Introduction
Lung cancer is the leading cause of
cancer-associated mortality in China and worldwide (1). In 2015, an estimated 733,300 new cases
of lung and bronchial cancer were diagnosed, and 610,200
mortalities were estimated to occur in China as a result of this
disease (2). Approximately 80% of
lung cancer cases involve non-small-cell lung cancer (NSCLC), which
has an overall 5-year relative survival rate of <20%, while an
exceptionally high mortality rate is reported for patients with
advanced NSCLC (3). During the past
decades, genomic medicine has increased our understanding of the
molecular characterization of cancer. The treatment strategy for
advanced NSCLC has changed from the traditional chemotherapy based
on pathologic histology to individualized precision treatment based
on the oncogenic drivers (3).
Fusions of the echinoderm microtubule-associated
protein-like 4 (EML4) gene and the anaplastic lymphoma kinase (ALK)
gene along with other ALK gene rearrangements (referred to as
ALK-positive) are detected in 3–7% of NSCLC patients. The EML4-ALK
translocation was first identified as an oncogene in a small
proportion of patients with NSCLC in 2007, and the chemical
inhibition of the EML4-ALK fusion protein demonstrated an
anti-tumor effect in vivo and in vitro (4,5).
Crizotinib, a multitargeted tyrosine kinase
inhibitor of ALK, MET and ROS1, had been originally developed as an
inhibitor of the c-MET growth factor receptor tyrosine kinase
(6). In 2011, this drug received
accelerated approval under the US Food and Drug Administration
(FDA) for treating ALK-positive advanced NSCLC patients based on
two single-arm clinical trials, namely PROFILE 1001 and PROFILE
1005 (7,8). Subsequently, crizotinib was evaluated in
several randomized clinical trials, demonstrating superior response
rate (RR) and progression-free survival (PFS) to chemotherapy, in
first- and second-line settings (PROFILE 1007 and PROFILE 1014)
(9,10). Recently, another first-line randomized
trial (PROFILE 1029) with the same design as PROFILE 1014,
confirmed that crizotinib was able to improve the objective RR
(ORR) and PFS compared with chemotherapy in the Asian populations
(11). To date, crizotinib has been
approved by the China FDA for the treatment of advanced or
metastasis ALK-translocated NSCLC.
To date, published real-world data on the treatment
patterns and outcomes of patients with ALK-positive advanced NSCLC
in China are limited. Therefore, the present study aimed to
characterize the treatment patterns and to estimate the survival of
patients in China with locally advanced or metastatic ALK-positive
NSCLC. Exploratory experimentation was used to investigate
independent prognostic factors associated with survival in this
cohort.
Patients and methods
Ethical approval
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards of the Research Ethics Committee of Zhejiang Cancer
Hospital and with the 2013 Declaration of Helsinki. Since this is a
retrospective study, patient informed consent was not required.
Patient enrollment
A total of 83 patients with locally advanced or
metastatic ALK-positive NSCLC who were treated at the Zhejiang
Cancer Hospital (Hangzhou, China) during the period of July 2010
and April 2017 were included in the present study. Exclusion
criteria in this study were as follows: (i) patients with other
types of malignancy; (ii) patients who did not pursue treatment
after diagnosis, and (iii) patients who were lost to follow up.
Patient data were collected including the following variables:
Gender, age, histological subtype, stage (8th edition of the
American Joint Committee on Cancer Tumor-Node-Metastasis staging
system) (12), smoking history,
metastatic sites, therapeutic regimens, efficacy of treatment, date
of progression, site of progression and date of mortality. ALK
rearrangements were detected by the Ventana ALK (D5F3) CDx
immunohistochemical assay (Ventana Medical Systems, Inc., Tucson,
AZ, USA), according to the manufacturer's protocol (13).
Follow-up procedures
Patients receiving chemotherapy were evaluated for
response every two treatment cycles during treatment and then every
2 months after treatment. Among the 50 patients receiving
first-line chemotherapy, 27 were treated with pemetrexed
(administered at a dose of 500 mg/m2 by intravenous
infusion on day 1 and every subsequent 21 days, for 4–6 cycles) in
addition to cisplatin (administered at a dose of 25
mg/m2 by intravenous infusion daily on days 1 to 3 and
every subsequent 21 days, for 4–6 cycles)/carboplatin [administered
at a dose of AUC (area under the curve)=5 by intravenous infusion
on day 1, and every subsequent 21 days, for 4–6 cycles], 11 were
treated with docetaxel (administered at a dose of 75
mg/m2 by intravenous infusion on day 1, and every
subsequent 21 days for 4–6 cycles) in addition to
cisplatin/carboplatin, and 12 were treated with gemcitabine
(administered at a dose of 1,000 mg/m2 on day 1 and day
8, and every subsequent 21 days for 4–6 cycles) in addition to
cisplatin/carboplatin.
