Introduction
Hepatic cancer (HCC) is the sixth most common type
of cancer, with ~782,000 new cancer cases in 2012 worldwide
(1). In the majority of cases, HCC
develops following cirrhosis of the liver, and is the primary cause
of mortality in these patients (1,2)
The incidence of HCC has doubled in the last 20
years in the United States and Europe, and has exhibited the
highest rate of increase among all cancer-associated causes of
mortality in recent years (3).
Approximately 90% of HCC cases are associated with known risk
factors (4); the most frequent causes
are chronic viral hepatitis [hepatitis C virus (HCV) and hepatitis
B virus (HBV)], alcohol consumption, and exposure to aflatoxins
(primarily in Africa and Asia), while other factors may also
increase the risk, including obesity, tobacco use and fatty liver
disease (5).
The most widely used technique in tumour staging is
the Barcelona Clinic Liver Cancer (BCLC) system, as this is the
only method that takes into account the spread of the tumour,
hepatic function and the presence of symptoms, and thus has high
predictive capacity (5). It
establishes tumour prognosis in four stages, with each one having
possible therapeutic indications. Patients with initial stage
cancer (BCLC stage A) are candidates for potentially curative
treatments, including surgical resection, percutaneous ablation and
transplant, whereas those with intermediate stage (BCLC stage B)
may be subject to palliative treatments, including arterial
chemoembolization. In addition, sorafenib is used as a systematic
treatment in the event of advanced HCC (BCLC stage C) (6).
The prognosis of most solid tumours depends on the
tumour stage; nevertheless, given that HCC is associated with
hepatic cirrhosis in the majority of cases, and that the degree of
hepatic function determines the therapeutic regimens and patient
survival, assessing the level of hepatic dysfunction and tumour
spreading is considered essential. Furthermore, the symptomatology
has been shown to be of high predictive value, and is also
indicative for selecting the most appropriate treatment.
With the current screening programmes performed, HCC
is able to be diagnosed at earlier stages, thus making it possible
to choose effective therapeutic methods. Surgical resection,
transplant and percutaneous ablation achieve high complete response
rates for tumours (7). Regarding
palliative therapy, the only treatments to demonstrate an increase
in survival rates have been chemoembolisation and sorafenib
(8,9).
Other therapies, including arterial embolisation without
chemotherapy, external radiotherapy and radioembolisation have been
demonstrated to exhibit antitumour activity, but have not
demonstrated efficacy in terms of survival (10,11).
Systematic chemotherapy with classic cytotoxic agents has not
revealed any benefit for patient survival and is associated with
high toxicity, and hormonal therapy, including the use of tamoxifen
and octreotide, and antiandrogens, appears to be ineffective
(7,9).
Sorafenib is an oral multikinase inhibitor with antiproliferative
and antiangiogenic activity, which has demonstrated efficacy in
improving the overall survival of patients with advanced HCC. It is
currently the only first-line treatment indicated for advanced or
intermediate-stage HCC following failure or contraindication of
standard therapies (12).
In an international, multicentre, randomised and
double-blind study (SHARP) (13),
sorafenib (400 mg/12 h) was evaluated compared with placebo
treatment in patients with advanced HCC exhibiting conserved
hepatic function (95% Child Pugh class A, 5% Child Pugh class B)
and practically asymptomatic conditions [Eastern Cooperative
Oncology Group Performance (ECOG) score 0–2]. This study revealed a
significant improvement in overall survival, as well as in median
time-to-radiological progression (mOS) of 10.7 vs. 7.9 months in
the placebo group.
A similarly designed clinical trial was performed in
Asia (8), involving HCC cases at more
advanced stages, with the large majority of cases involving tumours
occurring secondary to cirrhosis caused by the HBV. Sorafenib
exhibited benefits similar to those described in the SHARP study,
with a reduction in the risk of mortality, although it achieved a
lower median survival time (6.5 vs. 4.2 months in the placebo
group).
The monitoring of responses to treatments involves
the monitoring of patients by performing imaging tests, including
computed tomography or magnetic resonance imaging, and measuring
α-fetoprotein (AFP) levels. In order to define the stage of the
illness prior to and following treatment, it is recommended that
the Response Evaluation Criteria In Solid Tumors (RECIST) criteria
are used (14,15). In general, clinical trials use imaging
tests as a means of evaluating response to systematic treatments
(14). However, numerous patients
experience symptomatic improvement or evident improvement in
pathology without exhibiting significant radiological changes
(16). Numerous drugs exert
cytostatic rather than cytotoxic activity, and inhibit the growth
of tumours without causing a reduction in size (14). Furthermore, molecules, including
sorafenib, induce tumour necrosis, which is occasionally associated
with an increase in size or stabilisation of the tumour (14). The partial or complete response to
treatment (assessed according to the RECIST criteria) is the
primary independent predictive factor of survival in patients with
HCC (14). Nonetheless, only in few
cases is there a complete response to sorafenib, with a small
proportion achieving a partial response (12). Furthermore, between 45 and 75% of
patients exhibit stabilisation of the illness as their best
response (15,16). Thus, it is important to perform
studies that assess possible biomarkers or measures of response, to
allow us to determine probable response predictors (17).
