Introduction
Colorectal carcinoma is a common malignancy
worldwide (1,2). It is the second leading cause of
cancer-associated mortality and accounts for 10% of the
cancer-associated mortalities worldwide (1,2), even
though the screening techniques have improved markedly (3). Metastatic colorectal carcinoma is
frequently an incurable disease (4,5). The
underlying molecular mechanism involved in the progression of
metastatic colorectal carcinoma remains to be elucidated.
LIM and SH3 protein 1 (Lasp-1) is a specific focal
adhesion protein that serves an important role in the regulation of
cell proliferation and migration (6,7). It has
been reported to be upregulated in a number of malignant tumors,
including clear cell renal (8),
non-small cell lung (9), prostate
(10), gallbladder (11), bladder (12,13),
breast (14), hepatocellular
(15,16) and colorectal (17,18)
carcinomas. The role of Lasp-1 in metastasis and progression of
colorectal carcinomas has been suggested previously (17). It has been demonstrated that Lasp-1
promotes proliferation and metastasis and induces cell cycle arrest
at the G2/M phase by downregulating S100 calcium-binding
protein P via the phosphoinositide 3-kinase (PI3K)/protein kinase B
(AKT) pathway in gallbladder cancer (11). Lasp-1 overexpression has been
positively associated with lymph node metastasis and poor prognosis
in patients with cholangiocarcinoma (19). It has also been demonstrated that
knockdown of Lasp-1 in HCCC-9810 human cholangiocarcinoma cells
significantly increased cellular apoptosis and inhibited cell
migration, invasion and proliferation (19). Furthermore, silencing of the Lasp-1
gene has been demonstrated to impair cell proliferation and
migration of breast cancer cells (6).
Lasp-1 overexpression in SW480 colorectal carcinoma cellshas been
associated with an aggressive phenotype and poor prognosis in
patients with clear cell renal (20)
and colorectal (8) tumors.
Additionally, depletion of Lasp-1 expression suppressed the
transforming growth factor β (TGF-β) signaling pathway and
inhibited epithelial-mesenchymal transition (20). Lasp-1 is localized to multiple sites
of actin cytoskeleton assembly, including the focal adhesion,
lamellipodia and filopodia (21). The
expression and nuclear localization of Lasp-1 have been positively
associated with malignancy, tumor grade and metastatic lymph node
status (14). However, the
intracellular signaling pathways involved in the metastasis and
progression of colorectal carcinoma remain unclear. Lasp-1 induced
the phosphorylation of proteins involved in themitogen-activated
protein kinase (MAPK) signaling pathway (20). It has been demonstrated that microRNA
(miR)-133α downregulated the expression of Lasp-1, by inhibiting
the phosphorylation of extracellular-signal-regulated kinase
(ERK)1/2 and MAPK/ERK kinase (MEK), and suppressed tumor growth and
metastasis in liver and lung tumors (22). Further more, exogenous miR-133α
inhibited the MAPK signaling pathway and induced the expression of
epithelial markers (23). A similar
effect on epithelial-mesenchymal transition has been observed
following Lasp-1 targeting (22). The
present study provides novel evidence for the role of Lasp-1 and
the underlying molecular mechanism associated with the regulation
of colorectal carcinoma progression.
Materials and methods
Patients
Colorectal cancer and adjacent normal tissue
specimens were obtained from 20 patients (10 male and 10 female)
(47±5.6 years old) between October 2015 and October 2016 who were
treated in the Qianfoshan Hospital (Jinan, China). Written informed
consent was obtained from all patients prior to participation in
the study. The experimental protocol was approved by the ethics
committee of Shandong University (Jinan, China).
Cell culture
The human colorectal cancer cell line SW480 was
purchased from the Shanghai Cell Bank of Chinese Academy of
Sciences (Shanghai, China) and cultured in L-15 Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% fetal bovine serum
(FBS), both Thermo Fisher Scientific Inc. (Waltham, MA, USA), 100
units/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) in a humidified incubator at 37°C
and 5% CO2.
RNA interference
Small interfering RNA (siRNA) targeting the human
Lasp-1 gene and negative control (NC) siRNA were obtained from
Shanghai GenePharma Co., Ltd. (Shanghai, China). A total of three
Lasp-1 siRNA oligonucleotides (siRNA 1,
5′-UGUAGUUCUUCAUGUUCAGUGdTdT-3′; siRNA 2,
5′-UCAAACUCCUCCUUGUAGCGCdTdT-3′; siRNA 3,
5′-AUGGUAUUUUAUGUUACUGAUdTdT-3′) and one NC siRNA
(5′-CAGUCGCGUUUGCGACUGGdTdT-3′) (30 pmol per 6 wells) were used.
Cells were transfected using Lipofectamine RNAiMAX (Invitrogen;
Thermo Fisher Scientific, Inc.) for 48 h, according to the
manufacturer's protocol.
Lasp-1 overexpression
The coding region of the Lasp-1 primer was
synthesized by Sunshine Bio-Tech Co., Ltd. (Nanjing, China) and
cloned into the pIRES2 vector (pIRES2-Lasp-1; Addgene, Cambridge,
MA, USA). Cells in exponential phase were seeded in 96-well plates
at a density of 3×105 cells/well and cultured for 24 h
before transfection. Cells were divided into three groups: NC,
siRNA and siRNA+Lasp-1. The siRNA + Lasp-1 group was included as a
rescue group, and cells in this group were co-transfected with
siRNA and the Lasp-1-expressing vector. A 1 µl volume of
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was
diluted in 50 µl serum-free Opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.) for 5 min, prior to being mixed with NC siRNA,
Lasp-1 siRNA or Lasp-1 expression vector along with Lasp-1 siRNA
(30 pmol per 6 wells) to prepare the transfection solution. A total
of 100 µl transfection solution was added to each well and cultured
for 6 h. The medium was then changed to DMEM containing 10% FBS.