Patients receiving crizotinib were evaluated for
response 1 month after the initial treatment and then every 2
months during treatment. Brain or bone lesions that were detected
at the time of screening were evaluated in all subsequent tumor
assessments. For patients without brain or bone metastasis at
baseline assessment, brain and bone scanning was repeated every 6
months or when related symptoms appeared.
The response evaluation of the tumor to therapy was
based on computed tomography or magnetic resonance imaging
scanning. The short-term efficacy was defined based on version 1.1
of the Response Evaluation Criteria in Solid Tumors (RECIST)
guidelines (14). The long-term
efficacy was evaluated according to the PFS and overall survival
(OS). PFS1 was defined as the time from the initiation of treatment
to the radiological evidence of first progressive disease (PD).
PFS2 was defined as the time between the first and the second
RECIST-defined PD. OS was calculated from the initiation of
treatment to mortality.
Statistical analysis
Statistical analysis was performed using SPSS
version 22.0 software (IBM Corp., Armonk, NY, USA). Statistically
significant differences was indicated by P<0.05.
χ2-test was applied to examine the association between
the efficacy and therapeutic regimens. Survival rates were analyzed
using the Kaplan-Meier method and compared using the log-rank test.
Univariate and multivariate analysis were performed with the Cox
proportional hazard model.
Results
Patient characteristics
A total of 83 patients, treated at the Zhejiang
Cancer Hospital between July 2010 and April 2017, were enrolled
into the present study. The patient characteristics are listed in
Table I. The median age at diagnosis
was 50 years (ranging between 23 and 79 years), and more than half
of the patients were female (56.6%). The most common histological
type was adenocarcinoma (92.8%), and 61.4% of the patients were not
smokers. In total, 7 (8.4%) patients were diagnosed with stage IIIB
disease and 76 (91.6%) patients with stage IV disease. Among them,
34.9% displayed intrapulmonary metastasis, 10.8% had intracranial
metastasis, 20.5% had liver metastasis, 34.9% had bone metastasis,
and 32.5% presented pleural effusion. Until the last follow up
(June 7, 2017), the median follow-up time was 23.6 months (range,
1.2–66.9 months).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value (%) |
|---|
| Sex, n |
|
|
Female | 47 (56.6) |
|
Male | 36 (43.4) |
| Age, years |
|
|
Median | 50 |
|
Range | 23–79 |
| Histological type,
n |
|
|
Adenocarcinoma | 77 (92.8) |
|
Squamous cell carcinoma | 3 (3.6) |
|
Adenosquamous carcinoma | 3 (3.6) |
| Stagea |
|
|
IIIB | 7 (8.4) |
| IV | 76 (91.6) |
| Smoking history,
n |
|
|
Yes | 32 (38.6) |
| No | 51 (61.4) |
| Intrapulmonary
metastasis, n |
|
|
Yes | 29 (34.9) |
| No | 54 (65.1) |
| Intracranial
metastasis, n |
|
|
Yes | 9 (10.8) |
| No | 74 (89.2) |
| Liver metastasis,
n |
|
|
Yes | 17 (20.5) |
| No | 66 (79.5) |
| Bone metastasis,
n |
|
|
Yes | 29 (34.9) |
| No | 54 (65.1) |
| Pleural effusion,
n |
|
|
Yes | 27 (32.5) |
| No | 56 (67.5) |
Efficacy and survival of initial
therapy
The treatment efficacy of the 83 patients was listed
in Table II. Among these patients,
33 (39.8%) received crizotinib and 50 (60.2%) received chemotherapy
as the initial therapy. Among the 50 patients receiving first-line
chemotherapy, 27 were treated with pemetrexed (administered at a
dose of 500 mg/m2 by intravenous infusion on day 1,
every 21 days) plus cisplatin (administered at a dose of 25
mg/m2 by intravenous infusion daily on days 1 to 3,
every 21 days)/carboplatin (administered at a dose of AUC=5 by
intravenous infusion on day 1, every 21 days), 11 were treated with
docetaxel (administered at a dose of 75 mg/m2 by
intravenous infusion on day 1, every 21 days) plus
cisplatin/carboplatin, and 12 were treated with gemcitabine
(administered at a dose of 1,000 mg/m2 on day 1 and day
8, every 21 days) plus cisplatin/carboplatin. The ORR was
significantly higher in patients receiving crizotinib as compared
with those treated with chemotherapy (72.7 vs. 38%, respectively;
P=0.003).
 | Table II.Response to first-line therapy in the
included patients. |
Table II.