AFP is a serum glycoprotein that is elevated in over
half of patients with HCC (14). A
number of studies have demonstrated its value at the beginning of
diagnosis as a predictive factor of survival (18). Indeed, serum AFP level is used for
diagnosis, as a predictive marker and to evaluate response
following systematic chemotherapy or radiological therapies
(15). A previous study of 25
patients treated with systematic chemotherapy revealed that a
reduction in AFP level of >50% was associated with a good
clinical response to the treatment (19). Additionally, Chan et al
(20) confirmed that a decline in
basal AFP of >20% following 2–3 cycles of systemic chemotherapy
was predictive of response to treatment (20). However, only a small number of studies
have been performed to investigate its role as a possible predictor
of response following therapy with sorafenib in patients with HCC
(21–23).
At present the use of sorafenib is indicated for
patients with advanced HCC, for those who are not candidates for
chemoembolization, or for those with post-chemoembolisation
progression with conserved hepatic function (Child-Pugh class
A).
The aim of the present study was to assess the
effectiveness of sorafenib, and the value of AFP as a predictive
factor of response, as well as other prognostic factors associated
with overall survival, in patients diagnosed with advanced HCC
treated with sorafenib as the first-line treatment, or in patients
with advanced-stage disease following progression or resistance to
standard treatment.
Materials and methods
Study design
The present study was an observational retrospective
study of patients with HCC treated with sorafenib at the Central
University Hospital of Asturias (Oviedo, Spain). The study was
approved by the Ethical Research Committee of Central University
Hospital of Asturias. Written informed consent was obtained for use
of patient data, while maintaining patient anonymity and respecting
patient confidentiality throughout.
Study population
Patients
Patients with HCC treated at the Central University
Hospital of Asturias who started first-line treatment with
sorafenib for advanced C stage, or as second-line treatment for
intermediate stage B due to resistance to standard treatment or
progression, were enrolled. During the study period, 167 patients
began treatment with sorafenib with a median age of 65.6 years
(range, 23.9–80.6 years), and 147 (88%) men and 20 (12%) women.
Inclusion criteria
All patients diagnosed with HCC who started
treatment with sorafenib between January 2008 and December 2014
were included. The end date of the monitoring period was the 20th
of December 2015.
Exclusion criteria
All those being treated with sorafenib during a
clinical trial, along with those who had begun treatment prior to
2008, when access to the drug was granted on request for
compassionate use only were excluded. Those patients who lacked
records for the variables studied on the computer systems used were
also excluded, unless otherwise stated.
Treatments
All patients received 800 mg/day sorafenib (400 mg
twice/day) as an initial dosage. Dosage reductions took place in
accordance with the technical file upon signs of toxicity or
adverse effects. The patients continued treatment unless there was
clinical progression, unacceptable toxicity, or cases of patient
refusal or patient mortality.
Variable definitions
Main variables
To assess the effectiveness of the treatment, the
median overall survival (mOS) time, calculated as the interval (in
days) between the beginning of treatment and the date of mortality,
if mortality occurred, or the end date of monitoring for patients
who survived (20th of December 2015) were used. To evaluate the
possible predictive value of AFP in response to treatment, the
patients were classified into two groups: Those with AFP reduction
≤20% (non-responders) and those with AFP reduction >20%
(responders), measured at 6–8 weeks after starting treatment with
sorafenib.
Secondary variables
To assess other possible factors predictive of
survival, the ECOG score (0, 1 or 2), aetiology, stage according to
the BCLC system (BCLC stage B or C) and hepatic function based on
the Child-Pugh classification (A or B) for each patient were
determined. Additionally, whether or not at the beginning of the
treatment the patient presented vascular invasion and/or
extrahepatic extension was recorded. The analytical parameters
recorded were basal serum levels of albumin (>35 or ≤35 g/l),
basal serum AFP (>200 or ≤200 ng/dl) and serum AFP at 6–8 weeks.
Any other treatments administered prior to the beginning of
sorafenib treatment were also recorded.
Toxicity
The adverse effects and the duration of the
treatment were recorded. Any suspensions of treatment due to
toxicity or an unknown factor, including low patient compliance,
were also recorded.
Statistical analysis
A descriptive statistical analysis of the
demographic and clinical variables was performed using
centralisation and dispersion methods (median and interquartile
range) for the quantitative data, and frequencies and proportions
for the qualitative data. Duration of the treatment was determined
(in months), along with the time of appearance of any adverse
affects. In order to determine overall survival, Kaplan-Meier
curves were constructed and the differences between groups were
analysed via the log-rank test. To identify the possible predictive
factors associated with overall survival, the Cox proportional
hazards model was used to determine respective hazard quotients and
95% confidence intervals (CIs). The variables identified as
potential predictors of effectiveness in the univariate analysis
were subsequently included in the multivariate analysis. For all of
the analyses, P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed using
the SPSS statistical data package (version 19.0; IBM Corp., Armonk,
NY, USA).
