Introduction
Osteosarcoma (OS), which is the most common
histological form of primary bone cancer that arises from primitive
transformed cells of mesenchymal origin, exhibits osteoblastic
differentiation and produces malignant osteoid (1). OS is an aggressive malignant neoplasm
primarily identified in teenagers and young adults, with an
incidence of 4–5 cases/million people (2). OS is the eighth-most common form of
childhood cancer, comprising 2.4% of all malignancies in pediatric
patients and ~20% of all primary bone cancer cases in the United
States in 2008 (3). Despite the
success of chemotherapy and other therapeutic options that have
been reported for the treatment of OS, studies have demonstrated
that the 5-year overall survival rate remains ~70% (4–6).
Therefore, elucidation of the mechanism underlying the initiation
and progression of OS is urgent, and of great interest.
MicroRNAs (miRNAs/miRs) are a class of short (~22
nucleotides) and highly conserved non-coding RNA sequences, which
are able to regulate gene expression via targeting promoters of
mRNAs for transcriptional inhibition or translational repression
(7). miRNAs are important modulators
of key regulatory networks and signaling pathways through their
influence on target genes (8). The
accumulating scientific and clinical evidence has suggested that
miRNAs are involved in tumorigenesis and tumor progression, serving
the roles of tumor suppressors or oncogenes, and have become
potential biomarkers for tumor diagnosis, therapy and prognosis
(9).
miR-542-3p, located in Xq26.3, has been reported to
serve a crucial role in the initiation and development of several
types of cancer, including astrocytoma (10), neuroblastoma (11), hepatocellular carcinoma (12), breast cancer (13), colorectal cancer (14) and melanoma (15). miR-542-3p has also been reported to be
downregulated in non-small cell lung cancer and breast cancer, and
directly targets survivin or angiopoietin-2, respectively, leading
to cell growth, arrest and the inhibition of angiogenesis (16,17).
However, whether miR-542-3p is involved in the
tumorigenesis and tumor progression of OS remains unclear. In the
present study, the biological function and underlying mechanism of
miR-542-3p in OS were investigated, and the expression of
miR-542-3p was decreased in OS tissues and cell lines. Through
bioinformatics analysis and luciferase reporter assays, it was
identified that miR-542-3p directly targeted Smad2 mRNA and
negatively regulated the expression of Smad2 at the protein level.
The present study demonstrated that restoration of miR-542-3p was
able to suppress the growth and proliferation of OS cells through
directly targeting Smad2, and revealed the mechanism of its
tumor-suppressive role in OS pathogenesis. Therefore miR-542-3p may
serve as a promising therapeutic target of OS.
Materials and methods
Cell lines and tissue samples
The human osteoblastic cell line hFOB, OS cell lines
(U2OS, MG-63, and SAOS-2) and 293T cells were purchased from the
Cell Bank Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). All cell lines were maintained in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified 5% CO2 atmosphere.
A total of 48 OS tissues and self-matched non-tumor
tissues were collected from 48 patients with OS between February
2014 and September 2015. In total, 40 cases were <18 years old
and 8 cases were >18 years old, and 22 cases were women and 26
cases were men. All tissue samples were obtained via surgical
resection and stored in liquid nitrogen until use in further
analyses. All OS tissues were pathologically confirmed, and OS
specimens that received chemotherapy, radiotherapy, or other types
of therapy were excluded from the present study. The collection and
the use of all tissue samples in the present study were approved by
the Research Ethics Committee of Wenzhou Hospital of Integrated
Traditional Chinese and Western Medicine (Wenzhou, China). Written
informed consent was obtained from all participating patients.
RNA quantification and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from OS cell lines, OS
tissues and self-matched adjacent normal tissues using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Purified mRNA and miRNAs were quantified
by a RT-qPCR assay using an All-in-One™ miRNA RT-qPCR
Detection kit (GeneCopoeia, Inc., Rockville, MD, USA) according to
the manufacturer's protocol. All primers are listed in Table I. The reverse primer sequence of
miR-542-3p was provided by All-in-one miRNA RT-qPCR Detection kit
(GeneCopoeia, Inc.). U6 small RNA was used as an internal control
for normalization, miR-542-3p expression were calculated as
2−[(Ct miR-542-3p)-(Ct U6)], where Ct represents the
threshold cycle for each transcript.
 | Table I.Sequence of primers and RNA fragments
used in the present study. |
Table I.
Sequence of primers and RNA fragments
used in the present study.
| Name |
| Sequence (5′-3′) |
|---|
| U6 | F |
5′-GTGCTCGCTTCGGCAGCACAT-3′ |
|
| R |
5′-AATATGGAACGCTTCACGAAT −3′ |
| β-actin | F |
5′-AGAGCTACGAGCTGCCTGAC-3′ |
|
| R |
5′-AGCACTGTGTTGGCGTACAG-3′ |
| Smad2 (reporter) | F |
5′-AAACTAGTTCTTGTAGAGGTTGTGT-3′ |
|
| R |
5′-GGAAGCTTGCAGATTTCCTTCTGCC-3′ |
| Smad2 (pGL3) | F |
5′-AAAGGTACCACATGTCGTCCATCTTGCCATTCACG-3′ |
|
| R |
5′-AAACTCGAGTGGGACTTGATTGGTGAAGCTTTATGAC-3′ |
| miR-542-3p | F |
5′-TGTGACAGATTGATAACTGAAA-3′ |
Oligonucleotide transfection
RNA oligos were chemically synthesized and purified
by Shanghai Genepharma Co., Ltd., (Shanghai, China). The sense
sequence of the human miR-542-3p mimics was
5′-UGUGACAGAUUGAUAACUGAAA-3′, and the antisense sequence was
5′-UUUCAGUUAUCAAUCUGUCACA-3′. The sequences of the negative control
oligonucleotides were 5′-AAUUCUCCGAACGUGUCACTT-3′ and
5′-GUGACACGUUCGGAGAAUUTT-3′. In total, 1×105 cells were
seeded in a 6-well plate, and the transfections were performed with
INTERFER in reagent (Polyplus-transfection SA, Illkirch, France)
according to the manufacturer's protocol. The final concentration
of miRNA was 50 nM.