After 48 h of transfection, cells were collected with scraper and
washed by PBS. Cells were deposited by centrifugation at 350 × g
for 8 min at room temperature for further experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cultured cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). The RNA was reverse-transcribed at 42°C for 40 min into cDNA
using the Takara Reverse Transcription system kit (Takara Bio,
Inc., Otsu, Japan), according to the manufacturer's protocol. qPCR
was performed using the SYBR Green Premix kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's protocol. GAPDH was used as an internal reference
gene. The following primers were used: Lasp-1,
5′-GCTTCCATTGCGAGACCTG-3′ (forward) and 5′-TGCCACTACGCTGAAACCT-3′
(reverse); ERK1/2, 5′-GTGCCGTGGAACAGGTTGT-3′ (forward) and
5′-ATGGGCTCATCACTTGGGT-3′ (reverse); and GAPDH
5′-TGTTCGTCATGGGTGTGAAC-3′ (forward) and 5′-ATGGCATGGACTGTGGTCAT-3′
(reverse). qPCR was performed on target genes under the following
conditions: 95°C for 2 min, 35 cycles of 94°C for 30 sec, 58°C for
45 sec and 72°C for 35 sec. Data were collected and calculated for
CT values of all samples and standards based on fluorescent
quantification using GAPDH as the baseline. Standard curve was
firstly plotted using CT values of standards, followed by
semi-quantitative analysis by 2−ΔΔCq method (24).
Western blot analysis
Cells were lysed using the radioimmunoprecipitation
assay buffer. Total protein was quantified using the Bicinchoninic
Acid Protein Assay kit (Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. Protein samples (50 µg) were
boiled, then separated on 10% polyacrylamide gels and transferred
onto polyvinylidene difluoride membranes (Merck KGaA). Membranes
were blocked by 5% BSA for 1 h at room temperature and incubated
overnight at 4°C with anti-Lasp-1 (1:20,000; cat. no. ab156872),
anti-ERK1/2 (1:1,000; cat. no. ab17942), anti-phospho (p-)ERK1/2
(1:500; cat. no. ab214362), anti-GAPDH (1:2,000; cat. no. ab8245)
primary antibodies (Abcam, Cambridge, UK) followed by incubation
with horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:5,000; cat. no., ab181658, goat anti-HRP) at room temperature
for 1 h (Abcam). Immunoreactive bands were detected using enhanced
chemiluminescent HRP substrate (Merck KGaA).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde solution
for 20 min at room temperature and then washed with PBS. A solution
of 0.5% TritonX-100 in PBS was used for permeabilization of cells.
Blocking was performed using 10% FBS for 1 h at room temperature
and cells were incubated with anti-Lasp-1, anti-ERK or anti-phospho
(p-)ERK1/2 primary antibodies (1:200; cat. no. ab214362) overnight
at 4°C. After washing with PBS, the cells were incubated with
secondary antibody (1:500; cat. no. ab150077; goat anti-rabbit IgG
H&L (Alexa Fluor® 488); Abcam). Nuclei were
counterstained with DAPI for 5 min at room temperature. Staining
was observed using a fluorescence microscope (×400, magnification)
(Thermo Fisher Scientific Inc., Waltham, MA, USA).
Cell proliferation assay
Cells were seeded in 96-well plates at a density of
3×104 cells/0.1 ml and were cultured for 24, 48 and 72
h. Subsequently, 10 µl Cell Counting Kit-8 reagent (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) was added to each well. After 4
h of incubation at room temperature, the optical density at 450 nm
was determined. Results are reported as the mean ± standard
deviation (SD) of at least three independent experiments.
Flow cytometric analysis of
apoptosis
Cells transfected with the Lasp-1 or NC siRNA were
harvested with trypsin-EDTA and resuspended in DMEM supplemented
with 10% FBS. The cells (100 µl cell suspension, 1×106
cells/µl) were subsequently stained with fluorescein
isothiocyanate-conjugated annexin V and propidium iodide (Beyotime
Institute of Biotechnology, Haimen, China). Samples were incubated
for 20 min in the dark at 4°C and a Cell Analyzer instrument (Merck
KGaA) along with BD FACSDiva™ software (version 8.0.1;
BD, Franklin Lakes, NJ, USA) was used to evaluate cellular
apoptosis.
Flow cytometric analysis of cell
cycle
Cells transfected with the Lasp-1 or NC siRNA were
harvested with trypsin-EDTA after 72 h. Cells (1×106)
were resuspended in DMEM medium containing 10% FBS. The propidium
iodide/RNase staining kit (MultiSciences Biotech Co., Ltd.,
Hangzhou, China) was used according to the manufacturer's protocol.
The proportion of cells in each phase of the cell cycle
(G0/G1, S and G2/M) was determined
using a flow cytometer and BD FACS Diva™ software (BD
Biosciences, Franklin Lakes, NJ, USA).
Cell migration and invasion
assays
Migration and invasion assays were performed using
Transwell inserts (BD Biosciences). For invasion, chambers were
coated with Matrigel. Cells were seeded at a density of
2×105 cells/250 µl DMEM supplemented with 0.1% FBS.
