Introduction
Androgen deprivation therapy (ADT) has been widely
used for advanced prostate cancer since Huggins and Hodges
(1) first identified this therapy. Up
to 80% of patients respond favorably to ADT; nevertheless, the
therapy is not curative and the disease progresses to
castration-resistant prostate cancer (CRPC) within 1–2 years of
starting ADT (2,3). Docetaxel (DTX) was discovered in 2004
and until recently has been the only agent demonstrated to provide
a substantial survival benefit to patients with CRPC, despite the
development of resistance (or intolerance) to this therapy and
eventual disease progression (4,5).
The treatment paradigm for CRPC had been altered
markedly with the advent of a number of androgen receptor
(AR)-pathway-targeted agents [including enzalutamide (ENZ) and
abiraterone acetate (AA)] and new-generation chemotherapy
[including cabazitaxel (CBZ)] (6–10). AA is
an inhibitor of an androgen biosynthesis enzyme, cytochrome P450
17-α hydroxylase/17,20-lyase (CYP17A1), which is expressed in
testicular, adrenal and prostate tumor tissues, explaining why CRPC
tumor growth relies on androgen stimulation (6). AA suppresses androgen synthesis in the
testis, adrenal gland and prostate tumor tissues and decreases
circulating testosterone levels (11). In the COU-AA-301 clinical trial
(6), treatment with AA alongside
prednisone substantially increased the median overall survival (OS)
compared with placebo in patients with metastatic CRPC (mCRPC) who
had previously been treated with DTX. In the COU-AA-302 clinical
trial (7), treatment with AA in
addition to prednisone significantly increased median OS compared
with placebo in patients with metastatic DTX-naïve CRPC.
In Japan, AA is now widely used for patients with
CRPC since its approval in 2014 (12). However, few studies of Japanese
patients with CRPC treated with AA have been reported. There is
notable interest in confirming whether the efficacy of AA
demonstrated in trial settings is reproducible in routine clinical
practice, given the potential differences in the selection of
patients, ethnic differences and other factors in day-to-day
practice. AA is effective for DTX-naïve and post-DTX treated
patients with CRPC, yet the efficacy of AA varies substantially
between individuals (13). In
addition, there is cross-resistance among DTX, AA and ENZ (a
second-generation anti-androgen), and the sequence in which these
drugs are administered to the patient affects the efficacy of
treatment (14,15). Therefore, the identification of
biomarkers is necessary in order to predict the effect of AA. In
the present study, a collected database on patients with CRPC who
started AA treatment were retrospectively analyzed, with the aim of
evaluating outcomes in clinical practice in Japan and identifying
the prognostic factors for progression-free survival (PFS) and OS
in these patients.
Patients and methods
Patients and treatment
The present study was performed by the evaluation of
medical records of 93 consecutive Japanese patients with CRPC
(median age, 74 years; range, 55–93 years) who received AA (1,000
mg orally once daily at least 1 h before or 2 h after a meal,
alongside prednisone 10 mg daily) at 9 institutions (St. Maria
Hospital, Kurume University School of Medicine, Yame General
Hospital, Chikugo City Hospital, Kurume General Hospital, Kurume
University Medical Center, Takagi Hospital, Omuta City Hospital and
Saiseikai Omuta Hospital; all Fukuoka, Japan) between September
2014 and February 2017. A total of 93 patients who underwent
continuous AA treatment for >1 month were included to measure
efficiency. The present study was approved by the Research Ethics
Review Committee of St. Maria Hospital (Kurume, Japan), Kurume
University School of Medicine (Kurume, Japan), Yame General
Hospital (Yame, Japan), Chikugo City Hospital (Chikugo, Japan),
Kurume General Hospital (Kurume, Japan), Kurume University Medical
Center (Kurume, Japan), Takagi Hospital (Okawa, Japan), Omuta City
Hospital (Omuta, Japan) and Saiseikai Omuta Hospital (Omuta,
Japan). The requirement for informed consent was waived due to the
retrospective nature of the study.
Prostate-specific antigen (PSA) levels, full blood
counts and routine biochemical data were measured at least every
1–3 months, whereas radiologic examinations (computed
tomography/bone scans) were performed according to the discretion
of treating physicians. AA was discontinued subsequent to the
confirmation of disease progression if no earlier intolerable
adverse events occurred, including cardiac disorder and liver
dysfunction. Disease progression during AA treatment was identified
using a combination of PSA and radiographic progression. PSA
progression was defined according to the definition specified by
the Prostate Cancer Working Group 2 (16). Radiographic progression was defined
using the Response Evaluation Criteria in Solid Tumors (17). PSA response to AA was defined as a
decline in PSA of ≥50% from the PSA value immediately prior to the
introduction of AA. Patients were stratified using potential
predictors of outcome, including the following: Age, initial PSA
level, Gleason score (18), previous
prostatectomy or curative radiotherapy, PSA nadir level during
initial ADT, duration from ADT initiation to PSA nadir, duration of
initial ADT, number of previous anti-androgen agents, the presence
of visceral, lymph node and bone metastases, previous treatment
with estrogen preparations, baseline PSA prior to treatment with AA
and PSA response rate during treatment with AA. Cut-offs were
determined using the median values of all the data available for
each factor.
Statistical analysis
All continuous variables were expressed as median
and ranges (standard deviation). Patient characteristics were
compared using a Mann-Whitney U test or Fisher's exact test.
Time-to-event distributions were estimated using Kaplan-Meier
curves and significance was assessed using a log-rank test.
Univariate and multivariate analyses were performed using Cox
proportional hazards models. PFS was defined as the period between
the initial administration of AA and the time of diagnosis of
disease progression. OS was calculated from the initiation of CRPC
treatment until mortality from any cause. All analyses were
performed using EZR software version 1.27 (Saitama Medical Center,
Jichi Medical University, Saitama, Japan), which is a graphical
user interface for R and a modified version of R commander with
added statistical functions (19).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characteristics of the patients
Among the 93 patients (Table I), 78 were DTX-naïve and 15 had a
post-DTX status. The median age was 74 years (range, 55–93) and the
post-DTX group was significantly younger (P=0.0080). Radical
prostatectomy or radiation therapy had been performed in 17
patients in the DTX-naïve group and 3 patients in the post-DTX
group. The median initial PSA at the time of prostate cancer
diagnosis was 92.4 ng/ml (range, 3.13–12,534 ng/ml). The median
progression time to CRPC was 517 days (range, 118–5,944) for all
patients and the median lowest level of PSA during initial ADT was
0.4045 ng/ml (range, 0.01–352 ng/ml). These data did not differ
significantly between the DTX-naïve and post-DTX groups.