To construct a pGL3-Smad2 vector, the sequence of
Smad2 mRNA was amplified (primers are listed in Table I). The Smad2 fragments were inserted
into pGL3 by the designed cutting sites: KpnI and
XhoI. In total, 1×105 cells were seeded in a
6-well plate, the transfections were performed with
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
final concentration of plasmids was 100 ng.
Luciferase reporter assays
The pMIR-Smad2 luciferase reporter vector was
constructed by cloning a human Smad2 mRNA sequence into a
pMIR-Report construct (Ambion; Thermo Fisher Scientific, Inc.). A
60-bp Smad2 mRNA fragment (5,084–5,143 bp of Smad2 mRNA), which is
the predicted target of miR-542-3p, was amplified and cloned into
the luciferase reporter via SpeI and HindIII sites.
All sequences are listed in Table I.
Luciferase reporter assays were performed as follows:
5×103 293T cells were seeded in a 96-well plate, then
co-transfected with 50 nM single-stranded miR-542-3p mimics or
negative control oligonucleotides, 10 ng of pMIR-Smad2 luciferase
reporter, pMIR luciferase reporter and 3 ng of pRL-TK (Promega
Corporation, Madison, WI, USA) using the JetPRIME®
reagent (Polyplus-transfection SA). Cells were collected 36 h after
transfection and analyzed using a Dual-Luciferase Reporter assay
system (Promega Corporation). pRL-TK was cotransfected as an
internal control to correct the differences in both transfection
and harvest efficiencies. The firefly luciferase activity of each
sample was normalized to the Renilla luciferase
activity.
Cell proliferation assay
The proliferation of OS cells was measured by MTT
assay. A total of 5,000 cells were seeded into each well of 96-well
plates and transfected with miR-542-3p mimics or negative control
oligonucleotides at a final concentration of 50 nM. At 24, 48 and
72 h after transfection, the medium was replaced with 100 µl fresh
medium containing MTT (0.5 mg/ml), and the plates were incubated
for 4 h at 37°C. The medium was replaced by 100 µl DMSO
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and plates were
agitated at room temperature for 10 min. The absorbance was
measured at 570 nm.
Cell apoptosis analyses
Cell apoptosis analyses were performed using a
Phycoerythrin-Annexin V Apoptosis Detection kit I (BD Pharmingen;
BD Biosciences, San Jose, CA, USA). For cell apoptosis analysis,
8×105 cells were seeded in each well of 6-well plates.
At 78 h after transfection, cells were harvested and labeled with
Annexin V for 15 min. Subsequently, 50 µg/ml propidium iodide was
added for 1 h at 37°C to each sample prior to flow cytometry using
the BD LSR II (BD Biosciences).
Prediction of candidate miRNA
targets
The possible targets of miR-542-3p were screened by
the DIANA-TarBase web platform (version 7; http://diana.imis.athena-innovation.gr/), which
included >500,000 experimentally confirmed miRNA-mRNA
interactions utilizing cell types from 24 species (18).
Western blot analysis
MG-63 and U2OS cells were washed with pre-chilled
PBS three times. The cells were then solubilized in 1% Nonidet P-40
lysis buffer [20 mM Tris, pH 8.0, 137 mM NaCl, 1% Nonidet P-40, 10%
glycerol, 1 mM CaCl2, 1 mM MgCl2, 1 mM phenylmethylsulfonyl
fluoride, 1 mM sodium fluoride, 1 mM sodium orthovanadate, and
phosphatase inhibitor cocktail II (5 mg/ml, Sigma-Aldrich; Merck
KGaA)] for 0.5 h at 4°C, and protein concentration was determined
using a bicinchoninic acid assay (BCA Protein Assay Kit, Pierce;
Thermo Fisher Scientific, Inc.). Proteins (40 µg) were separated on
a 12% SDS-PAGE gel and then transferred to a nitrocellulose
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membrane was blocked with 5% non-fat milk in PBS with 0.1% Tween-20
for 2 h and washed three times with PBS with 0.1% Tween-20 at 4°C,
then incubated with anti-Smad2 antibody (1:1,000 dilution) or
anti-β-actin antibody (1:5,000 dilution) (Sigma-Aldrich; Merck
KGaA). Following extensive washing, a goat anti-mouse secondary
antibody (1:1,000 dilution) (Pierce; Thermo Fisher Scientific,
Inc.) was added to the system. The proteins were detected using
enhanced chemiluminescence reagents (Pierce; Thermo Fisher
Scientific, Inc.). Protein bands were quantified using Pearson's
correlation coefficient analysis (LabWorks software version 4.0;
Image Acquisition; UVP, Inc., Upland, CA, USA).