Response to first-line therapy in the
included patients.
| Parameter | Crizotinib (n=33),
n (%) | Chemotherapy
(n=50), n (%) | P-value |
|---|
| CR | 4 (12.1) | 0 (0.0) | 0.022 |
| PR | 20 (60.6) | 19 (38.0) | 0.036 |
| SD | 6 (18.2) | 19 (38.0) | 0.045 |
| PD | 3 (9.1) | 12 (24.0) | 0.073 |
| ORR | 24 (72.7) | 19 (38) | 0.003 |
| DCR | 30 (90.9) | 38 (76) | 0.143 |
Until the last follow-up, 67 patients had disease
progression. The median PFS1 was 18.5 months [95% confidence
interval (CI), 12.4–24.6 months] among patients in the crizotinib
group, as compared with 4.9 months (95% CI, 2.8–7.1 months) among
patients in the chemotherapy group [hazard ratio (HR) for
progression or mortality in crizotinib group, 0.345; 95% CI,
0.201–0.594; P<0.001; Fig. 1A].
Intracranial lesion progression or development of new intracranial
lesions was reported in significantly more patients in the
crizotinib group (n=10; 50%) as compared with those in the
chemotherapy group (n=4; 8.5%; P<0.001; data not shown).
Intrapulmonary lesion progression was reported in a significantly
higher number of patients in the chemotherapy group (n=17; 36.2%)
as compared with that in the crizotinib group (n=2; 10.0%;
P<0.05). There was no difference between the pleural lesion
progression in the crizotinib group (n=4; 20%) and the chemotherapy
group (n=13; 27.7%; P=0.760). In addition, there was no significant
difference in the OS between patients who received crizotinib and
those who received chemotherapy in the first-line setting (P=0.802;
Fig. 1B).
Survival of patients following the
first progression
Table III presents
the treatment patterns of the 83 patients. Of the 50 patients
receiving chemotherapy as the initial therapy, 47 experienced
disease progression until the final follow-up. Among them,
crizotinib was administered as second-line therapy in 22 (46.8%)
cases, the chemotherapy regimen was changed in 19 (40.4%) cases,
and 6 (12.8%) patients did not receive any other therapy beyond the
first progression. The median PFS2 was 16.4 months (95% CI,
6.4–26.4 months) among patients in the crizotinib group, as
compared with 3.5 months (95% CI, 1.0–6.1 months) among patients in
the chemotherapy group (P<0.001) and 3.2 months (95% CI, 1.0–5.3
months) among patients in the first-line treatment only group
(P=0.001; Fig. 2).
 | Table III.Treatment patterns of the 83
patients. |
Table III.
Treatment patterns of the 83
patients.
| Treatment |
|
|---|
|
|
|---|
| First line | Second line | Number of
patients |
|---|
| Crizotinib
(n=33) | Crizotinib | 13 |
|
| Chemotherapy | 6 |
|
| No treatment | 1 |
| Chemotherapy
(n=50) | Crizotinib | 22 |
|
| Chemotherapy | 19 |
|
| No treatment | 6 |
Of the 33 patients receiving crizotinib as the
initial therapy, 20 experienced disease progression until the final
follow-up. Among them, treatment was changed to chemotherapy in 6
(30.0%) cases, crizotinib treatment was continued along with local
radiotherapy in 13 (65%) cases and 1 (5%) case did not receive any
other therapy beyond the first progression. Of the 13 patients
continued on crizotinib and receiving local radiotherapy, the
median follow-up time beyond the first progression was 42.7 weeks
(95% CI, 0.6–122.1 weeks). Until the last follow up, the median
PFS2 of these patients had not been reached.
Survival of crizotinib patients
At the last follow up, 71 (85.5%) patients had
received crizotinib, and 12 (14.5%) patients were crizotinib naive.
