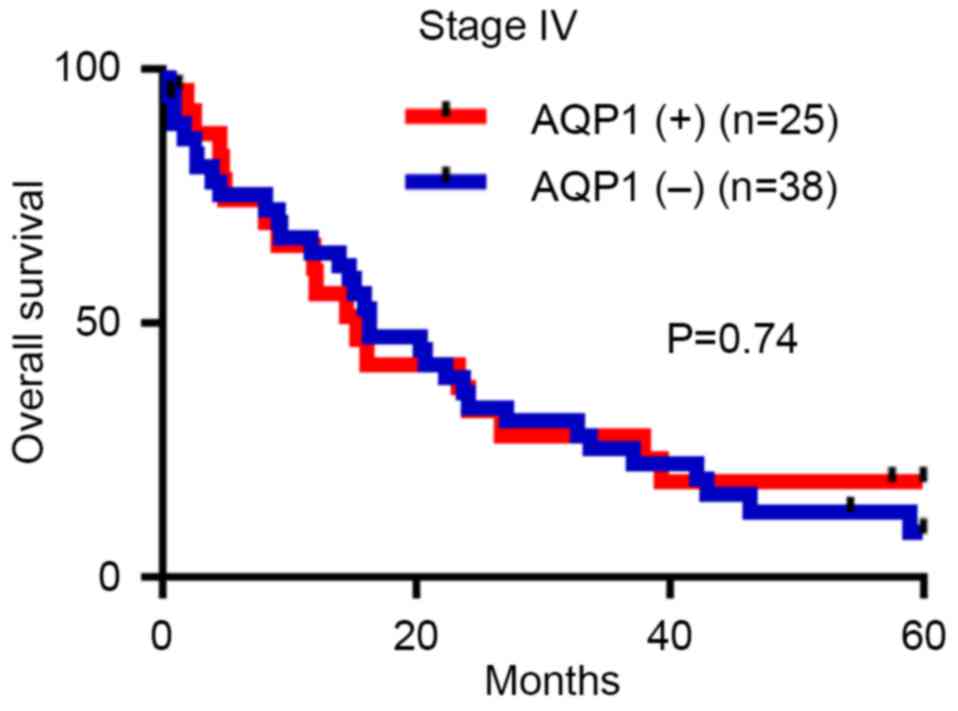
Although an overall survival analysis was conducted
in 63 patients with stage IV CRC, the majority of whom were treated
with one or more lines of chemotherapy, including 5-FU-based
regimens, AQP1 expression had no significant prognostic impact in
this subgroup (HR, 0.91; 95% CI, 0.51–1.61; P=0.74; Fig. 3).
Discussion
In the present study, the expression of AQP1 in 268
patients with stage 0–IV CRC was investigated using IHC. AQP1
expression was not detected in normal epithelial cells, whereas
~40% of tumors were identified as AQP1-positive. AQP1 expression
was significantly associated with advanced tumor stage, the
presence of lymph node metastasis and the presence of lymphatic
invasion and venous invasion. In a previous study by Yoshida et
al (20), tissue microarrays were
utilized to evaluate AQP1 expression in 120 patients with stage II
and III CRC disease. This study identified that 35.8% of patients
were AQP1-positive and that AQP1 expression was significantly
associated with lymph node metastasis and lymphovascular and
vascular invasion. The results are consistent with the present
study, suggesting that a tumor-promoting role of AQP1 may
particularly affect lymph node metastasis and vascular invasion. In
accordance with this, a previous study (23) demonstrated the involvement of AQP1 in
colorectal carcinogenesis, and indicated that AQP1 expression is
induced in early-stage disease and maintained throughout the late
stages of colon cancer development. In vitro studies have
also indicated that AQP1 increases the potential of invasion and
migration in CRC cell lines (12,22).
Notably, a significant association of AQP1 expression with
left-sided CRC was demonstrated in the present study, as well as in
that by Yoshida et al (20).
This may suggest that AQP1 has the potential to contribute to tumor
progression, particularly in left-sided colorectal tumors. However,
Kang et al (17) reported that
marked positive AQP1 expression was negatively associated with
lymph node metastasis in stage I–III CRC. Therefore, there are
still conflicting results regarding the role of AQP1 in lymph node
metastasis. Further studies are required to specifically elucidate
the clinical and biological significance of AQP1 in lymph node
metastasis in CRC.