Statistical analysis
All statistical analyses were performed using the
SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA). Data
are presented as the mean ± standard deviation. Data of miR-542-3p
expression levels in OS specimens compared to matched adjacent
normal tissues were subjected to analysis by paired Student's
t-test. Data of miR-542-3p expression levels in several cell lines
and MTT analysis were analyzed by one-way analysis of variance
followed by the Student-Newman-Keuls post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-542-3p is decreased
in OS
In order to identify the role of miR-542-3p in OS
carcinogenesis, the expression levels of miR-542-3p in 48 OS
samples and three OS cell lines were analyzed. Total RNAs were
extracted from OS tissues and adjacent normal tissues. The
expression levels of miR-542-3p were analyzed using RT-qPCR and
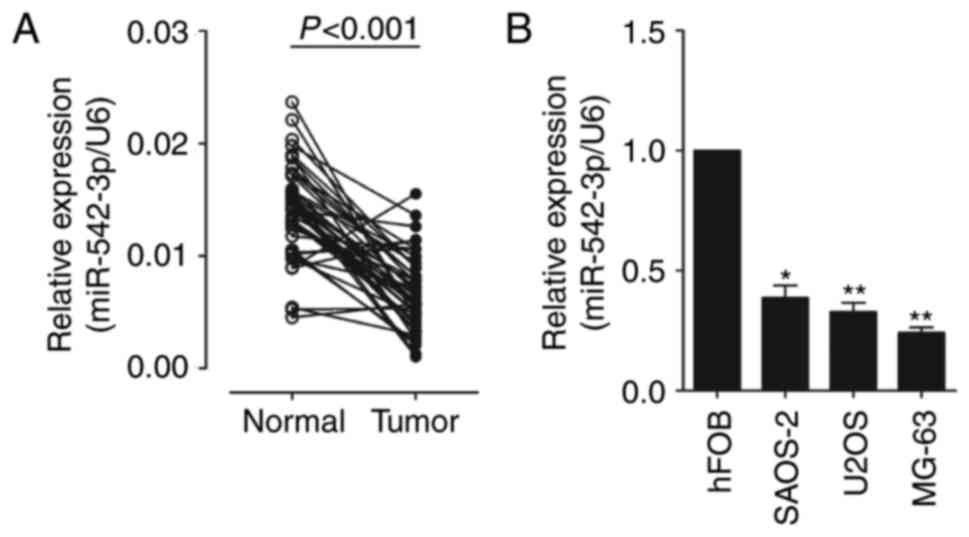
normalized to an endogenous control (U6 RNA). As shown in Fig. 1A, the expression of miR-542-3p was
significantly decreased in OS tissues vs. adjacent normal tissues
(0.0066±0.0033 vs. 0.0141±0.0040). The results also demonstrated
that the expression of miR-542-3p was significantly downregulated
in OS cell lines, U2OS, MG-6 and SAOS-2, compared with which in
human osteoblastic cell line hFOB (Fig.
1B). This indicates that miR-542-3p may function as an
oncosuppressor gene in OS carcinogenesis.
Overexpression of miR-542-3p inhibits
cell growth and induces apoptosis in OS cells
To further investigate the effect of miR-542-3p on
the proliferation ability of OS cells, gain of function studies
were performed in two human OS cell lines, MG-63 and U2OS.
miR-542-3p mimics or negative control oligonucleotides were
transiently transfected into MG-63 and U2OS cells. Expression of
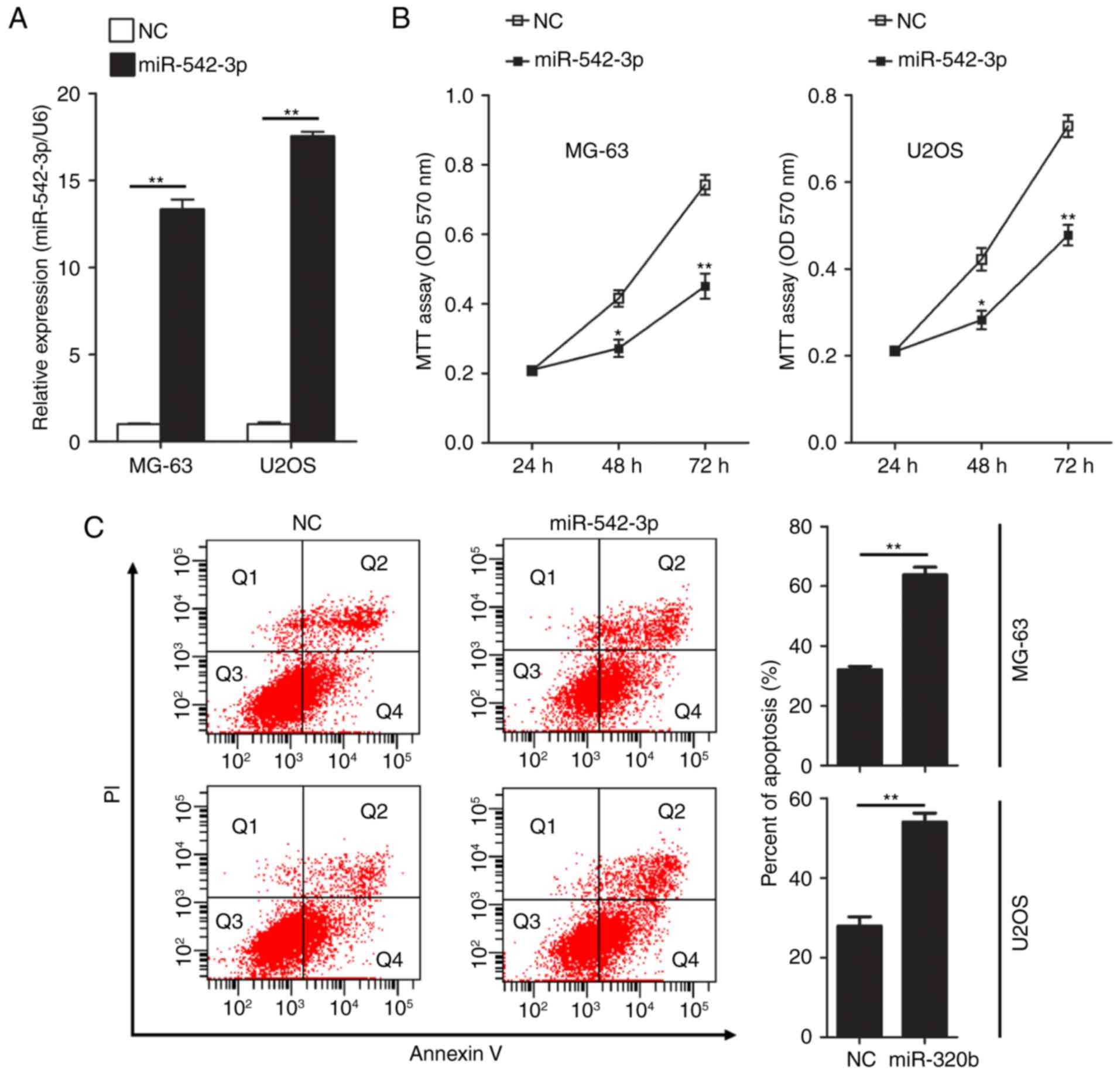
miR-542-3p was detected by RT-qPCR. The results confirmed that
