Introduction
Epidemiologic research has consistently indicated an
inverse association between the dietary levels of fruits and
vegetables consumed and the risk of certain types of cancer
(1,2).
It has been suggested that carotenoids are chemopreventive
constituents of fruit and vegetables (3,4).
Esophageal cancer is a prevalent type of malignancy with a high
mortality rate, which is increasing in incidence in China,
according to a 2011 study (5). The
northern area of Henan, China, including Linzhou, is an area of
high incidence of esophageal and cervical cancer, and the mortality
rate due to esophageal and gastric cancer is high in this region
compared with the rest of the world (6). The results of nutrition intervention
trials in Linxian demonstrated that the cancer-associated mortality
rate over a 5.25-year period was significantly reduced among trial
participants receiving β-carotene, vitamin E and selenium
supplementation (7). The anti-cancer
effects of crocetin, a carotenoid derived from saffron, have been
reported in KYSE-150 human esophageal squamous cell carcinoma
(ESCC) cells (8). Lycopene and
β-carotene also occur in fruits and vegetables in relatively high
concentrations. However, the effects of these carotenoids on the
viability of esophageal cancer cells remain uncharacterized,
despite the suspected etiological effects of chronic deficiencies
of multiple micronutrients (9).
Peroxisome proliferator-activated receptor gamma
(PPARγ) is a member of the nuclear hormone receptor superfamily.
PPARγ is a transcription factor that serves crucial roles in the
regulation of numerous physiological processes, including lipid
metabolism and adipogenesis. PPARγ is expressed in various human
tissues, and has been demonstrated to regulate cell proliferation,
differentiation, and apoptosis (10,11). A
deficiency in PPARγ may be a significant risk factor for
carcinogenesis (12). It is
established that PPARγ activation promotes antiproliferative,
antiangiogenic and pro-differentiation pathways (13). Various in vitro studies have
demonstrated that the activation of PPARγ leads to the growth
inhibition of numerous types of neoplastic cell, including human
esophageal adenocarcinoma cells (14–17).
However, in Barrett's esophagus and esophageal adenocarcinoma,
enhanced PPARγ expression has been associated with the development
of carcinoma cells (18).
Differential effects of the oral antidiabetic agent, pioglitazone,
on PPARγ activation and the growth of the OE33 human Barrett's
adenocarcinoma cancer cell line have been studied in vitro
and in vivo (19). PPARγ
activation by pioglitazone in vitro reduced OE33 cell growth
by the induction of apoptosis, whereas systemic pioglitazone
treatment of mice bearing transplantable Barrett's adenocarcinomas
derived from OE33 cells increased the rate of cell proliferation
(19).
Our previous studies demonstrated that the
upregulation of PPARγ expression may be associated with the
suppressive effects of β-carotene on the proliferation of MCF-7
breast cancer cells, and of carotenoids on the proliferation of
K562 chronic myelogenous leukemia cells (20–22).
However, to the best of our knowledge, the effects of lycopene and
β-carotene on the viability of human ESCC cells, and the role of
PPARγ in these effects, were not previously well defined.
The present study aimed to evaluate the inhibitory
effects of lycopene and β-carotene on the viability of the EC109
human ESCC cell line, and to elucidate the association between
PPARγ signaling and effect on viability. Subsequently, the
expression levels of cell proliferation regulators were explored in
the context of the carotenoid effects.
Materials and methods
Reagents and antibodies
The ESCC cell line, EC109, was a gift from the
College of Public Health, Zhengzhou University (Zhengzhou, China).
Fetal bovine serum (FBS) was purchased from Beijing Solarbio
Science & Technology Co., Ltd. (Beijing, China), and RPMI-1640
medium was purchased from Gibco (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). MTT, penicillin, streptomycin, and β-carotene
(purity, 97%) were all purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). 2-Chloro-5-nitro-N-phenylbenzamide (GW9662)
was purchased from Cayman Chemical Co. (Ann Arbor, MI, USA) and
lycopene was purchased from Chengdu Herbpurify Co., Ltd.
(http://www.herbpurify.com; Chengdu,
China; purity, 90%) identified by high performance liquid
chromatography following re-purification as described by the study
of Nguyen et al (23). Rabbit
anti-PPARγ (cat. no. H-100), mouse anti-p21 (cat. no. F-5), mouse
anti-cyclin D1 (cat. no. A-12) and mouse anti-COX-2 (cat. no. H-3)
antibodies were all purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Rabbit anti-β-actin (cat. no. D110007), and goat
anti-rabbit IgG (cat. no. D110058) and goat anti-mouse IgG (cat.
no. D110087) secondary antibodies were from Sangon Biotech Co.,
Ltd. (Shanghai, China).
Cell culture
EC109 cells were cultured in RPMI-1640 medium
supplemented with FBS (10%, v/v), penicillin (100 U/ml) and
streptomycin (100 µg/ml). The cells were incubated at 37°C in a
humidified atmosphere of 5% CO2. Cells were grown to
80–90% confluence and sub-cultured.
Drug treatment and MTT assays
EC109 cells were plated in 96-well plates at
5×103 cells/well for MTT assays, or 6-well plates at
1.5×105 cells/well for protein extraction. After 24 h,
β-carotene or lycopene were applied at final concentrations of 0,
1, 5, 10 or 20 µmol/l, and incubated for 0, 24, 48 or 72 h. Each
treatment was repeated in 6 separate wells. For GW9662 treatments,
GW9662 was added to the medium at 2 h prior to carotenoid
treatment. Dimethyl sulfoxide (DMSO) was used as a vehicle to
deliver lycopene, β-carotene and GW9662 to the cells. The
concentration of DMSO was consistent in all experiments, at 0.1%
(w/v). Cell viability was measured using an MTT assay, as
previously described (20).