Medium supplemented with 750 µl 10% FBS was added to the lower
chamber and served as a chemotactic agent. Following incubation at
37°C for 12 h, non-migrating cells were discarded from the upper
side of the membrane and cells on the lower side were fixed using
ice-cold methanol (−20°C) and then air-dried. Cells were stained
using crystal violet at 37°C for 30 min and imaged with Nikon
Eclipse te200 Inverted Phase Contrast Microscopeat (×100,
magnification; Nikon Corporation, Tokyo, Japan).
Statistical analysis
Results are presented as the mean ± SD. Statistical
analysis was performed using SPSS software (version 19.0; IBM
Corp., Armonk, NY, USA). Differences between groups were determined
using analysis of variance or the Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Lasp-1 and ERK1/2 are upregulated in
colorectal carcinoma
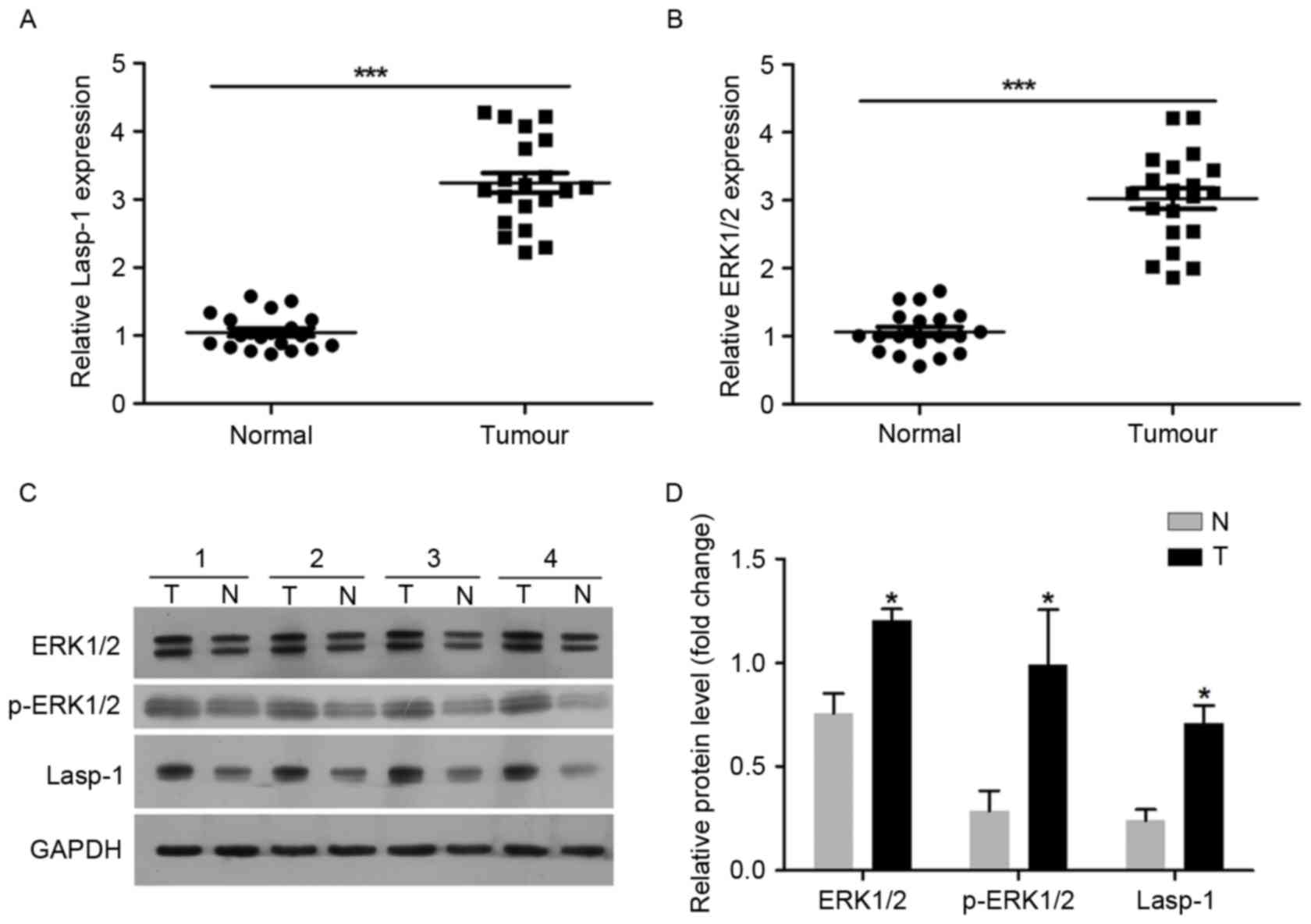
A total of 20 pairs of tumor samples (tumor, T) and
adjacent normal colorectal mucosa (normal, NT) tissues were used in
the present study. Lasp-1 and EKR1/2 expression levels were
investigated using RT-qPCR and western blot analysis (Fig. 1). It was demonstrated that Lasp-1 mRNA
expression was upregulated in tumor tissues compared with the
normal controls (Fig. 1A). ERK1/2
mRNA expression was also increased in tumor tissues compared with
the normal controls (Fig. 1B).
Regarding the protein level, Lasp-1, EKR1/2 and p-EKR1/2 were over
expressed in tumor samples (Fig. 1C and
D). These results suggest that the upregulation of Lasp-1 and
ERK1/2 may be associated with the progression of colorectal
carcinoma.