Results
Patient characteristics
All of the patients were diagnosed with advanced
HCC; 82.3% of cases also presented with a cirrhosis of the liver
and 61.7% exhibited advanced-stage HCC (BCLC stage C). Alcohol
consumption was the primary cause of HCC development (40.1%),
followed by chronic HCV infection (21.6%). At the start of
treatment, the majority of patients had preserved hepatic function,
with Child-Pugh class A (42.5% patients). Clinical data regarding
the spread of the disease was identified as incorrectly defined in
certain registries, and therefore it was only possible to analyse a
percentage of the total number of patients for this variable. Of
the 117 patients for whom the presence or absence of extrahepatic
extension was documented, this was positive in 46 cases (27.5% of
the total). Information on vascular invasion was only assessable
for 130 patients, with 80 cases (47.9% of the total) being positive
for vascular invasion. Regarding basal AFP serum levels, 49 (29.3%)
patients presented values >200 ng/ml. The demographical and
clinical characteristics of patients are outlined in Table I.
 | Table I.Baseline demographical and clinical
characteristics of patients. |
Table I.
Baseline demographical and clinical
characteristics of patients.
| Variable | Value |
|---|
| Total patients,
n | 167 |
| Age, years [median
(range)] | 65.6 |
|
| (23.9–80.6) |
| Sex, n (%) |
|
|
Male | 147 (88) |
|
Female | 20 (12) |
| Child-Pugh class, n
(%) |
|
| A | 71 (42.5) |
| B | 17 (10.4) |
| ECOG performance
status, n (%) |
|
| 0 | 71 (42.5) |
| 1 | 29 (17.4) |
| 2 | 4 (2.4) |
| Aetiology of HCC, n
(%) |
|
| VHB
cirrhosis | 5 (3) |
| VHC
cirrhosis | 36 (21.6) |
| VHC
cirrhosis and alcohol | 17 (10.2) |
| VHB
cirrhosis and alcohol | 4 (2.4) |
|
Alcoholic cirrhosis | 67 (40.1) |
|
Hepatitis C+HIV | 5 (3) |
|
Hemochromatosis | 5 (3) |
|
HCV+HBV | 1 (0.6) |
|
Other | 27 (16.1) |
| BCLC stage, n
(%) |
|
| B | 38 (22.8) |
| C | 103 (61.7) |
| Vascular invasion,
n (%) | 80 (47.9) |
|
Extrahepatic extension | 46 (27.5) |
| Liver
transplant | 7 (4.2) |
| Basal AFP level,
ng/ml [median (range)] | 76.6 |
|
| (1.2–233800.0) |
| Basal AFP >200
ng/ml, n (%) | 49 (29.3) |
| Basal AFP ≤200
ng/ml, n (%) | 118 (70.7) |
| Basal hepatic
enzymes, IU/l [median (range)] |
|
|
Aspartate
aminotransferase | 58 (19–344) |
| Alanine
aminotransferase | 44.5 (13–274) |
| Basal albumin
value, g/l [median (range)] | 39 (25–48) |
| Basal albumin ≤35
g/l, n (%) | 63 (37.7) |
| Basal albumin
>35 g/l, n (%) | 104 (62.3) |
| Basal bilirubin
value, mg/ml [median (range)] | 1 (0.2–4.4) |
| Basal bilirubin ≤1
mg/ml, n (%) | 80 (47.9) |
| Basal bilirubin
>1 mg/ml, n (%) | 87 (52.1) |
| AFP reduction at
6–8 weeks, n (%) |
|
| AFP
responders (>20%) | 28 (16.8) |
| AFP
non-responders (≤20%) | 139 (86.2) |
Treatment
Prior to beginning treatment with sorafenib, 53
(31.7%) patients had not received any treatment, 64 (38.3%)
patients had received locoregional therapy [transarterial
chemoembolization (TACE) and/or radiofrequency ablation], 18
(10.8%) had undergone surgical resection with curative intent prior
to sorafenib treatment and 7 (4.2%) had received liver transplants.
No information was available on previous treatments for 25
patients.
The median duration of treatment was 5 months.
During monitoring, 92 (55.1%) patients required a reduction in
dosage. Progression of the illness was the primary reason for
ending treatment in 94 (56.3%) patients (including those who
succumbed while being treated), followed by adverse effects (41
patients, 24.6%). A total of 2 patients were moved on to trials
with tivantinib, 2 (1.1%) with regorafenib, 1 with nivolumab (0.5%)
and another to treatment with tamoxifen (0.5%), following the
suspension of sorafenib.
Effectiveness and survival in
different subgroups
On analysis of overall survival, an mOS time of 11
months was determined (95% CI, 8.65–13.34). Two patients exhibited
complete responses for 17 and 24 months, respectively, with both
remaining alive until the date the monitoring ended.
Statistical analyses of survival was performed
between subgroups based on potential risk factors, including age,
Child Pugh classification, BCLC stage, ECOG score, basal AFP
>200 ng/ml and AFP reduction at 6–8 weeks.
With regards to the basal AFP levels, notable
differences in survival were observed, in that mOS time was
significantly lower in those with initial AFP levels >200 ng/ml
(mOS, 8 months vs. 14 months in patients with AFP levels ≤200
ng/ml) (P=0.01; Fig. 1).
Another clinical variable that was associated with
significantly higher mOS was ECOG performance status, with an mOS
of 16 months identified for patients with an ECOG of 0, compared
with an mOS of 4 months in patients with an ECOG of 2 (P=0.001).