Of the 71 patients receiving crizotinib, 33 (46.5%) received this
therapy in the first-line setting, 22 (31%) received this in the
second-line setting, and 16 (22.5%) received crizotinib in the
third-line or further setting. There was no significant difference
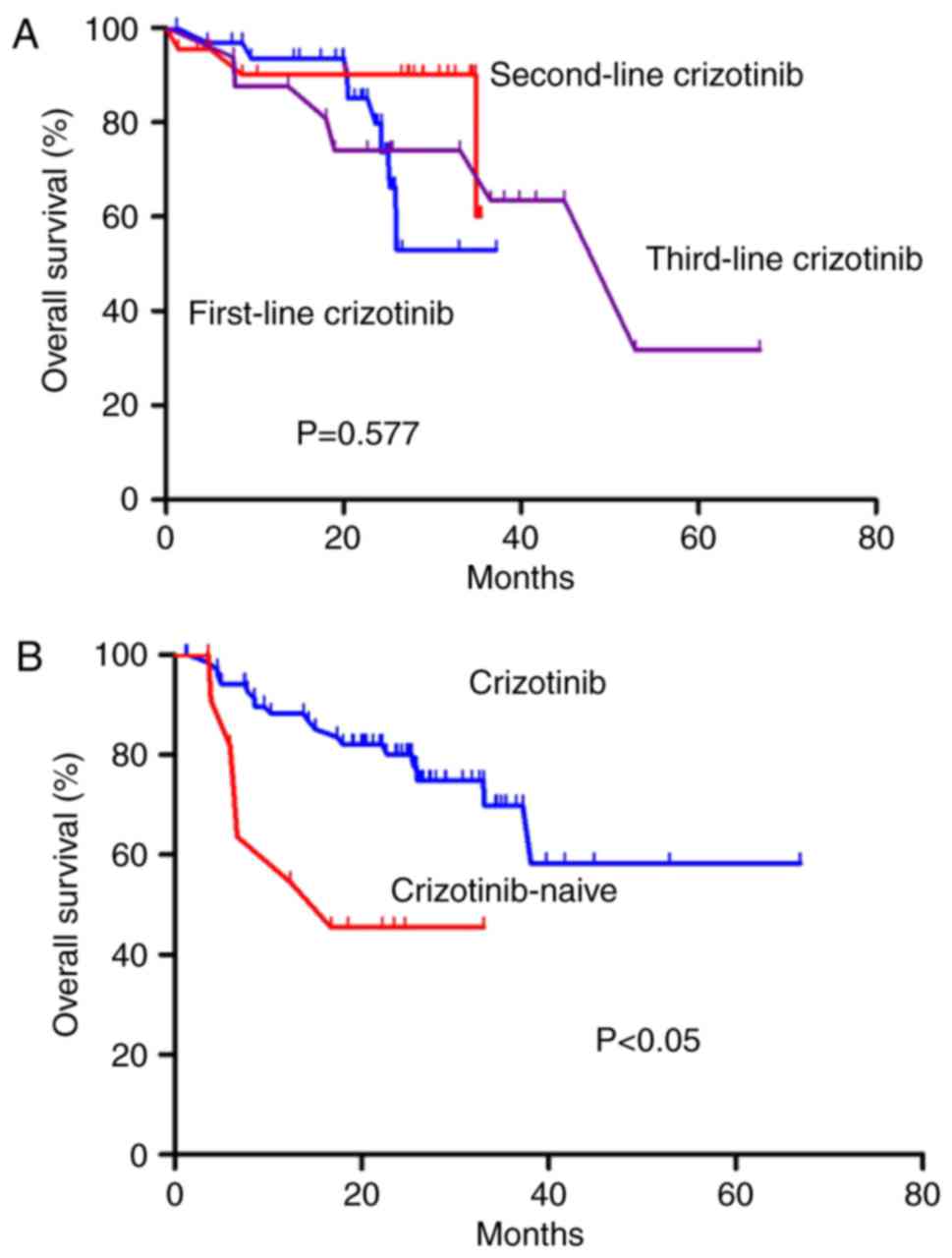
in OS among these three groups (P=0.577; Fig. 3A). However, patients receiving
crizotinib had an improved OS as compared with patients that were
crizotinib-naive (HR, 0.279; 95% CI, 0.107–0.727; P<0.05;
Fig. 3B).
Univariate analysis for OS
Factors that were analyzed by univariate analysis
are listed in Table IV. The gender
(P=0.028), smoking history (P=0.011), liver invasion at diagnosis
(P=0.023), bone invasion at diagnosis (P=0.01) and the use of
crizotinib (P=0.018) were significantly associated with the OS.
However, crizotinib used in the first-line setting, in the
second-line setting or after the second-line setting was not
associated with the OS.
 | Table IV.Univariate analysis for overall
survival. |
Table IV.