expression levels of miR-542-3p in MG-63 and U2OS cells were
significantly increased following transfection of miR-542-3p mimics
(Fig. 2A). Next, the functional role
of miR-542-3p in cell proliferation in OS cells was investigated by
performing MTT assays. Restoration of miR-542-3p expression in
MG-63 and U2OS cells resulted in significant suppression of cell
proliferation (Fig. 2B). Using flow
cytometry, the influence of overexpression of miR-542-3p on
apoptosis in U2OS and MG63 cells was evaluated following
transfection of miR-542-3p mimics. Overexpression of miR-542-3p
significantly increased the rate of apoptosis in MG-63 (63.77 vs.
32.07% in the control group; P<0.01) and U2OS cells (54.03 vs.
27.9% in the control group; P<0.01; Fig. 2C).
Smad2 as miR-542-3p direct target in
OS cells
After confirmation of function action of miR-542-3p
in OS cells, the underlying mechanism of the effects of miR-542-3p
in OS was investigated. Recently published DIANA-TarBase v7.0 data
included over half a million experimentally confirmed miRNA-mRNA
interactions utilizing cell types from 24 species (18). Following this analysis, many potential
miR-542-3p targeting genes were identified (data not shown). Among
these targeting genes, the present study focused on Smad2, which is
a member of the Smad family. Smad2 has been previously identified
in the regulation of cell proliferation and in apoptosis as a key
element in TGF-β signaling (19,20). In
addition, mirPath (http://www.microrna.gr/miRPathv2) was used to perform
the pathway analysis of the target genes of miR-542-3p. Enrichment
analysis identified the 12 most significant pathways, which
included the ‘TGF-β signaling pathway’ (P=0.003; Table II).
 | Table II.Pathway analysis of potential
miR-542-3p targeting genes. |
Table II.
Pathway analysis of potential
miR-542-3p targeting genes.