Knockdown of Lasp-1 suppresses the
expression and activation of ERK1/2 in SW480 cells
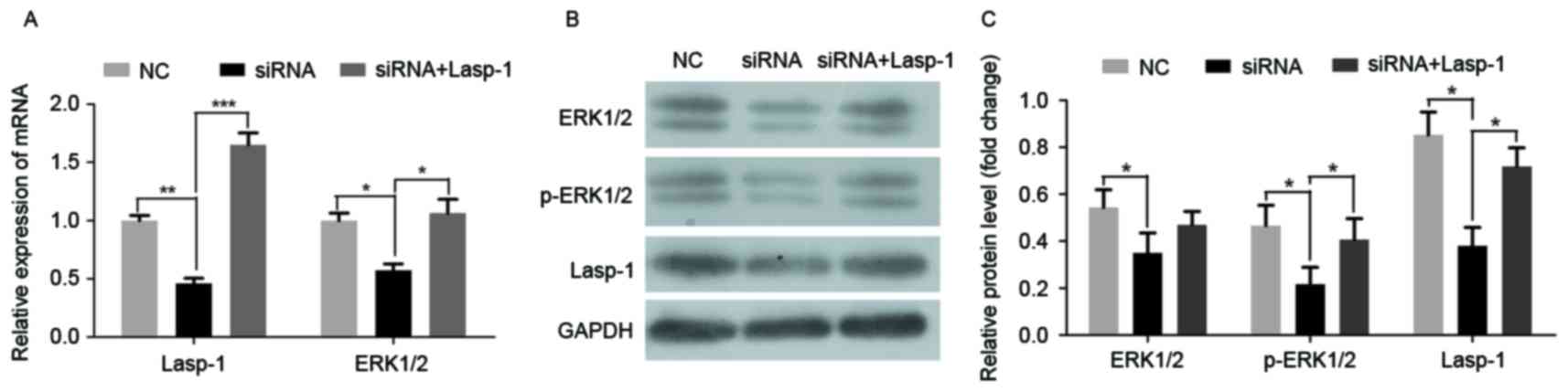
To investigate the role of Lasp-1 in the progression
of colorectal carcinoma and the involved underlying molecular
mechanism, Lasp-1 was knocked down by siRNA. Three siRNA
oligonucleotides were designed. Among them, siRNA 1 was the most
efficient (data not shown). It was then demonstrated that siRNA
targeting Lasp-1 significantly downregulated Lasp-1 and ERK1/2 mRNA
expression (Fig. 2A). Furthermore,
the protein levels of Lasp-1, ERK1/2 and p-ERK1/2 were also
suppressed by Lasp-1 knockdown (Fig. 2B
and C). By contrast, Lasp-1 and p-ERK1/2 mRNA and protein
levels were rescued in the siRNA group combined with Lasp-1
overexpression (Fig. 2A and C). Using
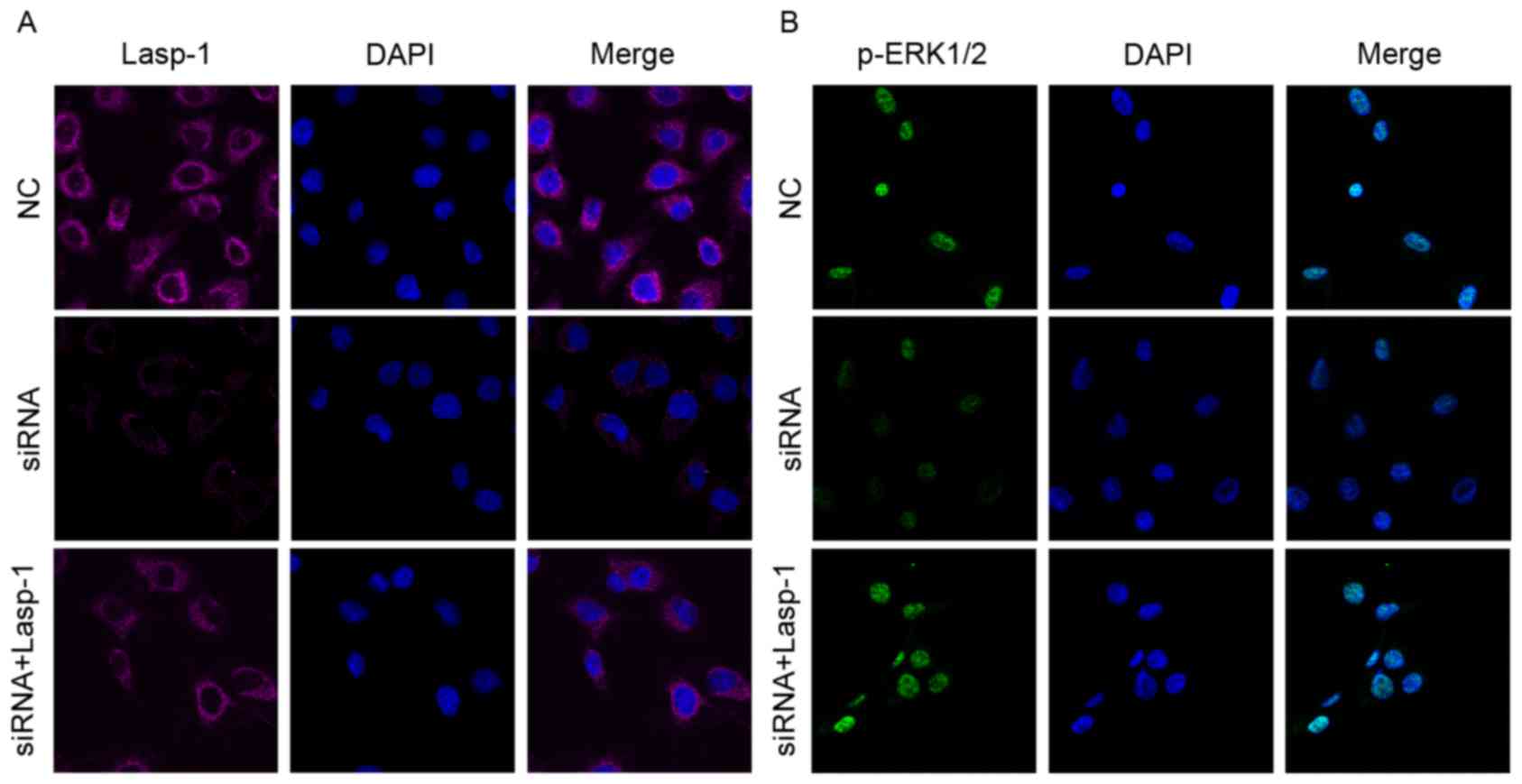
immunofluorescence staining, it was further confirmed that Lasp-1
and p-ERK1/2 were decreased following Lasp-1 knockdown (Fig. 3A and B). Notably, it was observed that
Lasp-1 inducedp-ERK1/2 expression, indicating that Lasp-1 regulates
ERK1/2 expression in the progression of colorectal carcinoma.