In terms of the prognostic significance of AQP1
expression, Yoshida et al (20) demonstrated positive AQP1 as a poor
prognostic factor for overall survival (OS) in patients with stage
II–III CRC using multivariate analysis, whereas Kang et al
(17) identified that AQP1 expression
had no significant survival impact on OS or DFS in patients with
stage I–III CRC. In the latter study, patients with marked positive
AQP1 expression typically had an improved DFS (17). The results of the present study are
similar to the latter study, as a non-significant trend of improved
DFS was observed in patients with stage II–III and AQP1-positive
CRC compared with that of patients that were AQP1-negative. As
there is inconsistency between previous studies and the current
study, the prognostic efficacy of AQP1 expression in stage II–III
CRC remains to be conclusively demonstrated.
In other types of malignancy, there has been
variability in the significance of AQP1 expression levels
associated with survival outcomes and tumor phenotype (15,16,24–27).
The association between high AQP1 expression and poor prognosis was
demonstrated in numerous types of solid cancer, including lung
(15,24), breast (16,25),
ovarian (26) and cutaneous melanoma
cancer (27). In accordance with
this, the upregulation of AQP1 was associated with aggressive
subtypes of brain tumors (28,29) and
cervical cancer (30). By contrast,
in renal cancer (31), mesothelioma
(32) and biliary tract cancer
(33), AQP1 expression was
demonstrated to be associated with improved survival rates. A
potential explanation for this discrepancy in clinical impact of
AQP1 between cancer types may be due to the multifaceted roles of
AQP1 across various organs and tumor cells.
Although a large number of previous studies have
analyzed the prognostic values of AQP1 in human cancer, only a
small number of in vitro studies have investigated the
association between AQP1 expression and response to chemotherapy
(18,19,34). In an
ovarian cancer cell line, AQP1 expression was demonstrated to be
associated with chemosensitivity to cisplatin (19). Pan et al (34) utilized a prostate cancer cell line and
reported the association between AQP1 expression and the response
to ginsenoside, which exerts antitumor effects. Liu et al
(18) indicated that AQP1 expression
was downregulated by combination therapy of celecoxib and afatinib
in a lung cancer cell line. In view of these results, it is
hypothesized that AQP1 may potentially modulate the sensitivity to
anticancer drugs. Therefore, the present study focused on
evaluating the prognostic value and also the potential contribution
of AQP1 expression to chemotherapeutic outcomes. In patients with
stage II–III CRC that were administered 5-FU-based adjuvant
chemotherapy, patients with AQP1-positive CRC had improved
therapeutic outcomes. By contrast, in AQP1-negative patients, the
chemotherapy-treated group demonstrated reduced DFS times compared
with the untreated group (treated with surgery alone). This may
suggest that AQP1-positive CRC may have a greater sensitivity to
5-FU-based adjuvant chemotherapy compared with AQP1-negative CRC.
Also, patients with AQP1-negative CRC may exhibit a poorer response
to chemotherapy and may even be harmed by 5-FU-based treatment as
demonstrated by the reduction in DFS in the AQP1negative CRC
chemotherapy treated group. However, there are no previous studies
that have addressed the biological roles of AQP1 in modulating the
sensitivity to 5-FU in any type of cancer. Also, there is currently
no sufficient explanation as to why AQP1-negative patients have a
poorer outcome following treatment with adjuvant chemotherapy,
suggesting the requirement for underlying mechanistic investigation
in future studies.
The present study demonstrated that AQP1 expression
was frequently identified in left-sided CRC, consistent with the
results of Yoshida et al (20). However, CRC with high-level
microsatellite instability (MSI), which has a hypermutable
phenotype due to the loss of DNA mismatch repair activity, occurs
predominantly in the right-sided colon (3). Also, it has been demonstrated that
patients with stage II–III MSI-CRC may not benefit from 5-FU-based
adjuvant chemotherapy (3). This
suggests that combining AQP1 expression with MSI status may provide
improved predictive ability compared with using single biomarkers
in stage II–III CRC, although MSI data was not available in the
present study.
The present study had several limitations, which
included its retrospective nature and the small number of patients
that were examined. Although all regimens of adjuvant therapy used
in this study included 5-FU-based drugs, ~10% of patients were
treated with the mFOLFOX6 regimen, as oxaliplatin-containing
regimens have been established as the standard of care for patients
with stage III CRC in the adjuvant setting (3,35). We were
not able to address the beneficial effect of adding oxaliplatin
compared to the conventional 5-FU-based therapy alone, due to the
small sample size of patients who were treated with oxaliplatin.