Significantly more patients in the post-DTX group had received
estrogen preparations (P=0.0172) and ENZ (P=0.0002) compared with
the DTX-naïve group (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Clinicopathological
factor | All patients
(n=93) | DTX-naive
(n=78) | Post-DTX
(n=15) | P-value |
|---|
| Median age, years
(range) | 74 (55–93) | 75 (55–93) | 71 (56–81) | 0.008 |
| Gleason score |
|
|
| 0.547 |
| ≤8,
n | 31 | 25 | 6 |
|
| ≥9,
n | 50 | 43 | 7 |
|
|
Unknown, n | 12 | 10 | 2 |
|
| Median iPSA, ng/ml
(range) | 92.40
(3.13–12,534) | 115.90
(3.13–12,534) | 43.90
(3.20–3,370) | 0.704 |
| Radical
therapy |
|
|
| 0.239 |
| None,
n | 73 | 61 | 12 |
|
|
Prostatectomy, n | 11 | 8 | 3 |
|
|
Radiation, n | 9 | 9 | 0 |
|
| Median progression
time to CRPC, days (range) | 517
(118–5,944) | 570
(119–5,944) | 317
(118–1,830) | 0.121 |
| Median PSA nadir
during initial ADT, ng/ml (range) | 0.40
(0.01–352) | 0.32
(0.01–84.7) | 0.81
(0.01–352) | 0.458 |
| Median time to PSA
nadir during initial ADT, days (range) | 200 (18–4,894) | 204 (19–4,894) | 164 (18–333) | 0.158 |
| Number of regimens
of anti-androgenic agent |
|
|
| 0.114 |
| ≤1,
n | 26 | 19 | 7 |
|
| ≥2,
n | 67 | 59 | 8 |
|
| Previous use of
estrogen preparations |
|
|
| 0.017 |
| Yes,
n | 3 | 21 | 9 |
|
| No,
n | 63 | 57 | 6 |
|
| Previous use of
ENZ |
|
|
| <0.001 |
| Yes,
n | 33 | 21 | 12 |
|
| No,
n | 60 | 57 | 3 |
|
| Bone
metastasis |
|
|
| 0.032 |
|
Positive, n | 63 | 49 | 14 |
|
|
Negative, n | 30 | 29 | 1 |
|
| Lymph node
metastasis |
|
|
| 1.000 |
|
Positive, n | 17 | 14 | 3 |
|
|
Negative, n | 76 | 64 | 12 |
|
| Visceral
metastasis |
|
|
| 0.661 |
|
Positive, n | 10 | 8 | 2 |
|
|
Negative, n | 83 | 70 | 13 |
|
| Median baseline PSA
prior to treatment with AA, ng/ml (range) | 9.86
(0.06–853.2) | 8.52
(0.06–853.2) | 25.10
(0.40–743.7) | 0.197 |
Radiographically confirmed metastases in bone, lymph
nodes and visceral organs were present in 67.7, 18.3 and 10.8% of
all patients, respectively. The rate of bone metastasis was
significantly higher in the post-DTX group (P=0.0315) compared with
the DTX-naïve group. The median baseline PSA prior to treatment
with AA was 9.855 ng/ml (range, 0.06–853.2 ng/ml). There was no
significant difference identified in the number of anti-androgen
treatments, rates of lymph node and visceral metastases, and
baseline PSA prior to treatment with AA between the DTX-naïve and
post-DTX groups.
PSA response
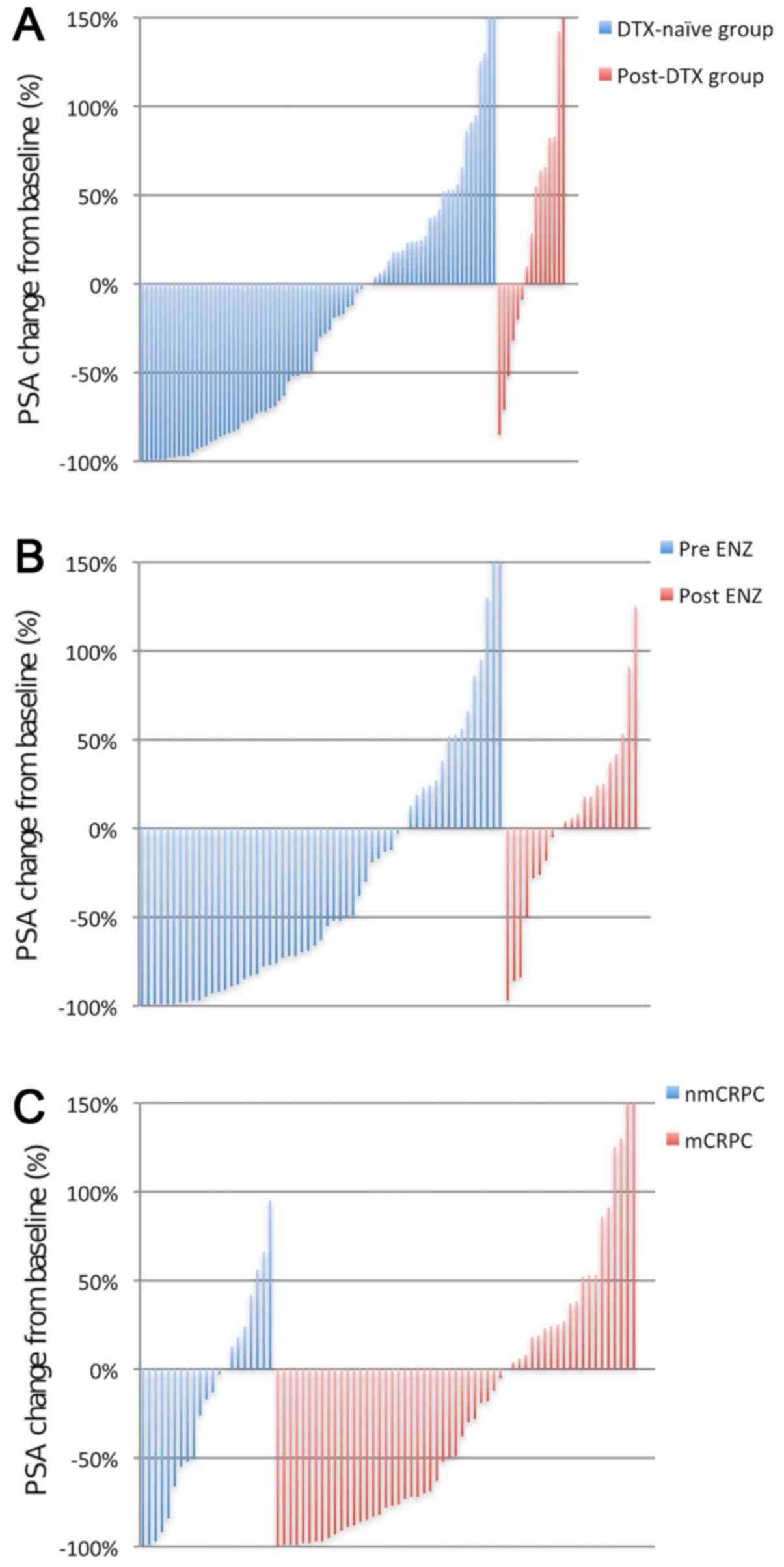
Maximum reduction in serum PSA level during
treatment with AA is presented in Fig.