Lasp-1 knockdown inhibits cellular
proliferation, induces apoptosis and cell cycle arrest in the
G0/G1 phase
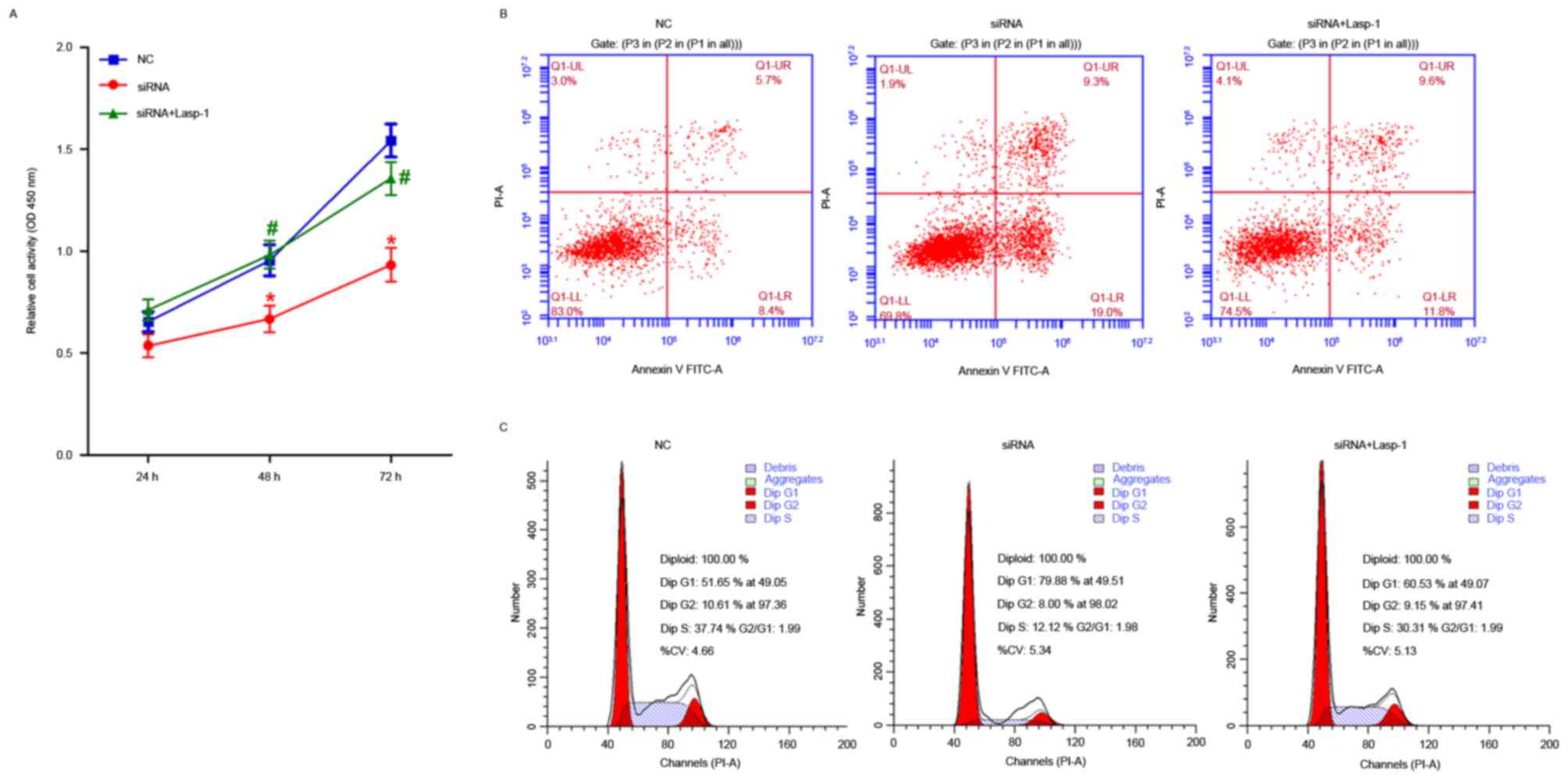
The effects of Lasp-1 knockdown on cellular
proliferation, induction of apoptosis and cell cycle arrest were
investigated (Fig. 4). Cellular
proliferation was impaired at 48 and 72 h after Lasp-1 knockdown
(Fig. 4A). Furthermore, increased
early-(19.0 vs. 8.4%) as well as late-(9.3 vs. 5.7%) stage
(Fig. 4B) apoptotic cell death was
detected. Furthermore, Lasp-1 silencing increased the proportion of
cells in the G0/G1 phase of the cell cycle
(79.88 vs. 51.65%) and decreased the proportion of cells in the S
phase (12.12 vs. 37.74%). By contrast, overexpression of
Lasp-1induced cellular proliferation and inhibited the induction of
apoptotic cell death, compared with cells treated with siRNA
(Fig. 4B and C). These results
indicate that cell-cycle arrest in the G0/G1
phase of the cell cycle was induced following Lasp-1 knockdown.