Western blot analysis
Western blotting was performed as previously
described (20). Briefly, subsequent
to 72 h carotenoid treatment, the cells were harvested and lysed in
ice-cold lysis buffer [50 mM Tris-Cl, 150 mM NaCl, 0.02% (w/v)
NaN3, 100 µg/ml PMSF, 1 µg/ml aprotinin, 1 µg/ml
pepstatin A, 2 µg/ml leupeptin, and 1% (v/v) Triton X-100]. The
protein concentration was determined using a BCA kit (Pierce;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. Proteins were separated by 10% SDS-PAGE and transferred
to nitrocellulose membranes (Pall Life Sciences, Port Washington,
NY, USA). The membranes were then incubated with primary antibodies
against PPARγ, p21, cyclin D1, COX-2 and β-actin, then subsequently
with secondary antibodies. The proteins were visualized by enhanced
chemiluminescence using the Super Signal West Pico Chemiluminescent
system (Pierce; Thermo Fisher Scientific, Inc.) and visualized by
autoradiography on Kodak-XAR film. The intensity of protein bands
was digitized and analyzed with ImageJ 1.46r software (National
Institutes of Health, Bethesda, MA, USA).
Statistical analysis
All experiments were performed ≥3 times. Statistical
analysis was performed using one-way analysis of variance followed