1. The PSA response rate to AA in the DTX-naïve group was
higher compared with that in the post-DTX group (47 vs. 20%). In
the DTX-naïve group, the PSA response rate to AA in the pre-ENZ
group was higher to that that in the post-ENZ group (58 vs. 19%).
In addition, the PSA response rate to AA in non-mCRPC and mCRPC
groups were similar (43 vs. 49%).
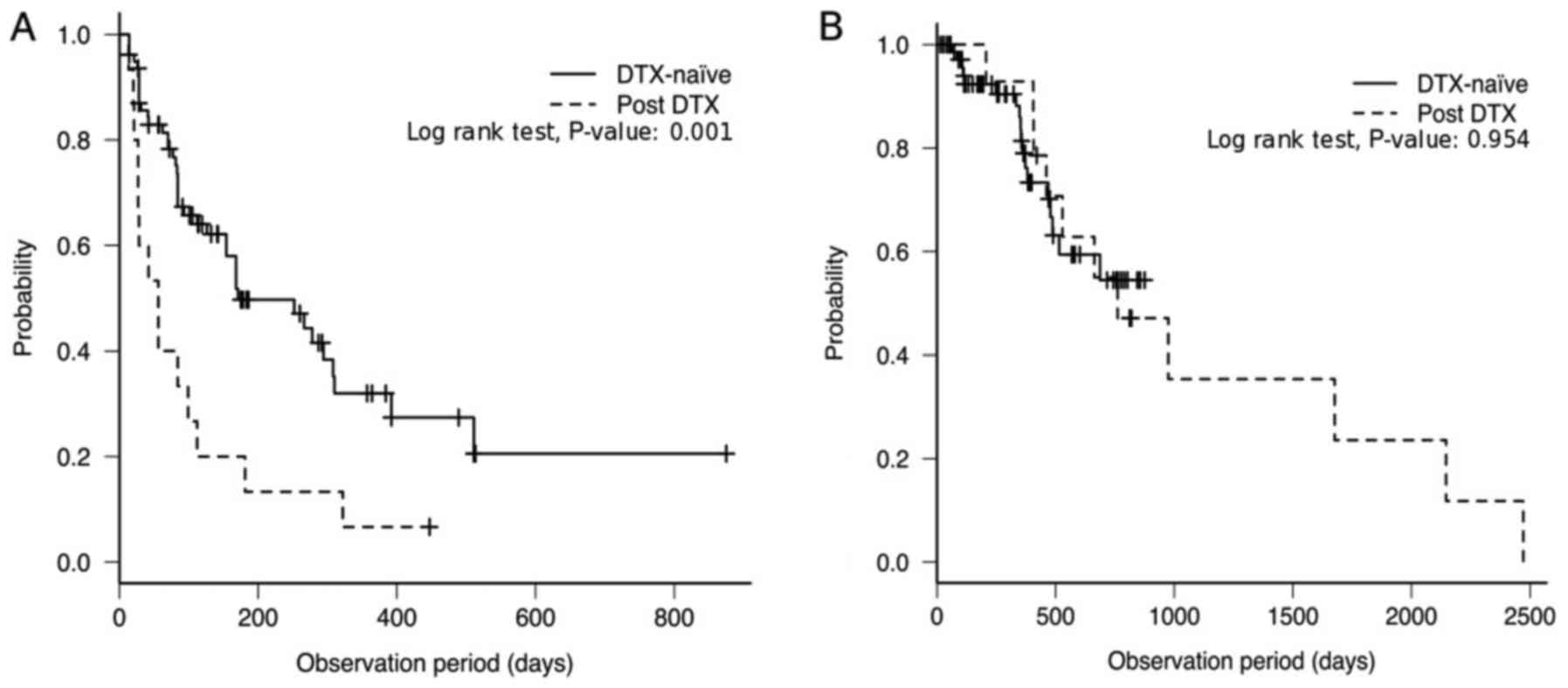
PFS during AA treatment and OS. Median PFS in the
DTX-naïve and post-DTX groups were 171 (95% CI, 126–308) and 56
(95% CI, 21–99) days, respectively. PFS in the DTX-naïve group was
significantly longer compared with that in the post-DTX group
(P=0.001; Fig. 2A). The DTX-naïve
group did not reach the median OS [95% CI, 478-not applicable (NA)
days]. Note that in the Kaplan-Meier method, if OS is >50% at
the last time point, then median OS is not defined and has ‘not
been reached’. A survival curve must reach the point at which 50%
of patients have died for a median OS to be defined. The median OS
in the post-DTX group was 761 days (95% CI, 407–2,146 days), and
there was no significant difference in OS between the DTX-naïve and
post-DTX groups (P=0.954; Fig. 2B).