Hepatic function was also an influential factor, with Child-Pugh
class A associated with a significantly higher mOS of 12 months,
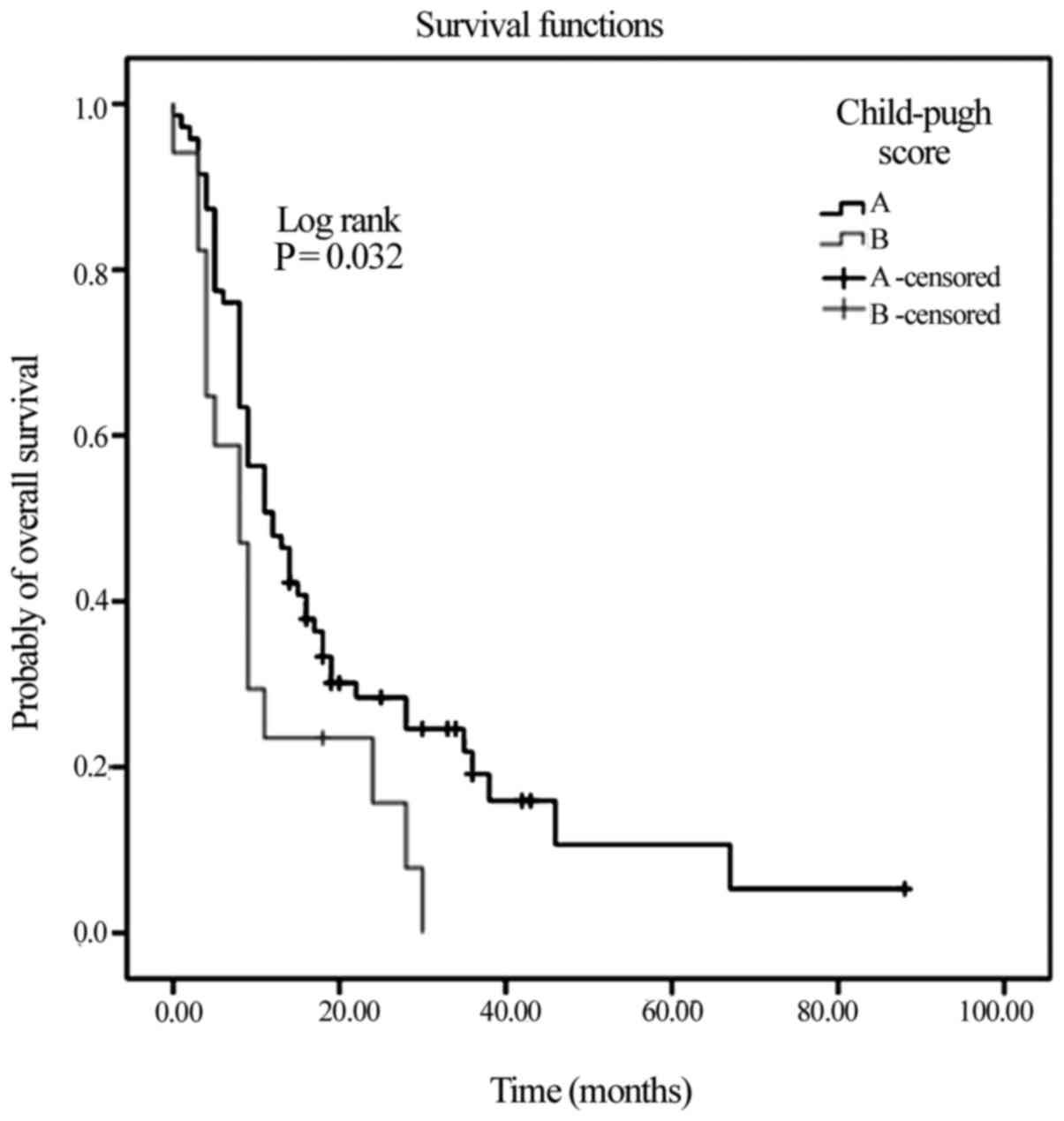
compared with 8 months for Child-Pugh class B (P=0.03; Fig. 2). Lastly, those patients aged >63
years exhibited a higher mOS time (14 months vs. 8 months in
patients ≤63 years old; P=0.03). Patients at BCLC stage B exhibited
increased mOS times compared with patients at BCLC stage C (15 vs.
10 months), without reaching statistical significance.
Role of AFP reduction as a predictive
factor of early response
Of the 167 patients, 28 belonged to the AFP
responders group, whereas 139 belonged to the non-responder group.