Univariate analysis for overall
survival.
| Characteristic | HR (95% CI) | P-value |
|---|
| Gender |
|
|
|
Male |
|
|
|
Female | 0.391
(0.167–0.915) | 0.028 |
| Age (years) |
|
|
|
<50 |
|
|
|
≥50 |
| 0.529 |
| Stage |
|
|
|
IIIB |
|
|
| IV |
| 0.931 |
| Smoking
history |
|
|
| No |
|
|
|
Yes | 2.957
(1.276–6.855) | 0.011 |
| Intrapulmonary
metastasis |
|
|
|
Yes |
|
|
| No |
| 0.666 |
| Intracranial
metastasis |
|
|
|
Yes |
|
|
| No |
| 0.561 |
| Liver
metastasis |
|
|
| No |
|
|
|
Yes | 2.934
(1.227–7.018) | 0.023 |
| Bone
metastasis |
|
|
| No |
|
|
|
Yes | 2.967
(1.299–6.775) | 0.010 |
| Pleural
effusion |
|
|
|
Yes |
|
|
| No |
| 0.832 |
| Crizotinib as
first-line therapy |
|
|
|
Yes |
|
|
| No |
| 0.802 |
| Crizotinib as
second-line therapy |
|
|
|
Yes |
|
|
| No |
| 0.138 |
| Crizotinib after
second-line therapy |
|
|
|
Yes |
|
|
| No |
| 0.943 |
| Use of
crizotinib |
|
|
| No |
|
|
|
Yes | 0.279
(0.107–0.727) | 0.018 |
Multivariate analysis
The Cox multivariate analysis identified the
following independent negative prognostic factors for OS: Smoking
history (HR=4.565), liver invasion at diagnosis (HR=4.294) and bone
invasion at diagnosis (HR=2.587). In addition, the use of
crizotinib (HR=0.319) was identified as positive prognostic factor
for OS (Table V).
 | Table V.Independent prognostic factors as
determined by multivariate analysis. |
Table V.
Independent prognostic factors as
determined by multivariate analysis.
| Factor | HR (95% CI) | P-value |
|---|
| Smoking
history | 4.565
(1.697–12.279) | 0.003 |
| Liver
metastasis | 4.294
(1.489–12.379) | 0.007 |
| Bone
metastasis | 2.587
(1.067–6.275) | 0.035 |
| Use of
crizotinib | 0.319
(0.110–0.928) | 0.036 |
The 2 year OS rate for patients who had never smoked
was 85%, with a rate of 54% for those who were former or current
smokers. Patients with liver invasion at diagnosis had a 2-year OS
rate of 81%, as compared with the rate of 49% for patients with
non-liver invasion. Furthermore, patients with bone invasion at
diagnosis had a 2-year OS rate of 83%, as compared with the rate of
59% for those with non-bone invasion. Patients who received
crizotinib had a 2-year OS rate of 80%, as compared with the rate
of 42% for those who were crizotinib-naive.
Discussion
Molecular targeted therapy of advanced NSCLC
represents the paradigm of personalized treatment of malignancies.
Several druggable cancer driver genes have been identified thus
far, with epidermal growth factor receptor mutation and ALK gene
rearrangements being the most characteristic features of the
disease (15). ALK rearrangements
occur in 3–7% of patients with NSCLC. Although the proportion of
ALK-positive patients is relatively low, the number of ALK-positive
patients in China is anticipated to be significant due to the large
population base and the high incidence of lung cancer. In China,
crizotinib has been approved as a first-line monotherapy for
advanced or metastatic patients with ALK-positive NSCLC, and as a
second-line monotherapy for those who have received prior
chemotherapy. However, to date, limited data have been published on
the treatment patterns and outcomes of Chinese ALK-positive NSCLC
patients.
In the current real-world study, the characteristics
of patients were consistent with those reported in previous studies
(16,17). More specifically, ALK rearrangement
occurred in relatively young patients, >50% of the patients had
no history of smoking and almost all patients were diagnosed with
adenocarcinoma. In addition, bone and pleural metastasis at
diagnosis was reported in approximately one third of the patients,
while brain metastasis at diagnosis was observed in ~10% of
patients in the present study. It has previously been reported that
ALK-positive NSCLC does not appear to be associated with an
increased risk of brain metastases at the first diagnosis (16). The incidence of intracranial invasion
in newly diagnosed ALK-positive advanced NSCLC patients has been
demonstrated to range from 20–30%, which was similar to that of
non-ALK-positive disease (18,19). In
the univariate analysis conducted in the current study, smoking
history was associated with worse OS. A study that provided a
50-year perspective on the evolution of smoking-associated risks in
the United States has demonstrated that, among patients with lung
cancer, smokers had an increased mortality rate as compared with
nonsmokers (20). Another study
revealed that the rate of mortality from any cause among current
smokers was ~3 times that reported among individuals who had never
smoked (21).