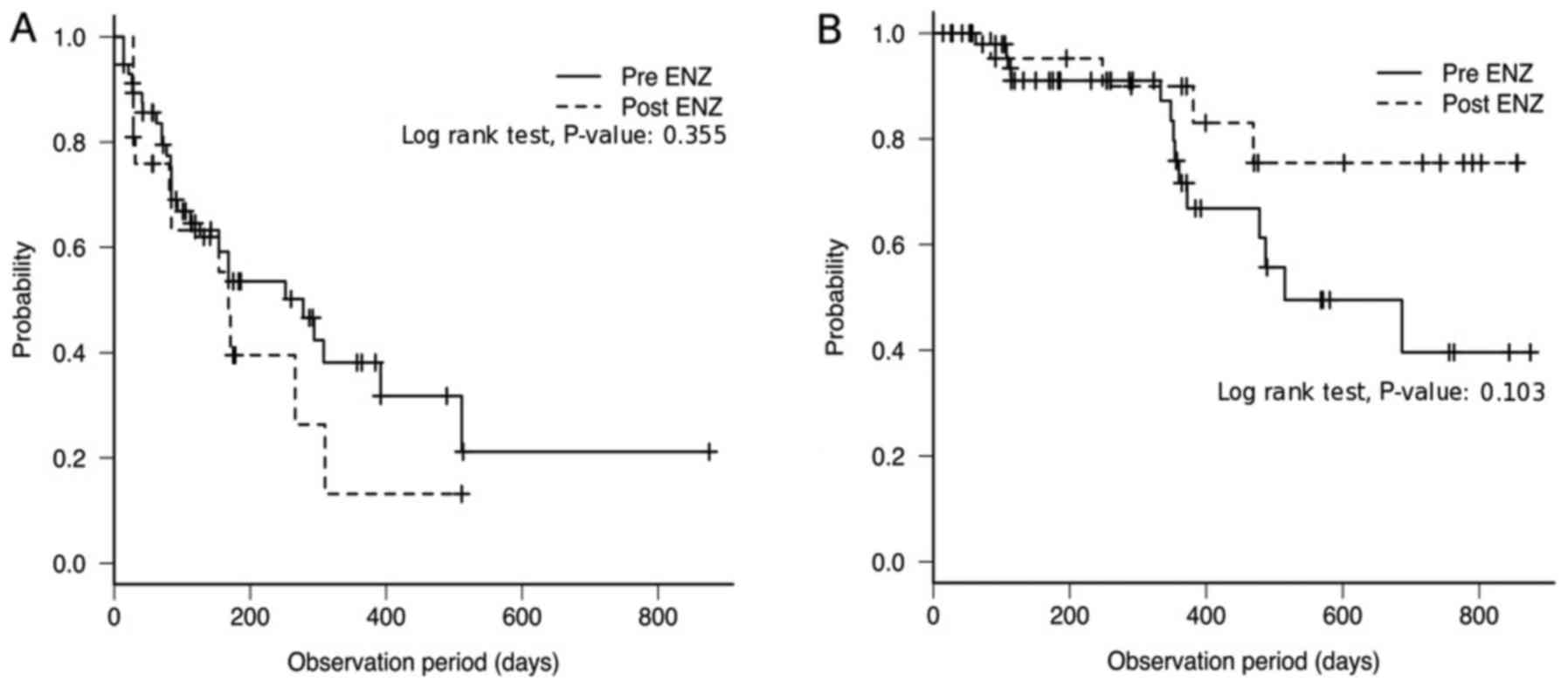
In the DTX-naïve group, the median PFS in the pre- and post-ENZ
groups were 278 (95% CI, 112–392) and 168 (95% CI, 81–310) days,
respectively, with no significant difference between them (P=0.355;
Fig. 3A). The median OS in the
pre-ENZ group was 515 (95% CI, 372-NA). The post-ENZ cohort did not
reach the median OS (95% CI, 469-NA days; Fig. 3B), as described above. There was no
significant difference in OS between the pre- and post-ENZ groups
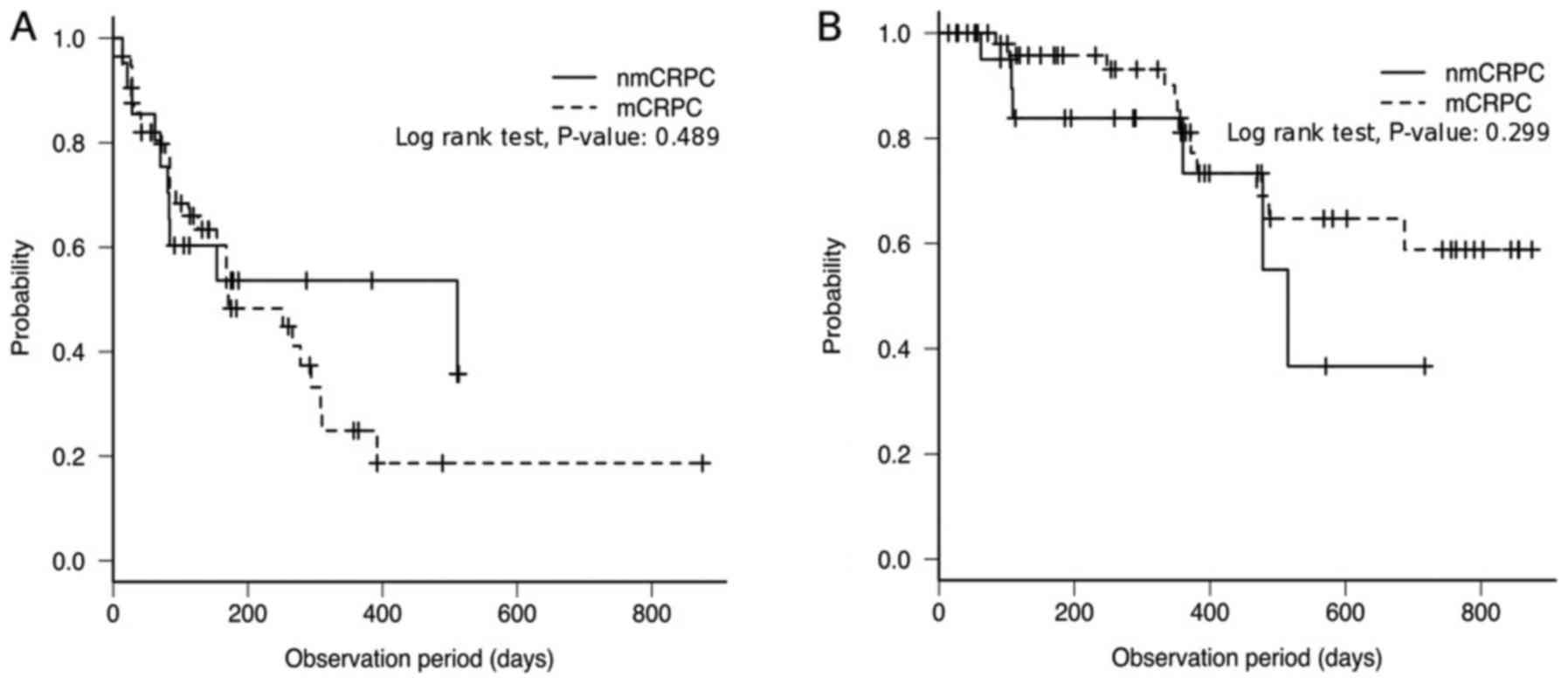
(P=0.103; Fig. 3B). Furthermore, the
median PFS in the nmCRPC and mCRPC groups were 511 (95% CI, 81-NA)
and 171 (95% CI, 126–294) days, respectively, with no significance
between them (P=0.489; Fig. 4A). The
median OS in the nmCRPC group was 515 (95% CI, 360-NA). The mCRPC
cohort did not reach the median OS (95% CI, 469-NA days). There was
no significant difference in OS between the nmCRPC and mCRPC groups
(P=0.299; Fig. 4B). Due to the small
number of patients that succumbed to disease, the DTX-naïve,
post-ENZ and mCRPC cohorts did not reach the median OS, for the
reason described above.