The AFP responder patients exhibited a difference in mOS compared
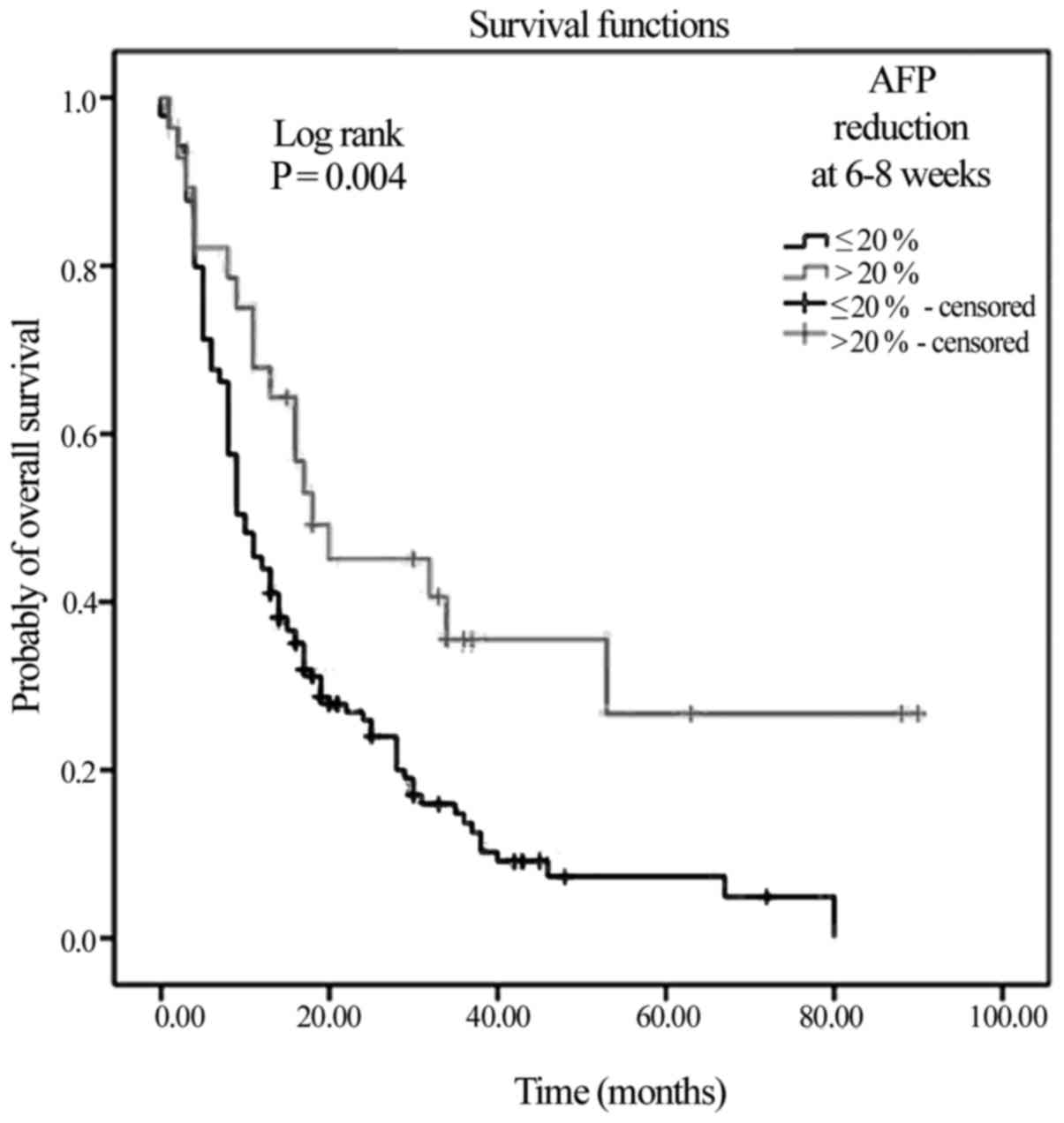
with the non-responders (18 vs. 10 months; Fig. 3; P=0.004).
A multivariate analysis was performed to assess the
potency of the AFP variable as an independent predictive factor, as
well as the value of the other clinical variables that were
significant in the univariate analysis (Table II). A reduction in AFP by >20% at
6–8 weeks was an independent factor associated with higher mOS time
(P=0.002).
 | Table II.Univariate and multivariate analysis
for the possible predictive factors of OS. |
Table II.
Univariate and multivariate analysis
for the possible predictive factors of OS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (>63 vs. ≤63
years) | 1.433
(1.015–2.023) | 0.041 | 3.895
(1.727–8.783) | 0.001 |
| ECOG performance
status (0 vs. 1) | 1.709
(1.050–2.781) | 0.006 | 1.120
(0.562–2.233) | 0.697 |
| ECOG performance
status (0 vs. 2) | 4.137
(0.462–11.707) | 0.007 | 1.913
(0.397–9.206) | 0.419 |
| Child-Pugh class (A
vs. B) | 1.787
(1.018–3.138) | 0.043 | 0.413
(0.157–1.089) | 0.074 |
| BCLC stage (B vs.
C) | 1.290
(0.850–1.958) | 0.231 |
|
|
| Basal AFP (≤200 vs.
>200 ng/ml) | 1.583
(1.103–2.272) | 0.013 | 2.694
(0.392–5.212) | 0.003 |
| AFP reduction
[Responders (>20%) vs. Non-responders (≤20)] | 2.032
(1.230–3.357) | 0.006 | 9.723
(2.252–41.969) | 0.002 |
Basal AFP levels of ≤200 ng/ml and age >63 years
were also identified as independent factors associated with higher
mOS time.