| KEGG pathway | Count (target
genes) | P-value |
|---|
| Prion diseases | 1 |
1.46×10−36 |
| Lysine
degradation | 4 | 3.56×10-3 |
| Biosynthesis of
unsaturated fatty acids | 1 |
3.56×10−3 |
| TGF-β signaling
pathway | 7 | 3.56×10-3 |
| Cell cycle | 10 |
4.64×10−3 |
| Ubiquitin mediated
proteolysis | 13 | 1.17×10-2 |
| Adherens
junction | 7 |
1.43×10−2 |
| Viral
carcinogenesis | 13 | 1.43×10-2 |
| Endocytosis | 13 |
2.28×10−2 |
| Central carbon
metabolism in cancer | 5 | 2.28×10-2 |
| ECM-receptor
interaction | 4 |
4.70×10−2 |
| Proteoglycans in
cancer | 13 | 4.70×10-2 |
Consequently, the present study established whether
Smad2 was a genuine target of miR-542-3p by performing a set of
experiments. To confirm whether miR-542-3p regulated the expression
of Smad2 gene, a luciferase reporter assay was performed in 293T
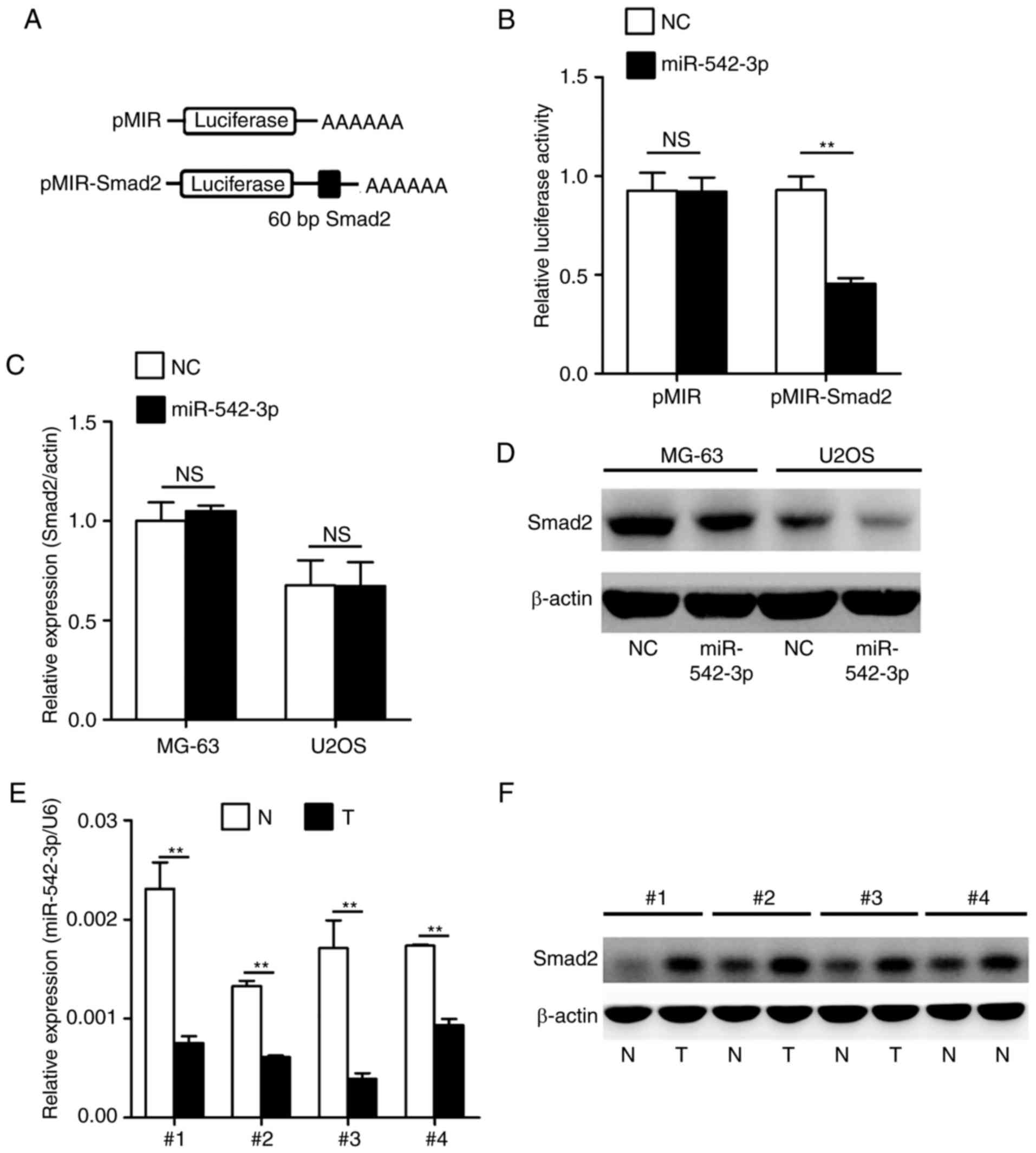
cells. As indicated in Fig. 3A, a
targeting sequence of Smad2 mRNA was cloned into a luciferase
reporter plasmid. These luciferase reporter plasmids were
co-transfected into 293T cells with miR-542-3p mimics or negative
control oligonucleotides. The luciferase activities in transfected
cells were measured after 36 h. As shown in Fig. 3B, overexpression of miR-542-3p caused
a significant decrease in luciferase activity in cells transfected
with the reporter plasmid containing the miR-542-3p-targeted
sequence of Smad2 mRNA, whereas overexpression of miR-542-3p
produced no significant change in luciferase activity in cells
transfected with the reporter plasmid without the targeted sequence
of Smad2. Subsequently, the present study investigated whether
endogenous Smad2 in OS cells was similarly regulated. MG-63 and
U2OS cells were transfected with miR-542-3p mimics or negative
control oligonucleotides. The mRNA and protein levels of Smad2 were
examined by RT-qPCR and western blotting, respectively. The level
of Smad2 protein was consistently and substantially downregulated
by miR-542-3p; however, the level of Smad2 mRNA was not affected by
miR-542-3p (Fig. 3C and D). Finally,
the expression levels of miR-542-3p and Smad2 were analyzed in four
representative OS tumor tissues and adjacent non-tumor tissues by
RT-qPCR and western blotting. The results confirmed that OS tumor
tissues with low expression of miR-542-3p exhibited markedly higher
Smad2 expression compared with adjacent non-tumor tissues (Fig. 3E and F).
Taken together, these results demonstrated that
Smad2 is a direct target of miR-542-3p in OS, and that miR-542-3p
directly regulates Smad2 expression at the protein level.