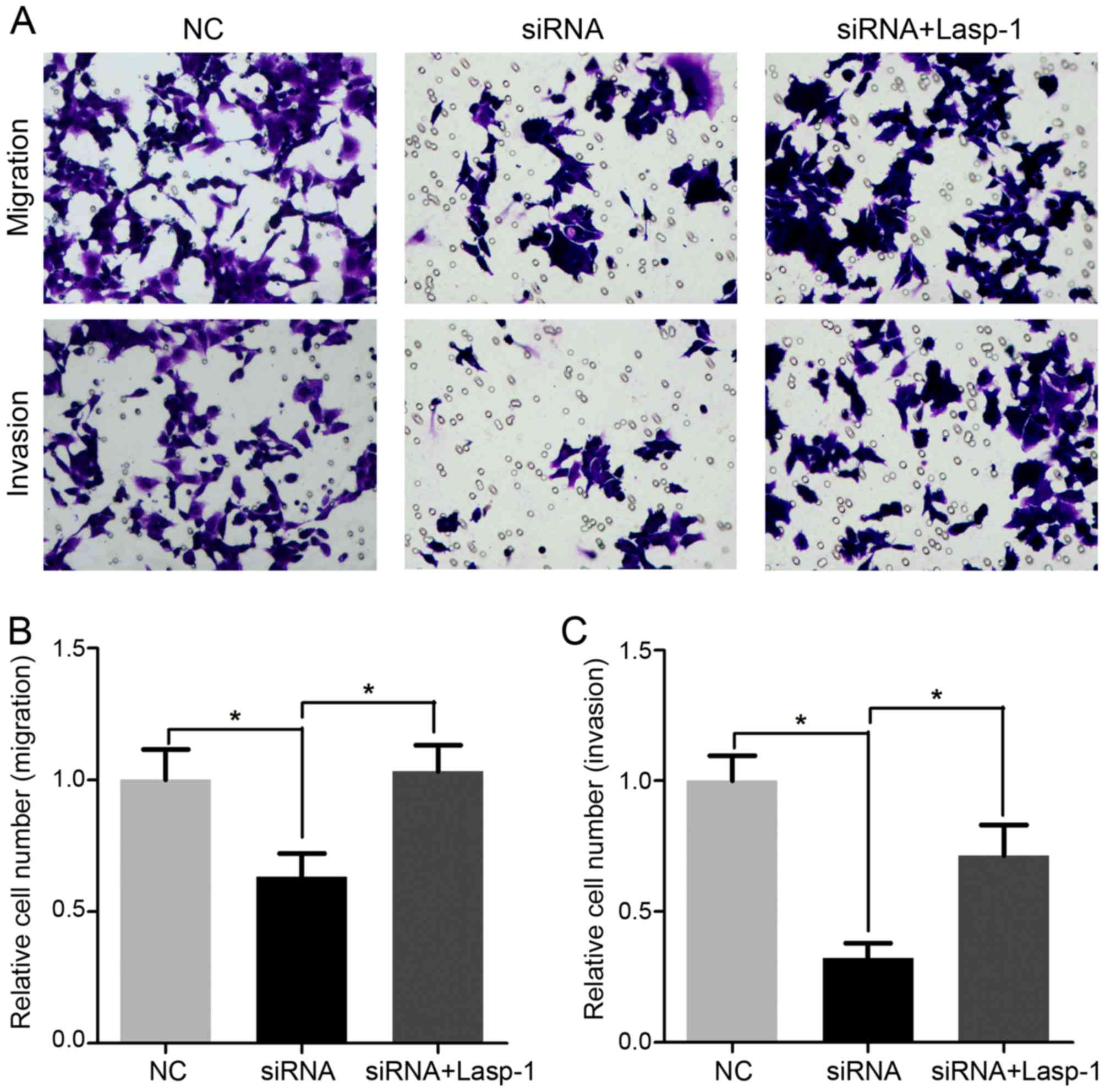
Decreased Lasp-1 expression inhibits
cellular migration and invasion
Cell migration and invasion following Lasp-1
knockdown were investigated using the Transwell assay (Fig. 5). It was demonstrated that cellular
migration and invasion were significantly inhibited in cells
treated with Lasp-1 siRNA compared with the NC group (Fig. 5B and C). Notably, migration and
invasion were increased following Lasp-1 overexpression. These
results indicated that Lasp-1 promotes metastasis in colorectal
carcinoma cells.