Toxicity
With regards to the toxicity of treatment, the
primary adverse reactions registered in the medical records were
hand-foot syndrome (35 patients, 21%), diarrhoea (39 patients,
23.4%), anorexia (29 patients, 17.4%) and arterial hypertension (30
patients, 18%). Seven cases of encephalopathy were registered
(4.2%), 1 patient suffered an ischaemic stroke (0.6%), 2 exhibited
angina with atrial fibrillation (1.2%), 1 exhibited myocardial
infarction (0.6%) and treatment was suspended for 2 patients (1.2%)
due to intense haemorrhoid bleeding.
Discussion
A number of studies have demonstrated that sorafenib
is effective in the treatment of HCC, particularly by increasing
the overall survival times compared with placebos, while having a
manageable toxicity profile (13,24,25). Thus,
it has become established as a first-line treatment for patients
with advanced HCC. In clinical practice, serum AFP is used as a
biomarker in HCC. There are studies that support the efficacy of
AFP level as a predictive factor and as a response factor following
surgical resection (16,26); nonetheless, its usefulness as a
predictive factor of response to treatment with sorafenib or other
anti-angiogenics drugs is not clearly established.
The results regarding the overall survival and
toxicity profile associated with sorafenib in the present study are
similar to those reported in the SHARP trial (13). This may be due to similarities in the
demographical and clinical characteristics collected from the
patients in the present study and those in the SHARP study
(13), which reported a higher
incidence in males (88%) and in those aged ~65 years (median, 65.6
years), involving patients in a good general state (92%, ECOG score
0–1) and with advanced-stage HCC (73%).
Regarding prior treatments, nearly half of the
patients (71/167, 42.5%) in the current study had been treated with
other optional palliative therapies, with a predominance of TACE at
a similar frequency to that in the SHARP trial, with the only
difference being that no patient in the present study had
previously received hormone therapy or systematic chemotherapy. A
sub-analysis of the SHARP trial performed by Bruix et al
(24) identified a tendency towards
improvement in overall survival in patients who received prior
therapies independently of the treatment received, with an mOS time
of 8.8 months for those treated with curative therapy and 9.9
months for those who received other palliative treatments. Indeed,
in the current study cohort, a difference in mOS time (24) between patients treated with curative
therapy (25 months) and those who had received other palliative
treatments was observed (11 months; 95% CI, 8.3–41.6 and 7.8–14.1,
respectively).
The present study cohort exhibited an overall mOS
time (11 months) similar to the SHARP trial (10.7 months) and
slightly lower compared with that in the study by Kudo et al
(26) (13.7 months). However, it was
higher compared with that in an extended population of the SHARP
study involving an Asian population (6.5 months), (8) as well as in other subsequent studies
performed (5.4 months) (27).
Previous data exhibit a tendency towards improvement in survival
for those patients with good liver function and general state (ECOG
performance status) (28–30). Regarding the parameters analysed, high
levels of basal AFP were an indicator of poor prognosis in the
present study, and have previously been associated with shorter
survival times (27,31). A sub-analysis of the SHARP trial
performed by Raoul et al (32)
analysed the influence of laboratory parameters, including hepatic
enzymes, AFP and bilirubin, on the response to treatment with
sorafenib. Patients who demonstrated elevated levels of these
markers had OS rate shorter (although the sorafenib cohort had
greater OS rate, compared with the placebo group), compared with
patients whose markers were normalised in both cohorts (32). Thus, the authors concluded that these
markers had predictive value in HCC, but were not predictive of
response to treatment with sorafenib in the HCC patients. In the
present study, when analysing mOS time according to subgroups, the
patients who presented AFP values of >200 ng/ml exhibited a
significantly lower mOS time, compared with the AFP ≤200 ng/ml
group. Another study performed on a Korean population by Lee et
al (33) revealed that liver
function (Child-Pugh class A) and low basal AFP levels were
independent risk factors associated with longer mOS times (P=0.02
and P=0.03, respectively) (33). In
the current group of patients, AFP levels >200 ng/ml was
identified as an independent predictor of shorter survival time in
the multivariate analysis; nevertheless, good liver function
(Child-Pugh class A), despite indicating improved survival (12 vs.
8 months in cases of Child Pugh class B) in the subgroup analysis
(P>0.05), was not deemed to be statistically significant as an
independent predictive factor on performance of the multivariate
test.
A number of studies performed with different
systemic chemotherapy treatments determined that a drop in AFP
level may be useful as a predictive marker for tumour response and
overall survival (17,20,34),
although no consensus was achieved regarding the amount of
reduction and the measurement times. Personeni et al
(21) classified those patients with
a reduction in AFP levels of ≥20% at 6–8 weeks as responders, and
those with an AFP of ≤20% as non-responders. Another study
restricted the definition of responder to a drop in AFP of >50%,
thus increasing the potency and specificity of AFP as a predictor
of response treatment (35). A
reduction in AFP measured at 6–8 weeks is considered relevant and
applicable in clinical practice for aggressive pathologies,
including HCC, with short mOS times of 4–6 months, with treatments
that achieve this also having a beneficial impact on medical costs
(8,27). This study demonstrated that an early
drop in AFP level (>20%) by 6–8 weeks of treatment with
sorafenib is associated with improved patient survival, with higher
greater mOS time observed for the AFP responder group (18 vs. 10
months in the non-responder group, log rank P=0.004), which is in
accordance with other previous studies (22,36),
despite a number of these not reaching statistical relevance
(28,37).