Although two randomized stage III clinical trials
(10,11) have demonstrated a higher ORR and
longer PFS of first-line crizotinib treatment when compared with
that in patients receiving chemotherapy, more than half (60.2%) of
the 83 patients selected chemotherapy as the initial therapy in the
present study. The main reason for this phenomenon is the fact that
crizotinib is not currently included in the medical insurance
catalogue in China, leading to certain patients unable to afford
such an expensive drug at the beginning of treatment. In the
current study, the ORR and PFS1 were also significantly improved in
the crizotinib group as compared with the chemotherapy group.
Notably, the PFS of patients receiving crizotinib as the initial
therapy (median PFS, 18.5 months) was longer compared with the PFS
results of the PROFILE 1014 (median PFS, 10.9 months) and PROFILE
1029 (median PFS, 11.1 months) trials. A similar PFS result (median
PFS, 17.6 months) was reported in another real-world study from a
single center in China (22). In the
previous clinical trials (10,11), brain
and bone scanning was repeated every 12 weeks to monitor for new
lesions in the follow-up schedule. By contrast, in the present
study, brain and bone scanning was repeated every 6 months or when
associated symptoms appeared in patients without brain or bone
metastasis at the baseline assessment. This may have resulted in an
artificially prolonged PFS in the crizotinib group since
intracranial progression occurred in half of the patients with
disease progression. In addition, according to previous studies,
different EML4-ALK variants may exhibit a different response to
crizotinib, of which the EML4-ALK variant 1 had significantly
longer PFS in comparison with other EML4-ALK variants (median, 31.1
vs. 5.7 months, respectively; P=0.003) (23,24). This
may be one of the reasons for the PFS difference between the
current study and previous clinical trials; however, further
investigation is required to test this hypothesis.
The estimation of OS in patients treated with
crizotinib has not yet been fully documented. In the present
analysis, there was no significant difference in OS between
patients receiving crizotinib and those receiving chemotherapy in
the first-line setting. Similar results were observed in the
PROFILE 1014 and PROFILE 1029 trials comparing crizotinib to
pemetrexed-plus-platinum as the first-line therapy, possibly due to
a cross-over in the chemotherapy arm. Indeed, in the current study,
38 (76.0%) of the 50 patients receiving chemotherapy as the initial
therapy were administered crizotinib as subsequent therapy. In
addition, there were no statistically significant difference in the
OS among patients using crizotinib in the first-line, second-line
and third-line or further setting in the present study. However,
patients receiving crizotinib exhibited improved OS in comparison
with patients who were crizotinib-naive. A retrospective analysis
comparing 30 crizotinib-treated ALK-positive NSCLC patients with 23
crizotinib-naive patients reported similar results, with the
patients in the crizotinib group exhibiting a longer OS (25). The current Cox multivariate analysis
also identified the use of crizotinib, but not the line of usage as
an independent prognostic factor for OS.
Despite a great response rate, the vast majority of
patients with ALK-rearranged NSCLC inevitably experienced acquired
resistance in ~1 year. The central nervous system (CNS) has been
reported to be a frequent site of acquired resistance to crizotinib
in patients with ALK-positive NSCLC (26–28). In
the present study, cerebral progression occurred in more patients
in the crizotinib group (n=10; 50%) in comparison with those in the
chemotherapy group (n=4; 8.5%). Consistently, the prevalence of CNS
metastases in crizotinib-refractory patients was approximately
twice as high as that of crizotinib-naive patients in previous
studies (29,30). This phenomenon can be largely
attributed to the following two reasons: Firstly, crizotinib is a
substrate of P-glycoprotein, a membranous transporter overexpressed
in the hematoencephalic barrier and responsible for the efflux of
the drug. Low cerebrospinal fluid-to-serum ratios have been
reported for crizotinib in the range between 0.06 and 0.26%
(31,32). Furthermore, crizotinib extended the
patient survival, which to a certain extent may be contributed to
the higher incidence of CNS metastases as compared with
chemotherapy.