Restoration of miR-542-3p inhibits
Smad2-induced OS cell proliferation
In order to further confirm that miR-542-3p exerts
tumor-suppressor activity in OS pathogenesis through targeting
Smad2, gain of function and rescue experiments were performed in OS
cells. It was investigated whether the proliferation of OS cells
was further promoted following transfection with Smad2 cDNA, and
whether this effect could be attenuated by miR-542-3p mimics. Smad2
cDNA was transiently transfected into MG-63or U2OS cells with and
without miR-542-3p mimics. Subsequently cell proliferation assays
were performed.
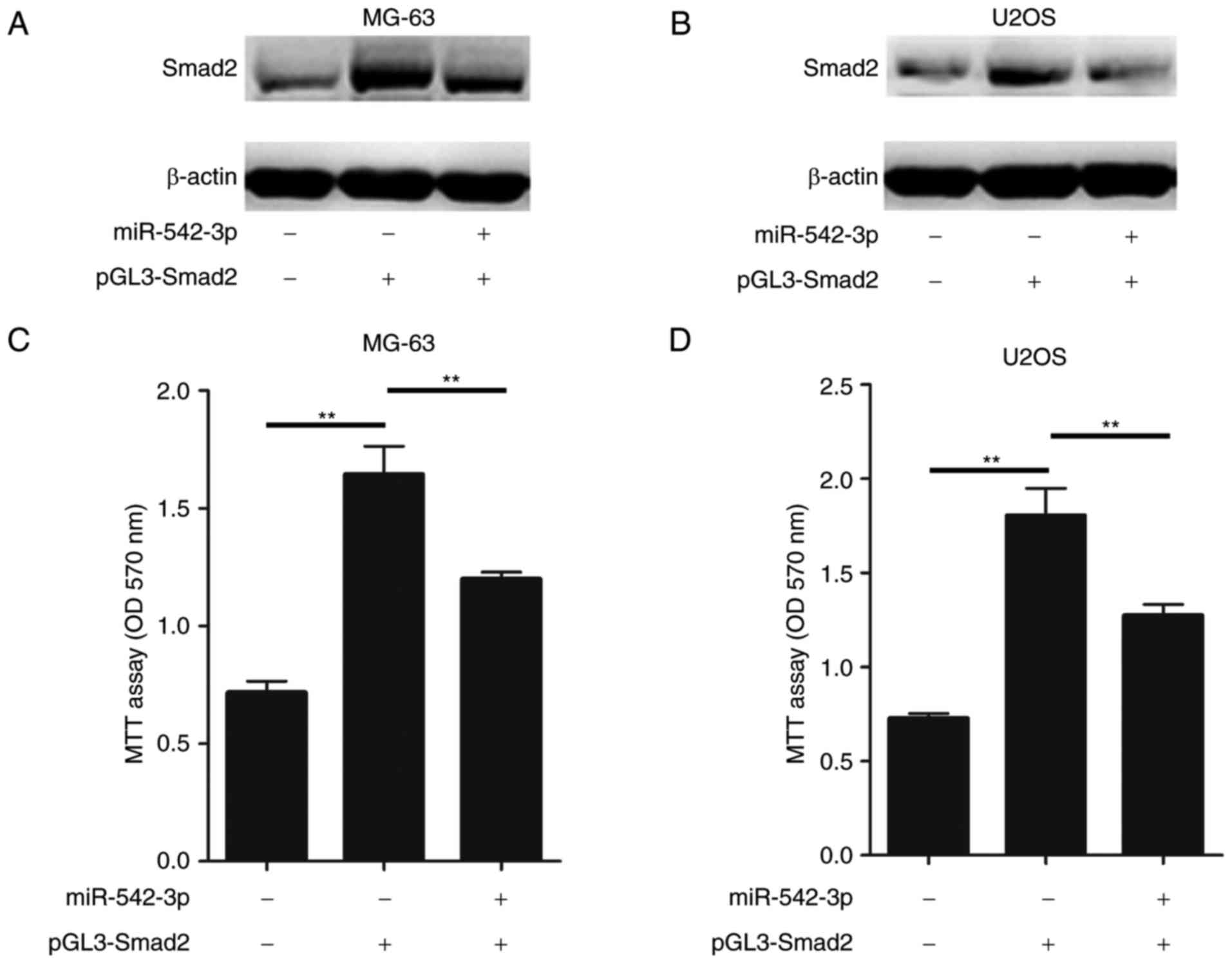
As hypothesized, ectopic expression of Smad2 in
MG-63 cells enhanced the accumulation of Smad2, while restoration
of the miR-542-3p expression in MG-63 cells markedly inhibited the
expression of Smad2 (Fig. 4A).
Furthermore, restoration of miR-542-3p inhibited the Smad2
expression in U2OS cells (Fig. 4B).
In conjunction with these results, the overexpression of miR-542-3p
significantly attenuated the Smad2-induced increase in cell
proliferation in MG-63 and U2OS cells (Fig. 4C and D). Taken together, these results
demonstrated that restoration of miR-542-3p was able suppress the
growth and proliferation of OS cells through directly targeting
Smad2.
Discussion
OS is the most frequently occurring type of primary
bone cancer. Although multiple treatments options, including
chemotherapy, are available, the 5-year survival rate of OS remains
poor due to the occurrence of drug resistance among patients
(21–23). The OS-associated high mortality rate
is attributed to the difficulty of early diagnosis and the lack of
efficient therapeutic approaches for OS. Therefore, it is essential
to elucidate the underlying mechanism that mediates the initiation
and progression of OS, and to identify diagnostic biomarker or
potential therapeutic targets for the treatment of this disease
(24).
miR-542-3p has been reported to serve crucial roles
in the initiation and development of multiple types of cancer.