In addition, a >20% reduction in AFP was a
significant independent predictive factor of response to treatment
in the univariate analysis and subsequent multivariate analysis.
Thus, >20% AFP reduction was useful as a predictive marker of
early response to sorafenib in patients with HCC, being associated
with a higher survival rate. This quickly detectable biomarker
requiring inexpensive methods may predict whether treatment is
effective, and ultimately identify patients who may benefit from a
continuation of treatment from those who should be referred for
alternative therapies.
Although high levels of AFP are associated with
negative prognoses, the value of AFP as an early response marker to
sorafenib in patients with HCC is not entirely clear. It is
necessary to perform prospective studies specifically designed to
assess the potency of AFP reduction in predicting patient prognosis
to validate the preliminary results of the present study.
Additionally, the current study included a number of limitations,
primarily deriving from its retrospective design: Data were not
available for all variables for the 167 patients, and were included
as missing in the statistical analysis, which thus resulted in a
loss of potency.
Acknowledgements
The authors thank Sabina Pérez Vicente (Results
Evaluation Unit, Institute of Biomedicine of Sevilla) for her help
with the statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AIPS conceived and designed the study, performed
acquisition, analysis and interpretation of data, and wrote the
manuscript. LVR assisted with the design of the study. ELL
participated in the data compilation. IZG reviewed the manuscript
and interpreted the data. MACH helped to design the methods of the
study. MIBP and JPD helped to interpret the data and reviewed
critically the intellectual content of the manuscript. All authors
read and approved the final manuscript
Ethics approval and consent to
participate
The study was approved by the Ethical Research
Committee of Central University Hospital of Asturias. Written
informed consent was obtained for use of patient data, while
maintaining patient anonymity and respecting patient
confidentiality throughout.
Consent for publication
All identifying information has been removed.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
International Agency for Research on
Cancer (IARC): Globocan 2012: Estimated Cancer Incidence, Mortality
and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Default.aspxApril
10–2015
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mittal S and El-Serag HB: Epidemiology of
hepatocellular carcinoma: Consider the population. J Clin
Gastroenterol. 47:S2–S6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
European Association for the Study of The
Liver; European Organisation for Research and Treatment of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reig M, Matilla A, Bustamante J, Castells
L, de La Mata M, Delgado M, Moreno JM, Forner A and Varela M:
Recommendations for the management of Sorafenib in patients with
hepatocellular carcinoma. Gastroenterol Hepatol. 33:741–752.
2010.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the asia-pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dawson LA: The evolving role of radiation
therapy in hepatocellular carcinoma. Cancer Radiother. 12:96–101.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han KH, Seong J, Kim JK, Ahn SH, Lee DY
and Chon CY: Pilot clinical trial of localized concurrent
chemoradiation therapy for locally advanced hepatocellular
carcinoma with portal vein thrombosis. Cancer. 113:995–1003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colagrande S, Regini F, Taliani GG, Nardi
C and Inghilesi AL: Advanced hepatocellular carcinoma and
sorafenib: Diagnosis, indications, clinical and radiological
follow-up. World J Hepatol. 7:1041–1053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lencioni R and Llovet JM: Modified recist
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawaoka T, Aikata H, Murakami E, Nakahara
T, Naeshiro N, Tanaka M, Honda Y, Miyaki D, Nagaoki Y, Takaki S, et
al: Evaluation of the mrecist and α-fetoprotein ratio for
stratification of the prognosis of
advanced-hepatocellular-carcinoma patients treated with sorafenib.
Oncology. 83:192–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takada J, Hidaka H, Nakazawa T, Kondo M,
Numata K, Tanaka K, Matsunaga K, Okuse C, Kobayashi S, Morimoto M,
et al: Modified response evaluation criteria in solid tumors is
superior to response evaluation criteria in solid tumors for
assessment of responses to sorafenib in patients with advanced
hepatocellular carcinoma. BMC Res Notes. 8:6092015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan
ST and Poon RT: The significance of early alpha-fetoprotein level
changes in predicting clinical and survival benefits in advanced
hepatocellular carcinoma patients receiving sorafenib. Oncologist.
16:1270–1279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nomura F, Ohnishi K and Tanabe Y: Clinical
features and prognosis of hepatocellular carcinoma with reference
to serum alpha-fetoprotein levels. Analysis of 606 patients.
Cancer. 64:1700–1707. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsumoto Y, Suzuki T, Ono H, Nakase A and
Honjo I: Evaluation of hepatoma chemotherapy by alpha-fetoprotein
determination. Am J Surg. 132:325–328. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB,
Ho WM, Lam KC, Chan AT, Mok TS and Yeo W: New utility of an old
marker: Serial alpha-fetoprotein measurement in predicting
radiologic response and survival of patients with hepatocellular
carcinoma undergoing systemic chemotherapy. J Clin Oncol.