Discussion
Colorectal carcinoma is the second leading cause of
cancer-associated mortality worldwide (1,2). The
results of the present study suggest that Lasp-1 regulates the
progression of colorectal carcinoma via activation of the MAPK
signaling pathway. The Lasp-1 contains a N-terminal LIM domain,
composed of two sequential zinc-binding modules with a typical LIM
motif, followed by a C-terminal Src homology region 3 (SH3) domain
and by tandem 35-residue nebulin-like repeats R1 and R2 (14,25). The
SH3 domain is shared with diverse structural and signaling proteins
(26). It has been suggested that LIM
domains bound to DNA are involved in homodimerization (27). The R1 and R2 repeats are involved in
protein-protein interactions and in cytoskeleton stability
(28,29). Lasp-1 protein interaction is essential
for cell motility, resulting in the activation of ERK1/2 (30). In the present study, it was
demonstrated that Lasp-1 and ERK1/2 were upregulated in tumor
tissues compared with adjacent normal colorectal mucosa tissues in
20 patients with colorectal carcinoma. Furthermore, decreased
Lasp-1 expression was associated with decreased ERK1/2 levels.
Lasp-1 phosphorylates a number of proteins involved
in the MAPK signaling pathway (20).
It has been demonstrated that miR-133α downregulated the expression
of Lasp-1, by inhibiting the phosphorylation of ERK and MEK,
resulting in suppressed tumor growth and metastasis in liver and
lung cancer cells (22). Exogenous
miR-133α inhibited the MAPK signaling pathway and increased the
expression of epithelial markers (23). A similar effect on
epithelial-mesenchymal transition has been observed following
Lasp-1 targeting (22). Consistently,
the results of the present study demonstrated that Lasp-1 mRNA was
upregulated in tumor samples, compared with adjacent normal
tissues. Additionally, Lasp-1 knockdown induced apoptotic cell
death and cell cycle arrest in the G0/G1
phase. Furthermore, it suppressed proliferation, migration and
invasion, suggesting a negative regulation of the progression and
metastatic potential of colorectal carcinoma.
Notably, Lasp-1 promotes proliferation and
metastasis in gallbladder cancer via the induction of cell cycle
arrest at the G2/M phase. Therefore, it is concluded
that Lasp-1 may promote cancer cell proliferation and metastasis
via modulating different phases of the cell cycle. A number of key
factors, including cyclin-dependent kinase, mitosis-promoting
factor, ataxia telangiectasia mutated serine/threonine kinase, p53
and retinoblastoma are involved in the regulation of cell cycle
progression (31–33). Additional studies are required to
validate the effect of Lasp-1 in a variety of tumors and
investigate the role of ERK1/2 and other MAPK-associated signaling
pathways, including the stress-activated protein kinase/c-Jun
N-terminal kinase signaling pathway and the p38 MAPK signaling
pathway in tumor progression.
The results of the present study demonstrated that
the role of Lasp-1 in the progression and metastasis of colorectal
carcinoma is associated with the MAPK signaling pathway. The data
of the present study elucidate the effect of Lasp-1 in the
progression and metastasis of colorectal carcinoma and suggest a
novel potential target for the treatment of patients with
metastatic colorectal carcinoma.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Quintero E, Carrillo M, Leoz ML, Cubiella
J, Gargallo C, Lanas A, Bujanda L, Gimeno-García AZ,
Hernández-Guerra M, Nicolás-Pérez D, et al: Risk of advanced
neoplasia in first-degree relatives with colorectal cancer: A large
multicenter cross-sectional study. PLoS Med. 13:e10020082016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krbal L, Hanušová V, Soukup J, John S,
Matoušková P and Ryška A: Contribution of in vitro comparison of
colorectal carcinoma cells from primary and metastatic lesions to
elucidation of mechanisms of tumor progression and response to
anticancer therapy. Tumour Biol. 37:9565–9578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tan WJ, Chew MH, Tan IB, Law JH, Zhao R,
Acharyya S, Mao YL, Fernandez LG, Loi CT and Tang CL: Palliative
surgical intervention in metastatic colorectal carcinoma: A
prospective analysis of quality of life. Colorectal Dis.
18:357–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grunewald TG, Kammerer U, Schulze E,
Schindler D, Honig A, Zimmer M and Butt E: Silencing of LASP-1
influences zyxin localization, inhibits proliferation and reduces
migration in breast cancer cells. Exp Cell Res. 312:974–982. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaman VSA, Poppe H, Houben R, Grunewald
TG, Goebeler M and Butt E: LASP1, a newly identified melanocytic
protein with a possible role in melanin release, but not in
melanoma progression. PLoS One. 10:e01292192015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang F, Zhou X, Du S, Zhao Y, Ren W, Deng
Q, Wang F and Yuan J: LIM and SH3 domain protein 1 (LASP-1)
overexpression was associated with aggressive phenotype and poor
prognosis in clear cell renal cell cancer. PLoS One. 9:e1005572014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zheng J, Wang F, Lu S and Wang X: LASP-1,
regulated by miR-203, promotes tumor proliferation and
aggressiveness in human non-small cell lung cancer. Exp Mol Pathol.