by Tukey's test. Data are presented as the mean ± standard error.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of carotenoids on EC109 cell
viability
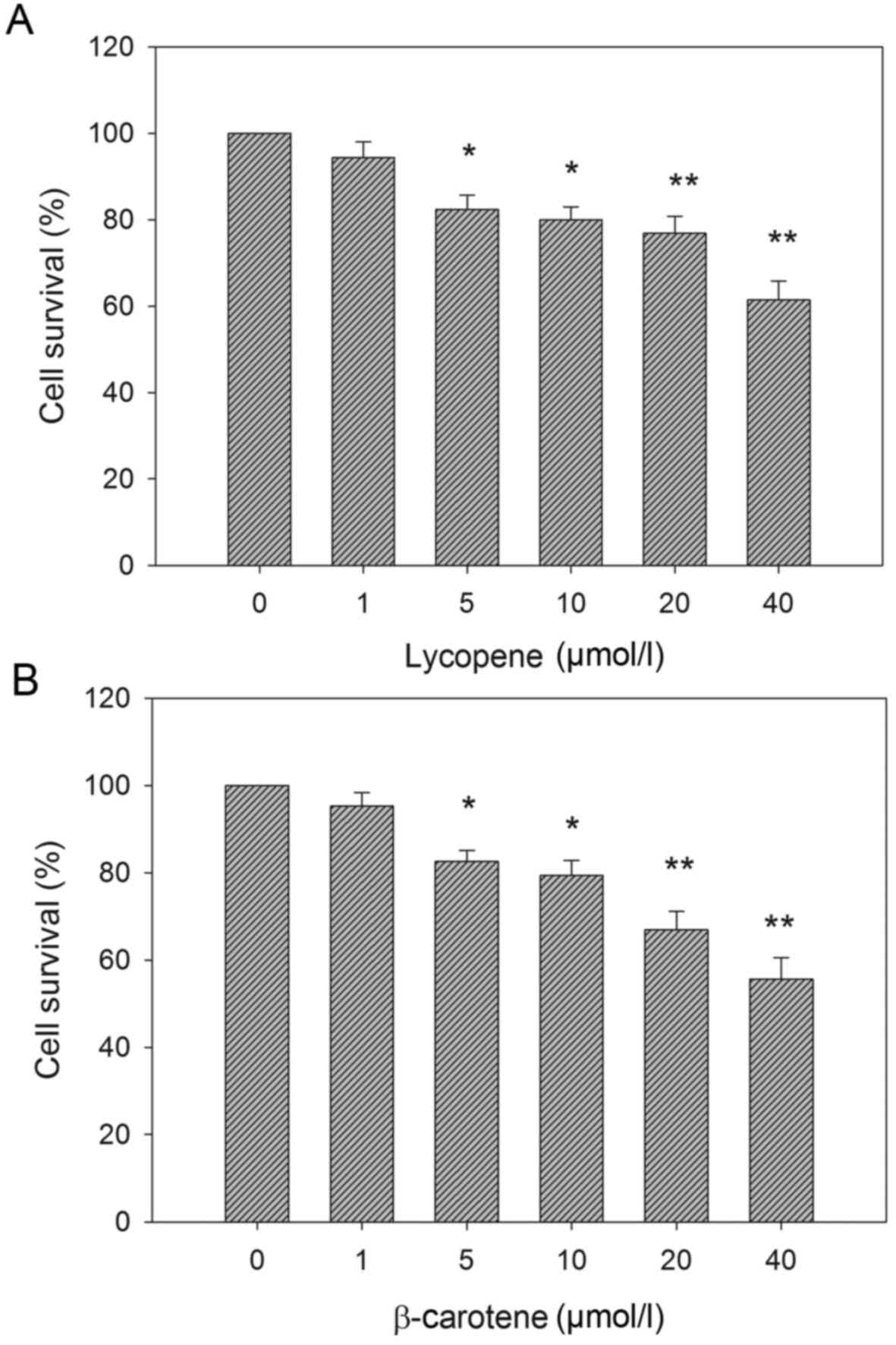
Fig. 1 demonstrates
the effect of altering carotenoid concentration on the viability of
EC109 cells, as determined by MTT assays. Treatment with lycopene
or β-carotene reduced the cell viability compared with the control
group. The suppression was dose-dependent; cell viability was
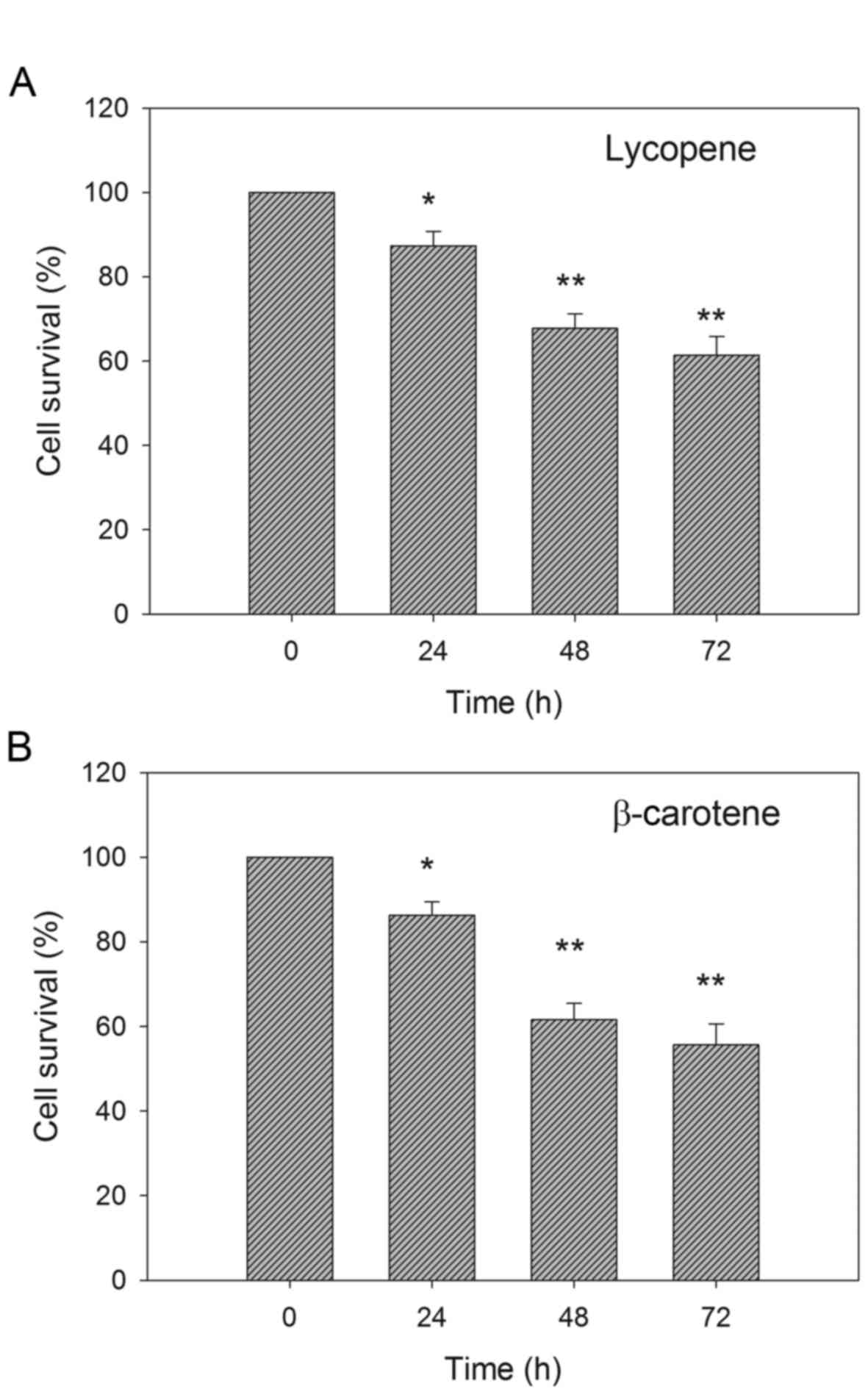
significantly reduced from 5 µM carotenoid treatment. Fig. 2 shows that the reduction in EC109 cell
viability was induced in a time-dependent manner. β-Carotene
appeared to be more effective than lycopene in reducing cell
viability.
Effects of carotenoids in combination
with GW9662 on EC109 cell viability
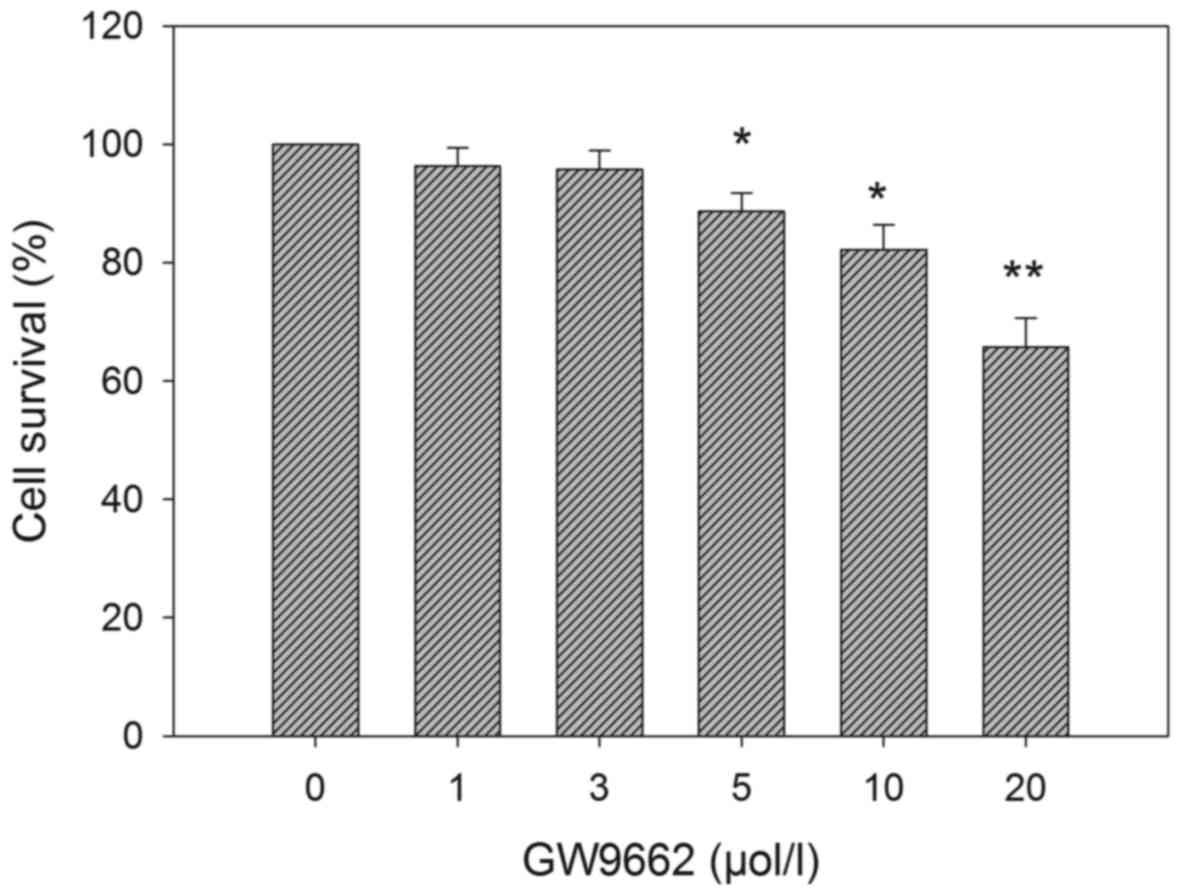
To evaluate the effect of GW9662, an irreversible
PPARγ antagonist, on the viability of EC109 cells, a dose-response
experiment was performed. GW9662 was used at a concentration of
1–20 µM for 72 h prior to an MTT assay. As shown in Fig. 3, GW9662 alone exerted a suppressive
effect on EC109 cell viability. At low concentrations (1–3 µM) the
suppressive effect of GW9662 was insignificant. However, at a
concentration ≥5 µM, GW9662 significantly reduced cell viability.