27:446–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Personeni N, Bozzarelli S, Pressiani T,
Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V,
Giordano L and Santoro A: Usefulness of alpha-fetoprotein response
in patients treated with sorafenib for advanced hepatocellular
carcinoma. J Hepatol. 57:101–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Zhao Y, Jia J, Chen H, Bai W, Yang
M, Yin Z, He C, Zhang L, Guo W, et al: The prognostic value of
alpha-fetoprotein response for advanced-stage hepatocellular
carcinoma treated with sorafenib combined with transarterial
chemoembolization. Sci Rep. 6:198512016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okuyama H, Ikeda M, Kuwahara A, Takahashi
H, Ohno I, Shimizu S, Mitsunaga S, Senda S and Okusaka T:
Prognostic factors in patients with hepatocellular carcinoma
refractory or intolerant to sorafenib. Oncology. 88:241–246. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruix J, Raoul JL, Sherman M, Mazzaferro
V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M,
Sangiovanni A, et al: Efficacy and safety of sorafenib in patients
with advanced hepatocellular carcinoma: Subanalyses of a phase III
trial. J Hepatol. 57:821–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iavarone M, Cabibbo G, Piscaglia F,
Zavaglia C, Grieco A, Villa E, Cammà C and Colombo M: SOFIA
(SOraFenib Italian Assessment) study group: Field-practice study of
sorafenib therapy for hepatocellular carcinoma: A prospective
multicenter study in Italy. Hepatology. 54:2055–2063. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kudo M, Imanaka K, Chida N, Nakachi K, Tak
WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al: Phase
III study of sorafenib after transarterial chemoembolisation in
Japanese and Korean patients with unresectable hepatocellular
carcinoma. Eur J Cancer. 47:2117–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Køstner AH, Sorensen M, Olesen RK, Grønbæk
H, Lassen U and Ladekarl M: Sorafenib in advanced hepatocellular
carcinoma: A nationwide retrospective study of efficacy and
tolerability. ScientificWorldJournal. 2013:9319722013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DA Fonseca LG, Barroso-Sousa R, Bento AD,
Blanco BP, Valente GL, Pfiffer TE, Hoff PM and Sabbaga J: Safety
and efficacy of sorafenib in patients with Child-Pugh B advanced
hepatocellular carcinoma. Mol Clin Oncol. 3:793–796. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakano M, Tanaka M, Kuromatsu R, Nagamatsu
H, Tajiri N, Satani M, Niizeki T, Aino H, Okamura S, Iwamoto H, et
al: Kurume liver cancer study group of japan. sorafenib for the
treatment of advanced hepatocellular carcinoma with extrahepatic
metastasis: A prospective multicenter cohort study. Cancer Med.
4:1836–1843. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Wu XL, Zeng WZ, Xu GS, Xu H, Weng
M, Hou JN and Jiang MD: Meta-analysis of the efficacy of sorafenib
for hepatocellular carcinoma. Asian Pac J Cancer Prev. 14:691–694.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH
and Cheng AL: Early alpha-fetoprotein response predicts treatment
efficacy of antiangiogenic systemic therapy in patients with
advanced hepatocellular carcinoma. Cancer. 116:4590–4596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raoul JL, Bruix J, Greten TF, Sherman M,
Mazzaferro V, Hilgard P, Scherubl H, Scheulen ME, Germanidis G,
Dominguez S, et al: Relationship between baseline hepatic status
and outcome, and effect of sorafenib on liver function: SHARP trial
subanalyses. J Hepatol. 56:1080–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee S, Kim BK, Kim SU, Park SY, Kim JK,
Lee HW, Park JY, Kim DY, Ahn SH, Tak WY, et al: Clinical outcomes
and prognostic factors of patients with advanced hepatocellular
carcinoma treated with sorafenib as first-line therapy: A korean
multicenter study. J Gastroenterol Hepatol. 29:1463–1469. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen LT, Liu TW, Chao Y, Shiah HS, Chang
JY, Juang SH, Chen SC, Chuang TR, Chin YH and Whang-Peng J:
Alpha-fetoprotein response predicts survival benefits of
thalidomide in advanced hepatocellular carcinoma. Aliment Pharmacol
Ther. 22:217–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raoul JL, Park JW, Kang YK, Finn RS, Kim
JS, Yeo W, Polite BN, Chao Y, Walters I, Baudelet C and Lencioni R:
Using modified recist and alpha-fetoprotein levels to assess
treatment benefit in hepatocellular carcinoma. Liver Cancer.
3:439–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chou WC, Lee CL, Yang TS, Huang CY, Teng
W, Tseng YT, Chen JS, Lin YC, Hou MM, Chang HH and Hsieh Chia-Hsun
J: Changes in serum α-fetoprotein level predicts treatment response
and survival in hepatocellular carcinoma patients and literature
review. J Formos Med Assoc. 117:153–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu XS, Qu K, Liu C, Zhang YL, Liu J, Song
YZ, Zhang P, Liu SN and Chang HL: Highlights for α-fetoprotein in
determining prognosis and treatment monitoring for hepatocellular
carcinoma. World J Gastroenterol. 18:7242–7250. 2012. View Article : Google Scholar : PubMed/NCBI
|