Prognostic factors for PFS and OS
Univariate analysis using a Cox proportional hazards
model revealed that the previous use of estrogen preparations
(P<0.001; HR, 0.363; 95% CI, 0.210–0.629), previous use of ENZ
(P=0.019; HR, 1.892; 95% CI, 1.106–3.236) and previous use of
docetaxel (P=0.002; HR, 0.381; 95% CI, 0.206–0.702) were
significant determinants of PFS, compared with no previous
treatment. Additionally, PSA response compared with no response
(P<0.001; HR, 7.224; 95% CI, 3.583–14.56) and a <10 ng/ml
baseline PSA compared with a ≥10 ng/ml baseline PSA prior to
treatment with AA (P<0.001; HR, 0.306; 95% CI, 0.171–0.547) were
significant determinants of PFS (Table
II). In multivariate analysis, a PSA response compared with no
response (P<0.001; HR, 6.085; 95% CI, 3.071–12.060) and a <10
ng/ml baseline PSA compared with a ≥10 ng/ml baseline PSA prior to
treatment with AA (P=0.001; HR, 0.369; 95% CI, 0.205–0.663) were
significant determinants of PFS (Table
II). For OS, univariate analysis identified previous use of
estrogen preparations compared with no use (P=0.027; HR, 0.409; 95%
CI, 0.186–0.902) and a <10 ng/ml baseline PSA compared with a
≥10 ng/ml baseline PSA prior to treatment with AA (P=0.024; HR,
0.385; 95% CI, 0.167–0.883) as significant determinants of OS. In
multivariate analysis, <10 ng/ml baseline PSA, compared with a
≥10 ng/ml baseline PSA, prior to treatment with AA (P=0.024; HR,
0.385; 95% CI, 0.167–0.883) was the only independent significant
predictor of OS (Table III).
 | Table II.Univariate and multivariate analysis
for progression-free survival. |
Table II.
Univariate and multivariate analysis
for progression-free survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Compared
groups | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | <75 vs. ≥75 | 1.12
(0.65–1.96) | 0.679 |
|
|
| Gleason score | ≤8 vs. ≥9 | 0.69
(0.39–1.27) | 0.233 |
|
|
| Prostatectomy or
radiation | Yes vs. no | 1.65
(0.82–3.34) | 0.162 |
|
|
| iPSA, ng/ml | <150 vs.
≥150 | 0.90
(0.52–1.57) | 0.717 |
|
|
| Time to CRPC,
days | <600 vs.
≥600 | 1.17
(0.67–2.03) | 0.582 |
|
|
| PSA nadir,
ng/ml | <0.5 vs.
≥0.5 | 0.60
(0.34–1.07) | 0.082 |
|
|
| Period from initial
ADT start to PSA nadir, days | <300 vs.
≥300 | 1.32
(0.74–2.35) | 0.342 |
|
|
| Number of
antiandrogen treatment lines | ≤1 vs. ≥2 | 1.24
(0.68–2.26) | 0.481 |
|
|
| Previous use of
estrogen preparations | Yes vs. no | 0.36
(0.21–0.63) | <0.001 | 0.58
(0.32–1.05) | 0.073 |
| Bone
metastasis | Yes vs. no | 0.53
(0.28–1.00) | 0.049 |
|
|
| Lymph node
metastasis | Yes vs. no | 0.73
(0.39–1.36) | 0.314 |
|
|
| Visceral
metastasis | Yes vs. no | 0.76
(0.34–1.69) | 0.501 |
|
|
| Previous use of
ENZ | Yes vs. no | 1.89
(1.10–3.24) | 0.019 | 0.86
(0.47–1.59) | 0.635 |
| Previous use of
DTX | Yes vs. no | 0.38
(0.21–0.70) | 0.002 | 1.12
(0.44–2.89) | 0.802 |
| Baseline PSA prior
to treatment with AA, ng/ml | <10 vs. ≥10 | 0.30
(0.17–0.55) | <0.001 | 0.37
(0.21–0.66) | 0.001 |
| PSA response
(%) | Yes vs. no | 7.22
(3.58–14.6) | <0.001 | 6.09
(3.07–12.0) | <0.001 |
 | Table III.Univariate and multivariate analysis
for overall survival. |
Table III.
Univariate and multivariate analysis
for overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Compared
groups | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years | <75 vs. ≥75 | 1.25
(0.55–2.86) | 0.597 |
|
|
| Gleason score | ≤8 vs. ≥9 | 0.78
(0.34–1.77) | 0.500 |
|
|
| Prostatectomy or
radiation | Yes vs. no | 1.08
(0.43–2.70) | 0.547 |
|
|
| iPSA, ng/ml | <150 vs.
≥150 | 0.85
(0.38–1.89) | 0.691 |
|
|
| Time to CRPC,
days | <600 vs.
≥600 | 1.27
(0.59–2.73) | 0.534 |
|
|
| PSA nadir,
ng/ml | <0.5 vs.
≥0.5 | 1.28
(0.56–2.91) | 0.554 |
|
|
| Period from initial
ADT start to PSA nadir, days | <300 vs.