Therefore, a final concentration of 3 µM was used in the subsequent
experiments of the present study.
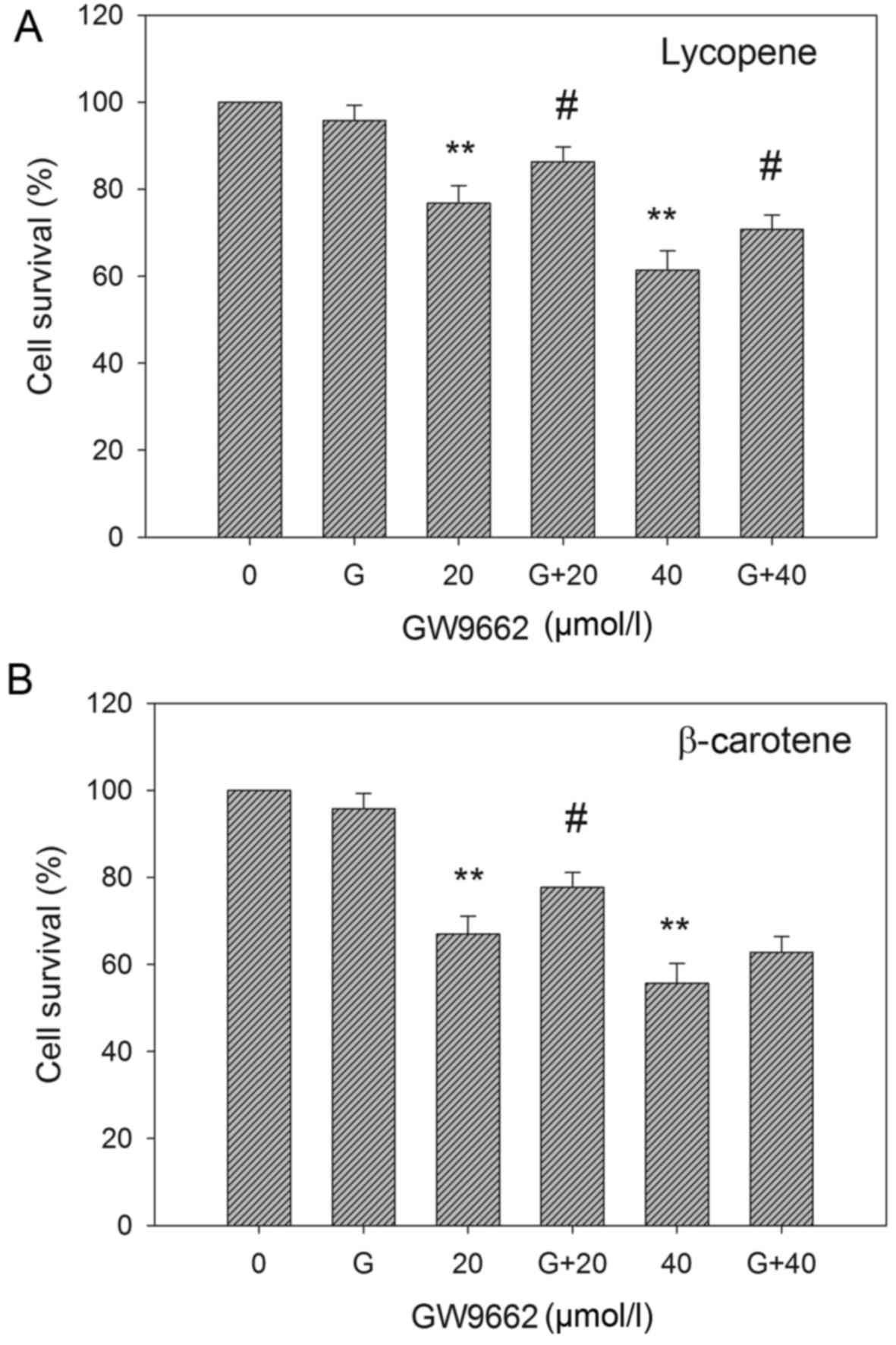
EC109 cells were treated with 20 and 40 µM lycopene
and β-carotene, subsequent to GW9662 pre-treatment for 2 h. As
shown in Fig. 4, GW9662 treatment
partly attenuated the reduced viability of EC109 cells induced by
carotenoid treatment. This implies that PPARγ was at least
partially involved in the suppression of EC109 cell viability by
lycopene and β-carotene.