Also, in patients with stage II CRC, the survival impact of high
risk characteristics of relapse was not examined due to the limited
clinical information and the lack of standardized criteria for the
identification of high-risk stage II patients, particularly in
patients with rectal cancer (36).
Therefore, the results from the present study are not definitive
and future studies are required to elucidate the clinical
implications of AQP1 expression in patients with stage II and III
CRC, by addressing the aforementioned limitations.
In conclusion, AQP1 expression was detected in ~40%
of patients with CRC and was significantly associated with
aggressive clinicopathological characteristics, including lymph
node metastasis, lymphatic invasion and venous invasion. In stage
II and III patients, AQP1 expression had no significant impact on
DFS. By contrast, in patients who received 5-FU-based adjuvant
chemotherapy following surgery, AQP1-positive CRC exhibited
improved therapeutic outcomes. Adjuvant chemotherapy may not be
advantageous for patients with AQP1-negative tumors. Therefore, the
results of the current study suggested that AQP1 expression may be
a potential predictive biomarker candidate for responsiveness to
5-FU-based adjuvant chemotherapy in stage II–III CRC. However,
further investigation using a large number of CRC cases as well as
functional studies are required to determine the clinical and
biological role of AQP1 expression in modulating the
chemosensitivity.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
AQP1
|
aquaporin 1
|
|
IHC
|
immunohistochemistry
|
|
5-FU
|
5-fluorouracil
|
|
DFS
|
disease-free survival
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gray R, Barnwell J, McConkey C, Hills RK,
Williams NS, Kerr DJ and Group QC: Adjuvant chemotherapy vs.
observation in patients with colorectal cancer: A randomised study.
Lancet. 370:2020–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agre P: The aquaporin water channels. Proc
Am Thorac Soc. 3:5–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kruse E, Uehlein N and Kaldenhoff R: The
aquaporins. Genome Biol. 7:2062006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Verkman AS: Aquaporins. Curr Biol.
23:R52–R55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ribatti D, Ranieri G, Annese T and Nico B:
Aquaporins in cancer. Biochim Biophys Acta. 1840:1550–1553. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Verkman AS, Anderson MO and Papadopoulos
MC: Aquaporins: Important but elusive drug targets. Nat Rev Drug
Discov. 13:259–277. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang L, Zhang Y, Wu X and Yu G:
Aquaporins: New targets for cancer therapy. Technol Cancer Res
Treat. 15:821–828. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Feng L, Zhu Z, Zheng M, Wang D,
Chen Z and Sun H: Aquaporins as diagnostic and therapeutic targets
in cancer: How far we are? J Transl Med. 13:962015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang Y: Aquaporin-1 activity of plasma
membrane affects HT20 colon cancer cell migration. IUBMB Life.
61:1001–1009. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei X and Dong J: Aquaporin 1 promotes the
proliferation and migration of lung cancer cell in vitro. Oncol
Rep. 34:1440–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu J and Verkman AS: Increased migration
and metastatic potential of tumor cells expressing aquaporin water
channels. FASEB J. 20:1892–1894. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yun S, Sun PL, Jin Y, Kim H, Park E, Park
SY, Lee K and Chung JH: Aquaporin 1 is an independent marker of
poor prognosis in lung adenocarcinoma. J Pathol Transl Med.
50:251–257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin F, Zhang H, Shao Y, Liu X, Yang L,
Huang Y, Fu L, Gu F and Ma Y: Expression of aquaporin1, a water
channel protein, in cytoplasm is negatively correlated with
prognosis of breast cancer patients. Oncotarget. 7:8143–8154. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang BW, Kim JG, Lee SJ, Chae YS and Jeong
JY, Yoon GS, Park SY, Kim HJ, Park JS, Choi GS and Jeong JY:
Expression of aquaporin-1, aquaporin-3 and aquaporin-5 correlates
with nodal metastasis in colon cancer. Oncology. 88:369–376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YH and Zhu WL: Effects of cetuximab
combined with afatinib on the expression of KDR and AQP1 in lung
cancer. Genet Mol Res. 14:16652–16661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xuejun C, Weimin C, Xiaoyan D, Wei Z,
Qiong Z and Jianhua Y: Effects of aquaporins on chemosensitivity to
cisplatin in ovarian cancer cells. Arch Gynecol Obstet.