In the current study, of the patients receiving
crizotinib as the initial therapy, 20 experienced disease
progression, 13 (65%) were continued on crizotinib and received
local radiotherapy beyond the first progression. In these 13
patients, the median follow-up time subsequent to the first
progression was 42.7 weeks. Until the last follow-up, the median
PFS2 of these patients had not been reached. Prior to
second-generation ALK inhibitors being available, treatment with
crizotinib was often continued beyond disease progression in
clinical practice and trials. For instance, in the phase 3
randomized trial PROFILE 1014 (10),
73% of patients with previously untreated ALK-positive NSCLC were
continued on crizotinib beyond disease progression for a median of
3.1 months. A retrospective analysis of patients in the PROFILE
1001 and PROFILE 1005 studies revealed that 62% (120/194) of the
patients continued crizotinib therapy after the RECIST-defined PD.
Among the 120 patients, 51% had brain metastases as the sole site
of PD, and the results revealed that this treatment strategy may
improve survival (33). The
therapeutic strategy of continuing crizotinib beyond disease
progression has also been supported by several retrospective
studies, in which continued ALK inhibition with crizotinib was
associated with clinical benefits and prolonged OS (34–36). In a
recently published physician survey and retrospective chart review
study in the US, the majority of physicians (75%) would add local
therapy and resume crizotinib when a new symptomatic isolated
lesion was detected in ALK-positve NSCLC treated with crizotinib
(37).
Mechanisms of acquired resistance to crizotinib
commonly include secondary mutations within the ALK tyrosine kinase
domain. Therefore, during the last decade more potent and
structurally different inhibitors have been developed (38,39). Since
the development of second-generation ALK-inhibitors, which were
demonstrated to be effective in crizotinib-resistant patients, are
not available in China, the continuation of crizotinib plus local
treatment may be a feasible option to maximize the overall clinical
benefits for patients with local-site progression post
crizotinib.
In conclusion, the present real-world study
demonstrated that the use of crizotinib improved the long-term
survival of advanced NSCLC patients with ALK rearrangement as
compared with crizotinib-naive patients. There was no difference in
the survival outcome between patients with initial use of
crizotinib and those with subsequent use of crizotinib beyond
first-line therapy. CNS was a frequent site of acquired resistance
to crizotinib, and the continuation of crizotinib plus local
treatment may improve the survival. However, head-to-head trials
comparing second- or third-generation ALK inhibitors with the
continuation of crizotinib beyond progression are necessary to
provide the optimum treatment strategy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Wu Jie Ping
Medical Foundation (grant no. wjpzlz-160830-01).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YJ contributed to the acquisition of data and
prepared the manuscript. YC performed the statistical analysis. XY
revised the manuscript critically for important intellectual
content. XS and XY designed the study.
Ethics approval and consent to
participate
All procedures performed in the present study
involving human participants were in accordance with the ethical
standards of the Research Ethics Committee of Zhejiang Cancer
Hospital (Zhejiang, China) and with the 2013 Declaration of
Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Siegel RL and Jemal A: Lung
cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boolell V, Alamgeer M, Watkins DN and
Ganju V: The evolution of therapies in non-small cell lung cancer.
Cancers. 7:1815–1846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soda M, Choi YL, Enomoto M, Takada S,
Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K,
Hatanaka H, et al: Identification of the transforming EML4-ALK
fusion gene in non-small-cell lung cancer. Nature. 448:561–566.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rikova K, Guo A, Zeng Q, Possemato A, Yu
J, Haack H, Nardone J, Lee K, Reeves C, Li Y, et al: Global survey
of phosphotyrosine signaling identifies oncogenic kinases in lung
cacner. Cell. 131:1190–1203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zou HY, Li Q, Lee JH, Arango ME, McDonnell
SR, Yamazaki S, Koudriakova TB, Alton G, Cui JJ, Kung PP, et al: An
orally available small-molecule inhibitor of c-Met, PF-2341066,
exhibits cytoreductive antitumor efficacy through antiproliferative
and antiangiogenic mechanisms. Cancer Res. 67:4408–4417. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Camidge DR, Bang YJ, Kwak EL, Iafrate AJ,
Varella-Garcia M, Fox SB, Riely GJ, Solomon B, Ou SH, Kim DW, et
al: Activity and safety of crizotinib in patients with ALK-positive
non-small-cell lung cancer: Updated results from a phase 1 study.