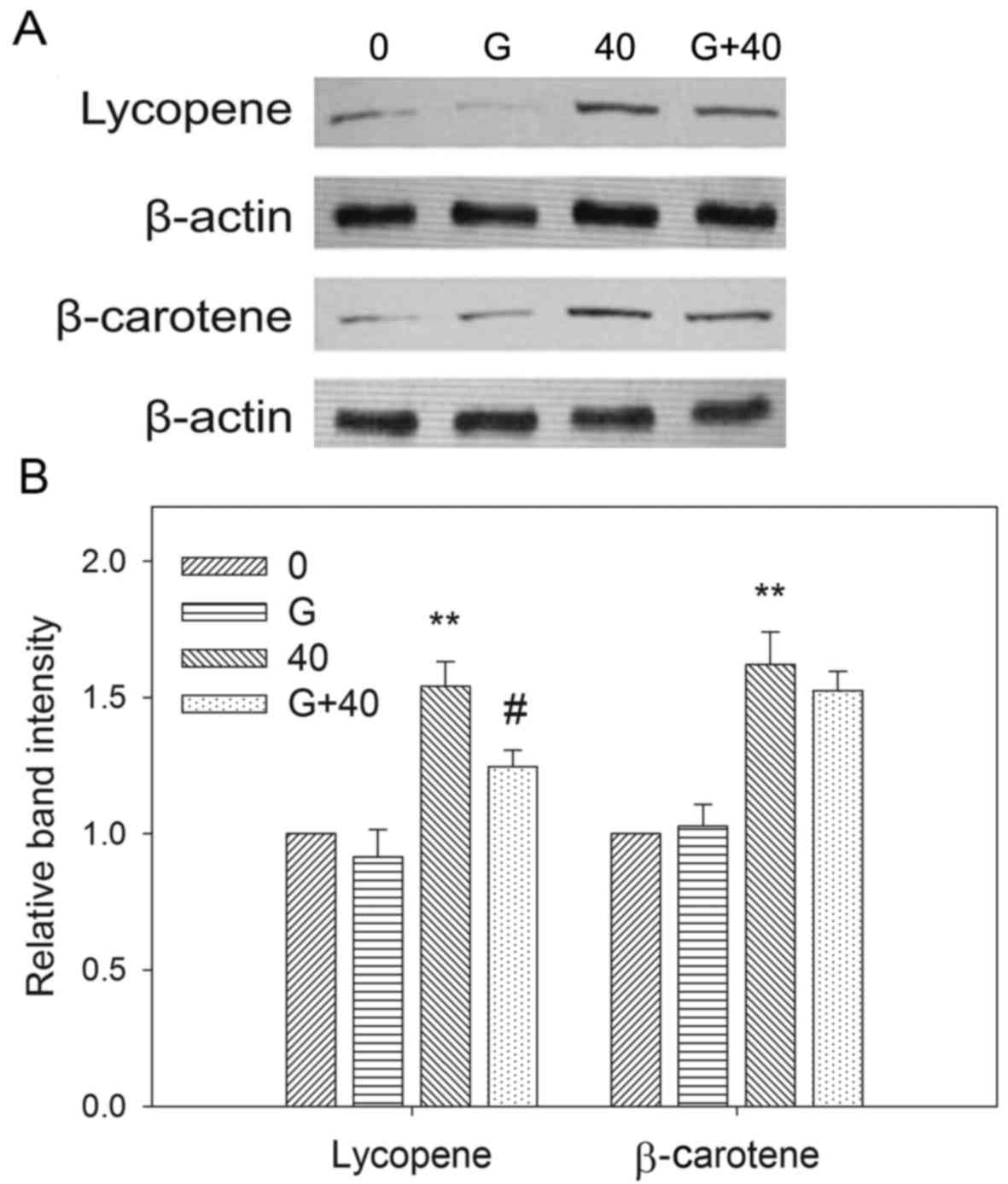
The effect of GW9662 on PPARγ
expression is mediated by carotenoids
The modulatory effects of carotenoids and GW9662 on
the expression of PPARγ were examined by investigating the changes
in the PPARγ protein levels following carotenoid treatment with
GW9662 pre-treatment. Fig. 5
demonstrates that EC109 cells treated with 40 µM lycopene or
β-carotene for 72 h exhibited significantly increased levels of
PPARγ protein compared with untreated cells. GW9662 treatment
attenuated the inhibitory effects of carotenoids on EC109 cell
viability (Fig. 4), and the increase
in PPARγ protein levels induced by carotenoids was reduced with
GW9662 pre-treatment in EC109 cells.