≥300 | 2.43
(0.99–5.99) | 0.053 |
|
|
| Number of
anti-androgen treatment lines | ≤1 vs. ≥2 | 1.29
(0.58–2.88) | 0.532 |
|
|
| Previous use of
estrogen preparations | Yes vs. no | 0.41
(0.19–0.90) | 0.027 | 0.5
(0.22–1.14) | 0.097 |
| Bone
metastasis | Yes vs. no | 1.05
(0.44–2.53) | 0.915 |
|
|
| Lymph node
metastasis | Yes vs. no | 1.28
(0.51–3.22) | 0.603 |
|
|
| Visceral
metastasis | Yes vs. no | 0.58
(0.22–1.55) | 0.278 |
|
|
| Previous use of
ENZ | Yes vs. no | 0.53
(0.24–1.19) | 0.125 |
|
|
| Previous use of
DTX | Yes vs. no | 1.03
(0.42–2.50) | 0.954 |
|
|
| Baseline PSA prior
to treatment with AA, ng/ml | <10 vs. ≥10 | 0.38
(0.17–0.88) | 0.024 | 0.38
(0.17–0.88) | 0.024 |
Discussion
In the present retrospective multicenter study, the
PSA response rate, PFS and OS were examined in DTX-naïve and
post-DTX patients with CRPC and in pre- and post-ENZ patients with
DTX-naïve CRPC. In the patients of the present study, 47% of
DTX-naïve patients exhibited a 50% decrease in PSA. The PFS in all
DTX-naïve patients was 171 days and OS did not reach the median. In
the COU-AA-302 clinical trial (7),
68% of all patients demonstrated a 50% PSA decline; additionally,
the median biochemical PFS was 11.1 months and the OS was 34.7
months. The clinical efficacy and PSA response rate in the
DTX-naïve patients in the present study was similar to that in the
COU-AA-302 clinical trial (7);
however, the OS and PFS were markedly shorter in the present
cohort. The poorer outcomes may be due to the higher proportion of
patients that underwent treatment with an anti-androgen regimen and
had a history of estramustine phosphate or ENZ use. In addition,
the DTX-naïve patients in the present study included cases with
visceral disease, which were excluded in the COU-AA-302 clinical
trial (7).
In post-DTX patients, 20% of patients exhibited a
50% PSA decline; in these patients, the median PFS was 56 days and
the median OS was 761 days. Of the patients in the COU-AA-301
clinical trial (6), 29% of all
patients exhibited a 50% decline in PSA levels; in these patients,
the median biochemical PFS was 8.5 months and the OS was 15.8
months. OS could not be compared between the two studies owing to
the difference in start dates; however, PFS in the post-DTX
patients group in the present study appeared to be shorter than
that in the COU-AA-301 clinical trial (6). This may also be due to the higher
proportion of patients with a high Gleason score and a history of
ENZ use. A number of previous studies in Japanese patients with
CRPC have also revealed a shorter PFS and OS compared with those in
phase III trials (20–22). It has been reported that the
differences in androgen receptor signaling between men of African
and Caucasian descent accounts for the differences in the
percentage of patients responding to AA, using the decline in PSA
level as a marker of responsiveness, although there were no
significant differences in PFS or OS (13). Similarly, it may be that the response
in Japanese patients may also differ.
The AR-signaling pathway is a target common to ENZ
and AA; however, these drugs differ in their mechanism of action.
ENZ inhibits AR signaling by the competitive inhibition of binding
of androgens, which inhibits the translocation of the ligand-bound
receptor to the nucleus and the subsequent binding to its response
elements in DNA. By contrast, AA inhibits CYP17A1, which is crucial
for testosterone synthesis, resulting in the potent suppression of
extragonadal androgen production (6,8). The
specific mechanisms of resistance to ENZ and AA have yet to be
clearly identified. However, Antonarakis et al (23) revealed that the detection of androgen
receptor splice variant-7 (AR-V7) mRNA in circulating tumor cells
was associated with poorer outcomes in patients with CRPC treated
with AA and ENZ. Additionally, prior treatment with ENZ and AA is
associated with AR-V7 positivity, and thus AR-V7 may be linked to
cross-resistance (23).
The outcome of AA treatment in DTX-naïve patients
with CRPC following progression during ENZ treatment is unknown. In
a study of outcomes of AA following the failure of ENZ in 14
DTX-naïve patients with mCRPC, Yamada et al (24) revealed a 50% PSA decline rate of 7%,
median PFS of 3.4 months and median OS from the initial treatment
of AA of 9.1 months. Miyake et al (22) compared the efficacies of sequential AA
and ENZ therapy in patients with DTX-naïve mCRPC. In 59 patients
receiving AA therapy following progression after treatment with
ENZ, the 50% PSA decline rate was 16.9%, median PFS was 2.6 months
and median OS from the initial treatment of ENZ was 22.1 months.
There are also a number of reports on AA in post-DTX patients with
CRPC following progression after treatment with ENZ (25,26). In 30
post-DTX patients treated with AA following the failure of ENZ
therapy, Noonan et al (25)
identified that 4% of all patients had a 50% PSA decline after
treatment with AA, median PFS of 15.4 weeks and median OS from the
initial treatment of AA of 50.1 weeks. In a similar study, in 38
post-DTX patients treated with AA following progression after
treatment with ENZ, the 8% of all patients had a 50% PSA decline
after treatment with AA the median PFS was 2.7 months and the
median OS from the initiation of AA treatment was 7.2 months
(26). Regardless of the history of
DTX treatment, these outcomes in post-ENZ patients were inferior to
those of pre-ENZ patients owing to the cross-resistance between ENZ
and AA therapies.
The group of patients who comprised the present
study population contains patients with CRPC, according to their
PSA levels, without radiological evidence of metastasis. The
provisional clinical opinion of the American Society of Clinical
Oncology differs on its consensus recommendations for treatment, as
in men with CRPC displaying no evidence of metastasis, second-line
hormonal therapy in those in the high risk group may be considered
but is not advised (27). In patients
with metastatic cancer, AA or ENZ is advised. A majority of
clinical studies on AA concern patients with mCRPC; however, it was
previously reported that treatment with ADT in combination with AA
was associated with significantly higher rates of OS and PFS
compared with ADT alone in men with castration-sensitive PC
(28,29). Although no difference was observed
between patients with nmCRPC and patients with mCRPC in the present
study owing to the limited number of patients, AA may be more
effective for patients with nmCRPC compared with patients with
mCRPC based on previous evidence.
The Eastern Cooperative Oncology Group performance
status (ECOG-PS), duration of ADT, PSA response and presence of
visceral metastasis have been reported to be prognostic factors for
patients with mCRPC receiving AA treatment (30–32). In a
study of 220 post-DTX patients treated with AA, Van Praet et
al (33) obtained an improved
estimate of OS using a multivariate model comprising 5 independent
risk factors: Hemoglobin <12 g/dl, >10 metastases, ECOG-PS,
radiographic progression and <90 months since diagnosis.
However, the majority of studies have focused on post-DTX patients
and there are few reliable prognostic models in DTX-naïve patients
with CRPC treated with AA. Poon et al (34) suggested that visceral metastasis and
the response of PSA were associated with PFS and OS in 58 DTX-naïve
patients with mCRPC treated with AA, similar to post-DTX patients
with mCRPC. In addition, Miyake et al (35) revealed that the time until PSA nadir
following treatment with AA was an independent predictor of PFS in
125 patients with DTX-naïve mCRPC receiving AA. In a study that
included DTX-naïve and post-DTX patients with CRPC, ‘duration of
primary ADT’ and ‘no prior chemotherapy’ were associated with the
duration of AA treatment (36).