290:525–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshida T, Hojo S, Sekine S, Sawada S,
Okumura T, Nagata T, Shimada Y and Tsukada K: Expression of
aquaporin-1 is a poor prognostic factor for stage II and III colon
cancer. Mol Clin Oncol. 1:953–958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union against Cancer: TNM classification of
malignant tumours. Wiley-Blackwell Chichester; West Sussex, UK;
Hoboken NJ: 2010
|
|
22
|
Dorward HS, Du A, Bruhn MA, Wrin J, Pei
JV, Evdokiou A, Price TJ, Yool AJ and Hardingham JE:
Pharmacological blockade of aquaporin-1 water channel by AqB013
restricts migration and invasiveness of colon cancer cells and
prevents endothelial tube formation in vitro. J Exp Clin Cancer
Res. 35:362016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moon C, Soria JC, Jang SJ, Lee J, Hoque
Obaidul M, Sibony M, Trink B, Chang YS, Sidransky D and Mao L:
Involvement of aquaporins in colorectal carcinogenesis. Oncogene.
22:6699–6703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Machida Y, Ueda Y, Shimasaki M, Sato K,
Sagawa M, Katsuda S and Sakuma T: Relationship of aquaporin 1, 3
and 5 expression in lung cancer cells to cellular differentiation,
invasive growth and metastasis potential. Hum Pathol. 42:669–678.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Otterbach F, Callies R, Adamzik M, Kimmig
R, Siffert W, Schmid KW and Bankfalvi A: Aquaporin 1 (AQP1)
expression is a novel characteristic feature of a particularly
aggressive subgroup of basal-like breast carcinomas. Breast Cancer
Res Treat. 120:67–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Willis S, Villalobos VM, Gevaert O,
Abramovitz M, Williams C, Sikic BI and Leyland-Jones B: Single gene
prognostic biomarkers in ovarian cancer: A meta-analysis. PLoS One.
11:e01491832016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imrédi E, Tóth B, Doma V, Barbai T, Rásó
E, Kenessey I and Tímár J: Aquaporin 1 protein expression is
associated with BRAF V600 mutation and adverse prognosis in
cutaneous melanoma. Melanoma Res. 26:254–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deb P, Pal S, Dutta V, Boruah D, Chandran
VM and Bhatoe HS: Correlation of expression pattern of aquaporin-1
in primary central nervous system tumors with tumor type, grade,
proliferation, microvessel density, contrast-enhancement and
perilesional edema. J Cancer Res Ther. 8:571–577. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saadoun S, Papadopoulos MC, Davies DC,
Bell BA and Krishna S: Increased aquaporin 1 water channel
expression in human brain tumours. Br J Cancer. 87:621–623. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shen Q, Lin W, Luo H, Zhao C, Cheng H,
Jiang W and Zhu X: Differential expression of aquaporins in
cervical precursor lesions and invasive cervical cancer. Reprod
Sci. 23:1551–1558. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Y, Murakami T, Sano F, Kondo K,
Nakaigawa N, Kishida T, Kubota Y, Nagashima Y and Yao M: Expression
of aquaporin 1 in primary renal tumors: A prognostic indicator for
clear-cell renal cell carcinoma. Eur Urol. 56:690–698. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kao SC, Armstrong N, Condon B, Griggs K,
McCaughan B, Maltby S, Wilson A, Henderson DW and Klebe S:
Aquaporin 1 is an independent prognostic factor in pleural
malignant mesothelioma. Cancer. 118:2952–2961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sekine S, Shimada Y, Nagata T, Moriyama M,
Omura T, Watanabe T, Hori R, Yoshioka I, Okumura T, Sawada S, et
al: Prognostic significance of aquaporins in human biliary tract
carcinoma. Oncol Rep. 27:1741–1747. 2012.PubMed/NCBI
|
|
34
|
Pan XY, Guo H, Han J, Hao F, An Y, Xu Y,
Xiaokaiti Y, Pan Y and Li XJ: Ginsenoside Rg3 attenuates cell
migration via inhibition of aquaporin 1 expression in PC-3M
prostate cancer cells. Eur J Pharmacol. 683:27–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
André T, Boni C, Navarro M, Tabernero J,
Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F
and de Gramont A: Improved overall survival with oxaliplatin,
fluorouracil and leucovorin as adjuvant treatment in stage II or
III colon cancer in the MOSAIC trial. J Clin Oncol. 27:3109–3116.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meropol NJ: Ongoing challenge of stage II
colon cancer. J Clin Oncol. 29:3346–3348. 2011. View Article : Google Scholar : PubMed/NCBI
|