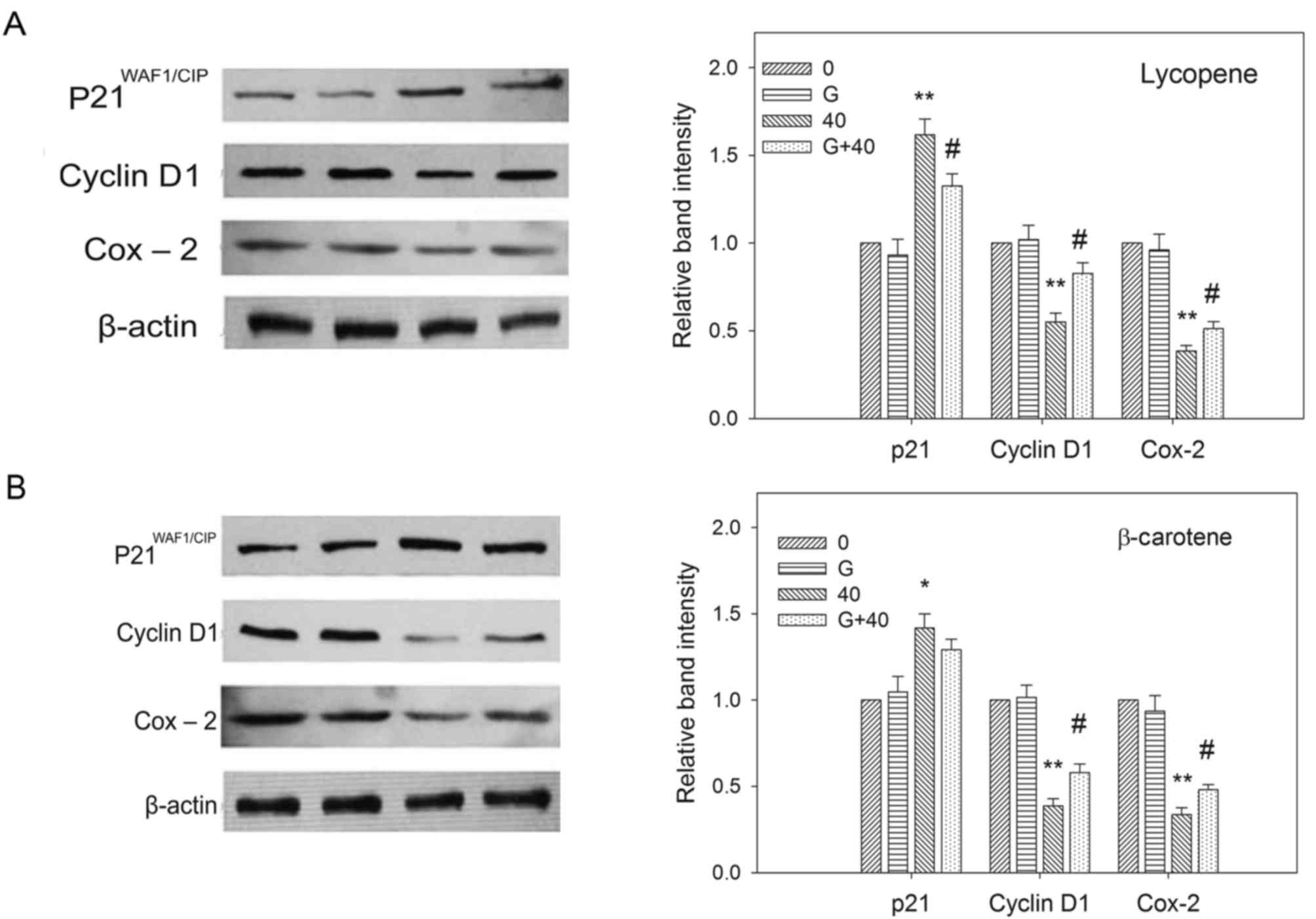
Effects of GW9662 on p21, cyclin D1
and COX-2 expression modulated by carotenoids
Our previous studies demonstrated that carotenoids
can modulate the expression of the cell cycle control protein
cyclin D1, the cyclin-dependent kinase inhibitor
p21WAF1/CIP1 and COX-2 (16–18).
GW9662 attenuated the modulation of p21 and cyclin D1 expression by
carotenoids, suggesting that p21 and cyclin D1 participate in the
PPARγ-dependent pathway, which executes the antiproliferative
effects of carotenoids (18). In
order to understand whether the growth-inhibitory effects of
carotenoids on EC109 cells is dependent on these three regulators,
the cells were treated with carotenoids in the absence or presence
of GW9662, and p21, cyclin D1 and COX-2 protein expression levels
were measured by western blotting. Fig.
6 shows that the protein levels of p21, cyclin D1 and COX-2
were altered by carotenoid and GW9662 treatment. P21 protein levels
were significantly increased by carotenoids in the absence of
GW9662. However, the inhibition of PPARγ by GW9662 resulted in a
significant reduction in p21 protein levels. The downregulation of
cyclin D1 and COX-2 expression induced by carotenoids was also
diminished in the presence of GW9662 pre-treatment.
Discussion
The antiproliferative effects of carotenoids have
been demonstrated in numerous tumors and cell lines. Our previous
results showed that β-carotene inhibited the growth of MCF-7 breast
cancer and H1299 lung cancer cells, and induced apoptosis (20), and that β-carotene, astaxanthin,
capsanthin and bixin suppressed the proliferation of K562 cells,
induced apoptosis, and affected cell cycle progression (21,22).
Crocetin has also been demonstrated to inhibit cell proliferation
and migration, and induce apoptosis, in ESCC KYSE-150 cells
(8). The present study demonstrates
that lycopene and β-carotene significantly decreased the viability
of EC109 cells in a dose- and time-dependent manner. To the best of
our knowledge, this is the first report of lycopene and β-carotene
inhibiting the viability of human ESCC cells. This may be helpful
for understanding the results of the nutrition intervention trials
in Linxian, and indicates the potent chemopreventive effects of
carotenoids on human ESCC.
It has been demonstrated that the activation of
PPARγ expression by agonists inhibited the growth of ESCC cells
(13–17). However, in Barrett's esophagus and
esophageal adenocarcinoma, enhanced PPARγ expression has been
associated with differential effects on the proliferation of
carcinoma cells (18,19). In the present study, the
antiproliferative effects of lycopene and β-carotene on ESCC EC109
cells were associated with the PPARγ pathway, as GW9662 treatment
not only attenuated the upregulation of PPARγ expression, but also
the reduction in EC109 cell viability induced by lycopene and
β-carotene. These data are in accord with our previous observations
that the upregulation of PPARγ expression contributed to the
inhibition of MCF-7 breast cancer and K562 cell growth by
carotenoids (16–18). PPARγ activation has also been
associated with the antiproliferative effects of lycopene in LNCaP
and DU145 human prostate cancer cells (24,25).