These studies reported that miR-542-3p suppressed tumor cell
growth, invasion and metastasis via targeting the AKT
serine/threonine kinase signaling pathway (10), the frizzled class receptor 7/Wnt
pathway (11), serine/threonine
protein kinases (15), survivin
(16) or angiopoietin-2 (17). Previous studies reported that
miR-542-3p expression was decreased in colorectal cancer cells, and
that the restoration of miR-542-3p was able to inhibit the growth
and invasion of colorectal cancer cells through decreasing the
expression of cortactin as a target (14). Another study indicated that miR-542-3p
suppressed cellular proliferation of bladder cancer cells through
post-transcriptionally regulating survivin (25). Kureel et al (26) also reported that miR-542-3p suppressed
osteoblast cell proliferation and differentiation, and inhibited
bone formation through targeting of bone morphogenetic protein 7
signaling (26). However, the
involvement of miR-542-3p is involved in the tumorigenesis and
progression of OS remains unclear.
In the present study, the biological function and
underlying mechanism of miR-542-3p in OS was investigated. This
demonstrated that the expression of miR-542-3p was significantly
decreased in OS tissues and cell lines, and that overexpression of
miR-542-3p in OS cells significantly inhibited cell proliferation,
and induced cell apoptosis. Through bioinformatics analysis, 190
potential miR-542-3p targeting genes were identified, with a member
of the Smad family, Smad2, serving as the primary focus.
Furthermore, mirPath was used to perform pathway analysis of the
predicted target genes of miR-542-3p. Enrichment analysis
identified the 12 most significant pathways, which included the
‘TGF-β signaling pathway’. The TGF-β signaling pathway is involved
in a number of cellular processes, including cell growth,
differentiation, apoptosis and homeostasis, amongst other cellular
functions. TGF-β signaling is mediated by a complex of
membrane-bound type I and type II receptors, and Smad proteins
function as intracellular mediators (27,28).
Binding of the TGF-β ligand with its receptors leads to the
phosphorylation of Smad2 and Smad3. This activity enables the
association of Smad2 and Smad3 with Smad4. Subsequently, the
complex of Smad2/3 and Smad4 is able to translocate to the nucleus,
and bind directly to Smad-binding elements, in addition to a number
of co-activators, to directly modulate TGF-β-regulated gene
expression. Tumor cells may exhibit resistance to TGF-β-induced
growth inhibition and apoptosis if the functional inactivation of
TGF-β receptors and Smads occurs (29,30). In
order to verify this, the present study used luciferase reporter
assays to establish that Smad2 is a genuine target of miR-542-3p.
Furthermore, it was confirmed that OS tumor tissues with low
expression of miR-542-3p exhibited markedly higher Smad2
expression. In order to demonstrate that miR-542-3p serves the role
of tumor suppression in OS pathogenesis through the targeting of
Smad2, gain of function and rescue experiments in OS cells were
performed. The present study demonstrated that restoration of
miR-542-3p was able to suppress the growth and proliferation of OS
cells through directly targeting Smad2.
In conclusion, the findings of the present study
suggest that miR-542-3p may serve as a tumor suppressor gene in OS
pathogenesis, and that miR-542-3p may be a promising therapeutic
target in the treatment of OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by Rising Star in
Medicine of Zhejiang (grant no. 201505403).
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YWu and JY contributed equally to the study. YWu and
YWa conceived and designed the study. YWu, JY, FL and FW performed
the experiments. YWu and JY wrote the paper. YWu, JY, FL, FW and
YWa reviewed and edited the manuscript. All authors read and
approved the manuscript.
Ethics approval and consent to
participate
The collection and the use of all tissue samples in
the present study were approved by the Research Ethics Committee of
Wenzhou Hospital of Integrated Traditional Chinese and Western
Medicine (Wenzhou, China). Written informed consent was obtained
from all participating patients.
Consent for publication
Written informed consent was obtained from all
participating patients.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
YWu, JY and YWa, Department of Orthopedic Surgery,
Wenzhou Hospital of Integrated Traditional Chinese and Western
Medicine, Wenzhou, Zhejiang 325000, P.R. China
References
|
1
|
Luetke A, Meyers PA, Lewis A and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ward E, DeSantis C, Robbins A, Kohler B
and Jemal A: Childhood and adolescent cancer statistics, 2014. CA
Cancer J Clin. 64:83–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kleinerman E: Maximum benefit of
chemotherapy for osteosarcoma achieved-what are the next steps?
Lancet Oncol. 17:1340–1342. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cates JM: Utility of examination of biopsy
tracts in osteosarcoma resection specimens. Am J Clin Pathol.
146:324–327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wilkins RM, Cullen JW, Odom L, Jamroz BA,
Cullen PM, Fink K, Peck SD, Stevens SL, Kelly CM and Camozzi AB:
Superior survival in treatment of primary non-metastatic pediatric
osteosarcoma of the extremity. Ann Surg Oncol. 10:498–507. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lionetti M, Agnelli L, Mosca L, Fabris S,
Andronache A, Todoerti K, Ronchetti D, Deliliers GL and Neri A:
Integrative high-resolution microarray analysis of human myeloma
cell lines reveals deregulated miRNA expression associated with
allelic imbalances and gene expression profiles. Genes Chromosomes
Cancer. 48:521–531. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Visone R and Croce CM: MiRNAs and cancer.