The present study revealed that a history of
anti-CRPC agent use, including DTX and ENZ, was associated with PFS
time in univariate analysis. In the present study, the response to
ENZ, DTX and estrogen preparations that were previously performed
were not assessed; however, they may be associated with the
therapeutic effect of AA.
The response of PSA was significantly associated
with PFS in multivariate analysis. In the exploratory analysis of
the COU-AA-302 clinical trial, the association of PSA response with
radiographic PFS (rPFS), OS time and time to PSA progression was
examined. Another study has revealed the presence of an association
between PSA response and treatment efficacy of AA (37). Additionally, Rescigno et al
(38) identified that patients that
did not achieve a 30% decline in PSA after 4 weeks of AA had a
lower likelihood of achieving a PSA response at 12 weeks and a
significantly decreased OS time, compared with patients that
achieve a 30% decline in a study of 274 patients with mCRPC treated
with AA prior to or following DTX treatment. If a PSA response is
associated with PFS or OS time, it may be an effective clinical
biomarker. However, a PSA flare, a phenomenon in which PSA is
temporarily elevated following the treatment of an
luteinizing-hormone-releasing hormone agonist or DTX treatment, may
also occur with AA treatment (39).
Therefore, when observing changes in PSA, the possibility of PSA
flares should be considered. The baseline PSA level prior to
treatment with AA was the only independent prognostic factor for
PFS and OS in the present study. In the exploratory analysis of the
COU-AA-302 clinical trial, associations were identified between
baseline PSA level and rPFS, OS and time until PSA progression,
including the PSA response (37).
These results indicate that long-term treatment efficacy may be
sustained by the administration of AA when PSA levels are low.
There are several limitations in the present study.
PFS was not analyzed separately for PSA and rPFS, and the limited
sample size prevented separate analyses for DTX-naive and post-DTX
patients. In addition, owing to the short observation period, the
median OS time was not reached for DTX-naive patients. Furthermore,
the group of patients that comprised the study population was not
representative of a typical patient with prostate cancer who has
failed treatment following primary curative therapy, which is due
to the fact that few patients in the present study underwent prior
prostatectomy radical or radiotherapy, and at least two-thirds of
patients had a Gleason score of at least 9. The PSA level at the
time of diagnosis was a median of 92 ng/ml, which does not
correspond to a typical screening population with PSA of >4
ng/ml. Thus, the study group appears to have a selection bias
towards high-grade cancer. Despite quality-control efforts, the
study is additionally limited by its retrospective design,
potential for selection bias and incomplete or variable
assessments. However the results of the present study reflect the
characteristics of AA treatment of CRPC in current clinical
practice in Japan.
In conclusion, the present study evaluated the
outcomes of patients with CRPC treated with AA in clinical practice
in Japan. The efficacy of AA appeared to be lower in the present
cohort than that in other studies, which may be due to a higher
proportion of patients with a high Gleason score, treatment with an
anti-androgen regimen, and a history of estramustine phosphate or
ENZ use in the present study. Analysis of prognostic factors
revealed that PSA response and baseline PSA levels were associated
with a longer PFS time and baseline PSA was associated with longer
OS time. In this context, long-term treatment efficacy may be
sustained by the administration of AA in patients with lower PSA
levels, and it may be better to change the agent at an early stage
if patients do not exhibit a response as measured by PSA
levels.
References
|
1
|
Huggins C and Hodges CV: Studies on
prostatic cancer. I. The effect of castration, of estrogen and
androgen injection on serum phosphatases in metastatic carcinoma of
the prostate. CA Cancer J Clin. 22:232–240. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walczak JR and Carducci MA: Prostate
cancer: A practical approach to current management of recurrent
disease. Mayo Clin Proc. 82:243–249. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chi KN, Bjartell A, Dearnaley D, Saad F,
Schröder FH, Sternberg C, Tombal B and Visakorpi T:
Castration-resistant prostate cancer: From new pathophysiology to
new treatment targets. Eur Urol. 56:594–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berthold DR, Pond GR, Soban F, de Wit R,
Eisenberger M and Tannock IF: Docetaxel plus prednisone or
mitoxantrone plus prednisone for advanced prostate cancer: Updated
survival in the TAX 327 study. J Clin Oncol. 26:242–245. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seruga B, Ocana A and Tannock IF: Drug
resistance in metastatic castration-resistant prostate cancer. Nat
Rev Clin Oncol. 8:12–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ryan CJ, Smith MR, de Bono JS, Molina A,
Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng
S, et al: Abiraterone in metastatic prostate cancer without
previous chemotherapy. N Engl J Med. 368:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beer TM, Armstrong AJ, Rathkopf DE, Loriot
Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J,
Chowdhury S, et al: Enzalutamide in metastatic prostate cancer
before chemotherapy. N Engl J Med. 371:424–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Attard G, Belldegrun AS and de Bono JS:
Selective blockade of androgenic steroid synthesis by novel lyase
inhibitors as a therapeutic strategy for treating metastatic
prostate cancer. BJU Int. 96:1241–1246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki M, Yuasa T, Yamamoto S, Hayashi
T, Ogawa M, Sakura M, Masuda H, Fukui I and Yonese J: Efficacy and
safety profile of enzalutamide for Japanese patients with
castration-resistant prostate cancer. Anticancer Res. 36:361–365.
2016.PubMed/NCBI
|
|
13
|
Ramalingam S, Humeniuk MS, Hu R, Rasmussen
J, Healy P, Wu Y, Harrison MR, Armstrong AJ, George DJ and Zhang T:
Prostate-specific antigen response in black and white patients
treated with abiraterone acetate for metastatic castrate-resistant
prostate cancer. Urol Oncol. 35:418–424. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mezynski J, Pezaro C, Bianchini D, Zivi A,
Sandhu S, Thompson E, Hunt J, Sheridan E, Baikady B, Sarvadikar A,
et al: Antitumour activity of docetaxel following treatment with
the CYP17A1 inhibitor abiraterone: Clinical evidence for
cross-resistance? Ann Oncol. 23:2943–2947. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van Soest RJ, Nieuweboer AJ, de Morrée ES,
Chitu D, Bergman AM, Goey SH, Bos MM, van der Meer N, Hamberg P, de
Wit R and Mathijssen RH: The influence of prior novel androgen
receptor targeted therapy on the efficacy of cabazitaxel in men
with metastatic castration-resistant prostate cancer. Eur J Cancer.