Together, these studies suggest that PPARγ may serve important
roles in the anti-cancer effects of carotenoids, which may be of
universal significance for chemoprevention strategies against
cancer. However, GW9662 pre-treatment did not abolish the
growth-inhibitory effects of the tested carotenoids on EC109 cells
completely, suggesting that the anti-cancer effects of the
carotenoids are separate from those of conventional
thiazolidinedione PPARγ agonists.
p21WAF1/CIP1 and cyclin D1 are well
characterized key regulators of cell cycle progression (26). P21 negatively modulates cell cycle
progression (27), and the
upregulation of p21 has been demonstrated to inhibit the
proliferation and colony formation of lung cancer cells (28). Cyclin D1 is overexpressed in various
types of human cancer, and its overexpression is positively
associated with tumor progression (26,28).
Cyclin D1 has also been associated with aggressive tumor behavior
in ESCC (29). COX-2 has been
demonstrated to be upregulated in several types of cancer,
including esophageal carcinomas, and serves an important role in
ESCC carcinogenesis (30,31). Our previous studies demonstrated that
the tested carotenoids upregulated the expression of p21 and
downregulated the expression of cyclin D1 and COX-2 in MCF-7 breast
cancer and K562 cells (20–22). GW9662 treatment significantly weakened
the regulatory effects of the carotenoids on cyclin D1 and p21
expression (22). The present study
demonstrated that lycopene and β-carotene upregulated the
expression of PPARγ and p21WAF1/CIP1, and downregulated
the expression of cyclin D1 and COX-2 in EC109 cells. The
modulatory effects on the expression of p21, cyclin D1 and COX-2 by
the carotenoids were attenuated by GW9662. This suggests that p21,
cyclin D1 and COX-2 may be involved in a PPARγ-dependent pathway
that executes the growth-inhibitory effects of lycopene and
β-carotene on EC109 cells. The activation of PPARγ has been
reported to be associated with an alteration in the cell cycle in
tumors, and may be associated with the altered expression of p21
and cyclin D1 (13–15,21,32–34).
Thus, carotenoids may exert antitumor effects on ESCC by
upregulating p21 and downregulating cyclin D1 and COX-2 through
activating PPARγ, which in turn reduces cell proliferation.
In summary, lycopene and β-carotene may act as
potent anti-proliferative agents in ESCC cells. The present study
may offer novel insights into improved dietary or supplementation
strategies and future therapeutic interventions in esophageal
cancer.
Acknowledgements
The authors would like to thank the College of
Public Health, Zhengzhou University (Zhengzhou, China) for gifting
the EC109 cells.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
NBN repurified and measured lycopene, performed
EC109 cell culture and drug treatment, measured cell viability and
protein levels, and made substantial contributions to the
experimental design and data collection. PL participated in
measurements of cell viability and protein levels, and made
substantial contributions to data analysis and interpretation, and
wrote the manuscript. WEZ made substantial contributions to
experimental design, data analysis and interpretation, and drawing
the conclusions. WEZ also participated in writing and submitting
the manuscript, revising it critically for important intellectual
content and gave final approval of the version to be published.
Each author agreed to be accountable for all aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work are appropriately investigated and resolved. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Baldrick FR, Woodside JV, Elborn JS, Young
IS and McKinley MC: Biomarkers of fruit and vegetable intake in
human intervention studies: A systematic review. Crit Rev Food Sci
Nutr. 51:795–815. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Boeing H, Bechthold A, Bub A, Ellinger S,
Haller D, Kroke A, Leschik-Bonnet E, Müller MJ, Oberritter H,
Schulze M, et al: Critical review: Vegetables and fruit in the
prevention of chronic diseases. Eur J Nutr. 51:637–663. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lampe JW: Health effects of vegetables and
fruit: Assessing mechanisms of action in human experimental
studies. Am J Clin Nutr. 70 3 Suppl:S475–S490. 1999. View Article : Google Scholar
|
|
4
|
Gandini S, Merzenich H, Robertson C and
Boyle P: Meta-analysis of studies on breast cancer risk and diet:
The role of fruit and vegetable consumption and the intake of
associated micronutrients. Eur J Cancer. 36:636–646. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin Y, Totsuka Y, Shan B, Wang C, Wei W,
Qiao Y, Kikuchi S, Inoue M, Tanaka H and He Y: Esophageal cancer in
high-risk areas of China: Research progress and challenges. Ann
Epidemiol. 27:215–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blot WJ, Li JY, Taylor PR, Guo W, Dawsey
S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY, et al: Nutrition
intervention trials in Linxian, China: Supplementation with
specific vitamin/mineral combinations, cancer incidence, and
disease-specific mortality in the general population. J Natl Cancer
Inst. 85:1483–1492. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li S, Jiang S, Jiang W, Zhou Y, Shen XY,
Luo T, Kong LP and Wang HQ: Anticancer effects of crocetin in human
esophageal squamous cell carcinoma KYSE-150 cells. Oncol Lett.
9:1254–1260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li JY, Taylor PR, Li B, Dawsey S, Wang GQ,
Ershow AG, Guo W, Liu SF, Yang CS, Shen Q, et al: Nutrition
intervention trials in Linxian, China: Multiple vitamin/mineral
supplementation, cancer incidence, and disease-specific mortality
among adults with esophageal dysplasia. J Natl Cancer Inst.
85:1492–1498. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feige JN, Gelman L, Michalik L, Desvergne
B and Wahli W: From molecular action to physiological outputs:
Peroxisome proliferator-activated receptors are nuclear receptors
at the crossroads of key cellular functions. Prog Lipid Res.
45:120–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Semple RK, Chatterjee VK and O'Rahilly S:
PPAR gamma and human metabolic disease. J Clin Invest. 116:581–589.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sporn MB, Suh N and Mangelsdorf DJ:
Prospects for prevention and treatment of cancer with selective
PPAR gamma modulators (SPARMs). Trends Mol Med. 7:395–400. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ondrey F: Peroxisome
proliferator-activated receptor gamma pathway targeting in
carcinogenesis: Implications for chemoprevention. Clin Cancer Res.