Am J Pathol. 174:1131–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Zhao J, Zhang N, Xu X, Li R, Yi Y,
Fang L, Zhang L, Li M, Wu J and Zhang H: MicroRNA-542-3p suppresses
tumor cell invasion via targeting AKT pathway in human astrocytoma.
J Biol Chem. 290:24678–24688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Althoff K, Lindner S, Odersky A, Mestdagh
P, Beckers A, Karczewski S, Molenaar JJ, Bohrer A, Knauer S,
Speleman F, et al: miR-542-3p exerts tumor suppressive functions in
neuroblastoma by downregulating Survivin. Int J Cancer.
136:1308–1320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu W, Dang S, Feng Q, Liang J, Wang Y and
Fan N: MicroRNA-542-3p inhibits the growth of hepatocellular
carcinoma cells by targeting FZD7/Wnt signaling pathway. Biochem
Biophys Res Commun. 482:100–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venkatadri R, Muni T, Iyer AK, Yakisich JS
and Azad N: Role of apoptosis-related miRNAs in resveratrol-induced
breast cancer cell death. Cell Death Dis. 7:e21042016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Long HC, Gao X, Lei CJ, Zhu B, Li L, Zeng
C, Huang JB and Feng JR: miR-542-3p inhibits the growth and
invasion of colorectal cancer cells through targeted regulation of
cortactin. Int J Mol Med. 37:1112–1118. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rang Z, Yang G, Wang YW and Cui F:
miR-542-3p suppresses invasion and metastasis by targeting the
proto-oncogene serine/threonine protein kinase, PIM1, in melanoma.
Biochem Biophys Res Commun. 474:315–320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon S, Choi YC, Lee S, Jeong Y, Yoon J
and Baek K: Induction of growth arrest by miR-542-3p that targets
survivin. FEBS Lett. 584:4048–4052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He T, Qi F, Jia L, Wang S, Song N, Guo L,
Fu Y and Luo Y: MicroRNA-542-3p inhibits tumour angiogenesis by
targeting angiopoietin-2. J Pathol. 232:499–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vlachos IS, Paraskevopoulou MD, Karagkouni
D, Georgakilas G, Vergoulis T, Kanellos I, Anastasopoulos IL,
Maniou S, Karathanou K, Kalfakakou D, et al: DIANA-TarBase v7.0:
Indexing more than half a million experimentally supported miRNA:
mRNA interactions. Nucleic Acids Res. 43:(Database Issue).
D153–D159. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brown KA, Pietenpol JA and Moses HL: A
tale of two proteins: Differential roles and regulation of Smad2
and Smad3 in TGF-beta signaling. J Cell Biochem. 101:9–33. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bao Y, Chen Z, Guo Y, Feng Y, Li Z, Han W,
Wang J, Zhao W, Jiao Y, Li K, et al: Tumor suppressor microRNA-27a
in colorectal carcinogenesis and progression by targeting SGPP1 and
smad2. PLoS One. 9:e1059912014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lagmay JP, Krailo MD, Dang H, Kim A,
Hawkins DS, Beaty O III, Widemann BC, Zwerdling T, Bomgaars L,
Langevin AM, et al: Outcome of patients with recurrent osteosarcoma
enrolled in seven phase II trials through children's cancer group,
pediatric oncology group, and children's oncology group: Learning
from the past to move forward. J Clin Oncol. 34:3031–3038. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baumann S and Hennet T: Collagen
accumulation in osteosarcoma cells lacking GLT25D1 collagen
galactosyltransferase. J Biol Chem. 291:18514–18524. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Angelini A, Mavrogenis AF, Trovarelli G,
Ferrari S, Picci P and Ruggieri P: Telangiectatic osteosarcoma: A
review of 87 cases. J Cancer Res Clin Oncol. 142:2197–2207. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buondonno I, Gazzano E, Jean SR, Audrito
V, Kopecka J, Fanelli M, Salaroglio IC, Costamagna C, Roato I,
Mungo E, et al: Mitochondria-targeted doxorubicin: A new
therapeutic strategy against doxorubicin-resistant osteosarcoma.
Mol Cancer Ther. 15:2640–2652. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Wang S, Han F, Li J, Yu L, Zhou
P, Chen Z, Xue S, Dai C and Li Q: MicroRNA-542-3p suppresses
cellular proliferation of bladder cancer cells through
post-transcriptionally regulating survivin. Gene. 579:146–152.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kureel J, Dixit M, Tyagi AM, Mansoori MN,
Srivastava K, Raghuvanshi A, Maurya R, Trivedi R, Goel A and Singh
D: miR-542-3p suppresses osteoblast cell proliferation and
differentiation, targets BMP-7 signaling and inhibits bone
formation. Cell Death Dis. 5:e10502014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Massagué J: G1 cell-cycle control and
cancer. Nature. 432:298–306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mishra L, Derynck R and Mishra B:
Transforming growth factor-beta signaling in stem cells and cancer.
Science. 310:68–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bierie B and Moses HL: Tumour
microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer.
Nat Rev Cancer. 6:506–520. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-beta family signalling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|