51:2562–2569. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the prostate cancer clinical
trials working group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, National cancer
institute of the United States, National cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL: ISUP Grading Committee: The 2005 international society
of urological pathology (ISUP) consensus conference on gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsubara N, Uemura H, Satoh T, Suzuki H,
Nishiyama T, Uemura H, Hashine K, Imanaka K, Ozono S and Akaza H: A
phase 2 trial of abiraterone acetate in Japanese men with
metastatic castration-resistant prostate cancer and without prior
chemotherapy (JPN-201 study). Jpn J Clin Oncol. 44:1216–1226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satoh T, Uemura H, Tanabe K, Nishiyama T,
Terai A, Yokomizo A, Nakatani T, Imanaka K, Ozono S and Akaza H: A
phase 2 study of abiraterone acetate in Japanese men with
metastatic castration-resistant prostate cancer who had received
docetaxel-based chemotherapy. Jpn J Clin Oncol. 44:1206–1215. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyake H, Hara T, Terakawa T, Ozono S and
Fujisawa M: Comparative assessment of clinical outcomes between
abiraterone acetate and enzalutamide in patients with
docetaxel-naive metastatic castration-resistant prostate cancer:
Experience in real-world clinical practice in Japan. Clin
Genitourin Cancer. 15:313–319. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonarakis ES, Lu C, Wang H, Luber B,
Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et
al: AR-V7 and resistance to enzalutamide and abiraterone in
prostate cancer. N Engl J Med. 371:1028–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamada Y, Matsubara N, Tabata KI, Satoh T,
Kamiya N, Suzuki H, Kawahara T, Uemura H, Yano A and Kawakami S:
Abiraterone acetate after progression with enzalutamide in
chemotherapy-naïve patients with metastatic castration-resistant
prostate cancer: A multi-center retrospective analysis. BMC Res
Notes. 9:4712016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Noonan KL, North S, Bitting RL, Armstrong
AJ, Ellard SL and Chi KN: Clinical activity of abiraterone acetate
in patients with metastatic castration-resistant prostate cancer
progressing after enzalutamide. Ann Oncol. 24:1802–1807. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loriot Y, Bianchini D, Ileana E, Sandhu S,
Patrikidou A, Pezaro C, Albiges L, Attard G, Fizazi K, De Bono JS
and Massard C: Antitumour activity of abiraterone acetate against
metastatic castration-resistant prostate cancer progressing after
docetaxel and enzalutamide (MDV3100). Ann Oncol. 24:1807–1812.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Virgo KS, Basch E, Loblaw DA, Oliver TK,
Rumble RB, Carducci MA, Nordquist L, Taplin ME, Winquist E and
Singer EA: Second line hormonal therapy for men with
chemotherapy-Naïve, castration-resistant prostate cancer: American
Society of Clinical Oncology provisional clinical opinion. J Clin
Oncol. 35:1952–1964. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
James ND, de Bono JS, Spears MR, Clarke
NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones
RJ, et al: Abiraterone for prostate cancer not previously treated
with hormone therapy. N Engl J Med. 377:338–351. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lolli C, Caffo O, Scarpi E, Aieta M,
Conteduca V, Maines F, Bianchi E, Massari F, Veccia A, Chiuri VE,
et al: Systemic immune-inflammation index predicts the clinical
outcome in patients with mCRPC treated with abiraterone. Front
Pharmacol. 7:3762016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Facchini G, Caffo O, Ortega C, D'Aniello
C, Di Napoli M, Cecere SC, Della Pepa C, Crispo A, Maines F, Ruatta
F, et al: Very early PSA response to abiraterone in mCRPC patients:
A novel prognostic factor predicting overall survival. Front
Pharmacol. 7:1232016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi KN, Kheoh T, Ryan CJ, Molina A,
Bellmunt J, Vogelzang NJ, Rathkopf DE, Fizazi K, Kantoff PW, Li J,
et al: A prognostic index model for predicting overall survival in
patients with metastatic castration-resistant prostate cancer
treated with abiraterone acetate after docetaxel. Ann Oncol.
27:454–460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Van Praet C, Rottey S, Van Hende F,
Pelgrims G, Demey W, Van Aelst F, Wynendaele W, Gil T, Schatteman
P, Filleul B, et al: Which factors predict overall survival in
patients with metastatic castration-resistant prostate cancer
treated with abiraterone acetate post-docetaxel? Clin Genitourin
Cancer. 15:502–508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poon DM, Chan K, Lee SH, Chan TW, Sze H,
Lee EK, Lam D and Chan MF: Abiraterone acetate in metastatic
castration-resistant prostate cancer-the unanticipated real-world
clinical experience. BMC Urol. 16:122016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyake H, Hara T, Tamura K, Sugiyama T,
Furuse H, Ozono S and Fujisawa M: Independent association between
time to prostate-specific antigen (PSA) nadir and PSA
progression-free survival in patients with docetaxel-naïve,
metastatic castration-resistant prostate cancer receiving
abiraterone acetate, but not enzalutamide. Urol Oncol. 35:432–437.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McKay RR, Werner L, Fiorillo M,
Nakabayashi M, Kantoff PW and Taplin ME: Predictors of duration of
abiraterone acetate in men with castration-resistant prostate
cancer. Prostate Cancer Prostatic Dis. 19:398–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ryan CJ, Londhe A, Molina A, Smith MR, De
bono JS, Mulders P, Rathkopf DE, Saad F, Logothetis C, Fizazi k, et
al: Relationship of baseline PSA and degree of PSA decline to
radiographic progression-free survival (rPFS) in patients with
chemotherapy-naive metastatic castration-resistant prostate cancer
(mCRPC): Results from COU-AA-302. J Clin Oncol. 31:2013.
|
|
38
|
Rescigno P, Lorente D, Bianchini D,
Ferraldeschi R, Kolinsky MP, Sideris S, Zafeiriou Z, Sumanasuriya
S, Smith AD, Mehra N, et al: Prostate-specific antigen decline
after 4 weeks of treatment with abiraterone acetate and overall
survival in patients with metastatic castration-resistant prostate
cancer. Eur Urol. 70:724–731. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueda Y, Matsubara N, Tabata KI, Satoh T,
Kamiya N, Suzuki H, Kawahara T and Uemura H: Prostate-specific
antigen flare phenomenon induced by abiraterone acetate in
chemotherapy-naive patients with metastatic castration-resistant
prostate cancer. Clin Genitourin Cancer. 15:320–325. 2017.
View Article : Google Scholar : PubMed/NCBI
|