15:2–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rumi MA, Sato H, Ishihara S, Ortega C,
Kadowaki Y and Kinoshita Y: Growth inhibition of esophageal
squamous carcinoma cells by peroxisome proliferator-activated
receptor gamma ligands. J Lab Clin Med. 140:17–26. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashimoto Y, Shimada Y, Itami A, Ito T,
Kawamura J, Kawabe A, Kaganoi J, Maeda M, Watanabe G and Imamura M:
Growth inhibition through activation of peroxisome
proliferator-activated receptor gamma in human oesophageal squamous
cell carcinoma. Eur J Cancer. 39:2239–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takashima T, Fujiwara Y, Hamaguchi M,
Sasaki E, Tominaga K, Watanabe T, Oshitani N, Higuchi K and Arakawa
T: Relationship between peroxisome proliferator-activated receptor
gamma expression and differentiation of human esophageal squamous
cell carcinoma. Oncol Rep. 13:601–606. 2005.PubMed/NCBI
|
|
17
|
Sawayama H, Ishimoto T, Watanabe M,
Yoshida N, Sugihara H, Kurashige J, Hirashima K, Iwatsuki M, Baba
Y, Oki E, et al: Small molecule agonists of PPAR-γ exert
therapeutic effects in esophageal cancer. Cancer Res. 74:575–585.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Wang R, Zhang Z, Li D and Yut Y:
Enhanced PPAR gamma expression may correlate with the development
of Barrett's esophagus and esophageal adenocarcinoma. Oncol Res.
19:141–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Taie OH, Graf T, Illert B, Katzenberger
T, Mörk H, Kraus MR, Barthelmes HU, Scheurlen M and Seufert J:
Differential effects of PPAR gamma activation by the oral
antidiabetic agent pioglitazone in Barrett's carcinoma in vitro and
in vivo. J Gastroenterol. 44:919–929. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cui Y, Lu Z, Bai L, Shi Z, Zhao WE and
Zhao B: Beta-Carotene induces apoptosis and up-regulates peroxisome
proliferator-activated receptor gamma expression and reactive
oxygen species production in MCF-7 cancer cells. Eur J Cancer.
43:2590–2601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Zhao WE, Hu L, Zhao L and Huang
J: Carotenoids inhibit proliferation and regulate expression of
peroxisome proliferators-activated receptor gamma (PPARγ) in K562
cancer cells. Arch Biochem Biophys. 512:96–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao H, Gu H, Zhang H, Li JH and Zhao WE:
PPARγ-dependent pathway in the growth-inhibitory effects of K562
cells by carotenoids in combination with rosiglitazone. Biochim
Biophys Acta. 1840:545–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nguyen BN and Zhao WE: Isolation of
lycopene from the crude extract of red watermelon flesh by
high-speed counter-current chromatograghy. J Zhengzhou Univ (Energ
Sci). 37:43–46. 2016.
|
|
24
|
Yang CM, Lu IH, Chen HY and Hu ML:
Lycopene inhibits the proliferation of androgen-dependent human
prostate tumor cells through activation of PPARγ-LXRα-ABCA1
pathway. J Nutr Biochem. 23:8–17. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang CM, Lu YL, Chen HY and Hu ML:
Lycopene and the LXRα agonist T0901317 synergistically inhibit the
proliferation of androgen-independent prostate cancer cells via the
PPARγ-LXRα-ABCA1 pathway. J Nutr Biochem. 23:1155–1162. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chellappan SP, Giordano A and Fisher PB:
Role of cyclin dependent kinases and their inhibitors in cellular
differentiation and development. Curr Top Microbiol Immunol.
227:57–103. 1998.PubMed/NCBI
|
|
27
|
Gartel AL and Tyner AL: The
growth-regulatory role of p21 (WAF1/CIP1). Prog Mol Subcell Biol.
20:43–71. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei J, Zhao J, Long M, Han Y, Wang X, Lin
F, Ren J, He T and Zhang H: p21WAF1/CIP1 gene
transcriptional activation exerts cell growth inhibition and
enhances chemosensitivity to cisplatin in lung carcinoma cell. BMC
Cancer. 10:6322010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Xiao W, Ma J, Zhang Y, Li R, Ye J,
Wang X, Zhong X and Wang S: Dual high expression of STAT3 and
cyclin D1 is associated with poor prognosis after curative
resection of esophageal squamous cell carcinoma. Int J Clin Exp
Pathol. 7:7989–7998. 2014.PubMed/NCBI
|
|
30
|
Yang GZ, Li L, Ding HY and Zhou JS:
Cyclooxygenase-2 is overexpressed in Chinese esophageal squamous
cell carcinoma, and correlated with NF-kappa B: An
immunohistochemical study. Exp Mol Pathol. 79:214–218. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Wu YD, Li P, Tu J, Niu YL, Xu CM
and Zhang ST: Effects of cyclooxygenase-2 on human esophageal
squamous cell carcinoma. World J Gastroenterol. 17:4572–4580. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugimura A, Kiriyama Y, Nochi H, Tsuchiya
H, Tamoto K, Sakurada Y, Ui M and Tokumitsu Y: Troglitazone
suppress cell growth of myeloid leukemia cell lines by induction of
p21WAF1/CIP1 cyclin-dependent kinase inhibitor. Biochem
Biophys Res Commun. 261:833–837. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han S, Sidell N, Fisher PB and Roman J:
Up-regulation of p21 gene expression by peroxisome
proliferator-activated receptor gamma in human lung carcinoma
cells. Clin Cancer Res. 10:1911–1919. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Smith AG, Beaumont KA, Smit DJ, Thurber
AE, Cook AL, Boyle GM, Parsons PG, Sturm RA and Muscat GE: PPAR
gamma agonist attenuate proliferation and modulate Wnt/beta-catenin
signaling in melanoma cells. Int J Biochem Cell Biol. 41:844–852.
2009. View Article : Google Scholar : PubMed/NCBI
|