100:116–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hailer A, Grunewald TG, Orth M, Reiss C,
Kneitz B, Spahn M and Butt E: Loss of tumor suppressor mir-203
mediates overexpression of LIM and SH3 protein 1 (LASP1) in
high-risk prostate cancer thereby increasing cell proliferation and
migration. Oncotarget. 5:4144–4153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Z, Chen Y, Wang X, Zhang H, Zhang Y,
Gao Y, Weng M, Wang L, Liang H, Li M, et al: LASP-1 induces
proliferation, metastasis and cell cycle arrest at the G2/M phase
in gallbladder cancer by down-regulating S100P via the PI3K/AKT
pathway. Cancer Lett. 372:239–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Payton S: Bladder cancer: LASP-1-a
promising urine marker for detection of bladder cancer. Nat Rev
Urol. 9:2402012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ardelt P, Grünemay N, Strehl A, Jilg C,
Miernik A, Kneitz B and Butt E: LASP-1, a novel urinary marker for
detection of bladder cancer. Urol Oncol. 31:1591–1598. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orth MF, Cazes A, Butt E and Grunewald TG:
An update on the LIM and SH3 domain protein 1 (LASP1): A versatile
structural, signaling, and biomarker protein. Oncotarget. 6:26–42.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salvi A, Bongarzone I, Ferrari L, Abeni E,
Arici B, De Bortoli M, Scuri S, Bonini D, Grossi I, Benetti A, et
al: Molecular characterization of LASP-1 expression reveals
vimentin as its new partner in human hepatocellular carcinoma
cells. Int J Oncol. 46:1901–1912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Li W, Jin X, Cui S and Zhao L: LIM
and SH3 protein 1, a promoter of cell proliferation and migration,
is a novel independent prognostic indicator in hepatocellular
carcinoma. Eur J Cancer. 49:974–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao L, Wang H, Liu C, Liu Y, Wang X, Wang
S, Sun X, Li J, Deng Y, Jiang Y and Ding Y: Promotion of colorectal
cancer growth and metastasis by the LIM and SH3 domain protein 1.
Gut. 59:1226–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Wang H, Sun X and Ding Y:
Comparative proteomic analysis identifies proteins associated with
the development and progression of colorectal carcinoma. FEBS J.
277:4195–4204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang H, Li Z, Chu B, Zhang F, Zhang Y, Ke
F, Chen Y, Xu Y, Liu S, Zhao S, et al: Upregulated LASP-1
correlates with a malignant phenotype and its potential therapeutic
role in human cholangiocarcinoma. Tumour Biol. 37:8305–8315. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang H, Shi J, Luo Y, Liao Q, Niu Y, Zhang
F, Shao Z, Ding Y and Zhao L: LIM and SH3 protein 1 induces
TGFβ-mediated epithelial-mesenchymal transition in human colorectal
cancer by regulating S100A4 expression. Clin Cancer Res.
20:5835–5847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin YH, Park ZY, Lin D, Brahmbhatt AA, Rio
MC, Yates JR III and Klemke RL: Regulation of cell migration and
survival by focal adhesion targeting of Lasp-1. J Cell Biol.
165:421–432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, An H, Wang B, Liao Q, Li W, Jin X,
Cui S, Zhang Y, Ding Y and Zhao L: miR-133a represses tumour growth
and metastasis in colorectal cancer by targeting LIM and SH3
protein 1 and inhibiting the MAPK pathway. Eur J Cancer.
49:3924–3935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu L, Zhang Y, Wang H, Zhang G, Ding Y and
Zhao L: Tumor suppressor miR-1 restrains epithelial-mesenchymal
transition and metastasis of colorectal carcinoma via the MAPK and
PI3K/AKT pathway. J Transl Med. 12:2442014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomasetto C, Moog-Lutz C, Régnier CH,
Schreiber V, Basset P and Rio MC: Lasp-1 (MLN 50) defines a new LIM
protein subfamily characterized by the association of LIM and SH3
domains. FEBS Lett. 373:245–249. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kay BK: SH3 domains come of age. FEBS
Lett. 586:2606–2608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hammarström A, Berndt KD, Sillard R,
Adermann K and Otting G: Solution structure of a
naturally-occurring zinc-peptide complex demonstrates that the
N-terminal zinc-binding module of the Lasp-1 LIM domain is an
independent folding unit. Biochemistry. 35:12723–12732. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pappas CT, Bliss KT, Zieseniss A and
Gregorio CC: The Nebulin family: An actin support group. Trends
Cell Biol. 21:29–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mihlan S, Reiß C, Thalheimer P, Herterich
S, Gaetzner S, Kremerskothen J, Pavenstädt HJ, Lewandrowski U,
Sickmann A and Butt E: Nuclear import of LASP-1 is regulated by
phosphorylation and dynamic protein-protein interactions. Oncogene.
32:2107–2113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raman D, Sai J, Neel NF, Chew CS and
Richmond A: LIM and SH3 protein-1 modulates CXCR2-mediated cell
migration. PLoS One. 5:e100502010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Celeghini C, Voltan R, Rimondi E, Gattei V
and Zauli G: Perifosine selectively induces cell cycle block and
modulates retinoblastoma and E2F1 protein levels in p53 mutated
leukemic cell lines. Invest New Drugs. 29:392–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park SJ, Yang SW and Kim BC: Transforming
growth factor-β1 induces cell cycle arrest by activating atypical
cyclin-dependent kinase 5 through up-regulation of Smad3-dependent
p35 expression in human MCF10A mammary epithelial cells. Biochem
Biophys Res Commun. 472:502–507. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chaudhary P, Sharma R, Sahu M, Vishwanatha
JK, Awasthi S and Awasthi YC: 4-Hydroxynonenal induces G2/M phase
cell cycle arrest by activation of the ataxia telangiectasia
mutated and Rad3-related protein (ATR)/checkpoint kinase 1 (Chk1)
signaling pathway. J Biol Chem. 288:20532–20546. 2013. View Article : Google Scholar : PubMed/NCBI
|