Lancet Oncol. 13:1011–1019. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crino L, Kim D, Riely GJ, Janne PA,
Blackhall FH, Camidge DR, Hirsh V, Mok T, Solomon BJ, Park K, et
al: Initial phase II results with crizotinib in advanced
ALK-posivie non-small cell lung cancer (NSCLC): PROFILE 1005. ASCO
Meet Abstr. 29 15 Suppl:75142011.
|
|
9
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cacner. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu S, Mok T, Lu Y, Zhou J, Shi Y and
Sriuranpong V: Phase 3 study of first-line crizotinib vs
pemetrexed-cisplatin/carboplatin (PCC) in East Asian patients (pts)
with ALK+ advanced non-squamous non-small cell lung cancer (NSCLC).
ASCO Meet Abstr. 34 15 Suppl:90582016.
|
|
12
|
Rami-Porta R, Bolejack V, Giroux DJ,
Chansky K, Crowley J, Asamura H and Goldstraw P: International
Association for the Study of Lung Cancer Staging and Prognostic
Factors Committee, Advisory Board Members and Participating
Institutions: The IASLC lung cancer staging project: The new
database to inform the eighth edition of the TNM classfication of
lung cancer. J Thorac Oncol. 9:1618–1624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wynes MW, Sholl LM, Dietel M, Schuuring E,
Tsao MS, Yatabe Y, Tubbs RR and Hirsch FR: An international
interpretation study using the ALK IHC antibody D5F3 and a
sensitive detection kit demonstrates high concordance between ALK
IHC and ALK FISH and between evaluators. J Thorac Oncol. 9:631–638.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melosky B: Current treatment algorithms
for patients with metastatic non-small cell, non-squamous lung
cancer. Front Oncol. 7:382017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodig SJ, Mino-Kenudson M, Dacic S, Yeap
BY, Shaw A, Barletta JA, Stubbs H, Law K, Lindeman N, Mark E, et
al: Unique clinicopathologic features characterize ALK-rearranged
lung adenocarcinoma in the western population. Clin Cancer Res.
15:5216–5223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang YG, Jin ML, Li L, Zhao HY, Zeng X,
Jiang L, Wei P, Diao XL, Li X, Cao Q and Tian XX: Evaluation of ALK
rearrangement in Chinese non-small cell lung cancer using FISH,
immunohistochemistry, and real-time quantitative RT-PCR on
paraffin-embedded tissues. PLoS One. 8:e648212013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doebele RC, Lu X, Sumey C, Maxson DA,
Weickhardt AJ, Oton AB, Bunn PA Jr, Barón AE, Franklin WA, Aisner
DL, et al: Oncogene status predicts patterns of metastatic spread
in treatment-naïve non-small cell lung cancer. Cancer.
118:4502–4511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seto T, Kiura K, Nishio M, Nakagawa K,
Maemondo M, Inoue A, Hida T, Yamamoto N, Yoshioka H, Harada M, et
al: CH5424802(RO5424802) for patients with ALK-rearranged advanced
non-small-cell lung cancer (AF-001JP study): A single-arm,
open-label, phase 1–2 study. Lancet Oncol. 14:590–598. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Thun MJ, Carter BD, Feskanich D, Freedman
ND, Prentice R, Lopez AD, Hartge P and Gapstur SM: 50-year trends
in smoking-related mortality in the United States. N Engl J Med.
368:351–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jha P, Ramasundarahettige C, Landsman V,
Rostron B, Thun M, Anderson RN, McAfee T and Peto R: 21st-century
hazards of smoking and benefits of cessation in the United States.
N Engl J Med. 368:341–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen G, Chen X, Zhang Y, Yan F, Fang W,
Yang Y, Hong S, Miao S, Wu M, Huang X, et al: A large,
single-center, real-world study of clinicopathological
characteristics and treatment in advanced ALK-positive
non-small-cell lung cancer. Cancer Med. 6:953–961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshida T, Oya Y, Tanaka K, Shimizu J,
Horio Y, Kuroda H, Sakao Y, Hida T and Yatabe Y: Differential
crizotinib response duration among ALK fusion variants in
ALK-positive non-small-cell lung cancer. J Clin Oncol.
34:3383–3389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cha YJ, Kim HR and Shim HS: Clinical
outcomes in ALK-rearranged lung adenocarcinomas according to ALK
fusion variants. J Transl Med. 14:2962016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shaw AT, Yeap BY, Solomon BJ, Riely GJ,
Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, et
al: Effect of crizotinib on overall survival in pateints with
advanced non-small-cell lung cancer harbouring ALK gene
rearrangement: A retrospective analysis. Lancet Oncol.
12:1004–1012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Costa DB, Shaw AT, Ou SH, Solomon BJ,
Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, et al:
Clinical experience with crizotinib in patients with advanced
ALK-rearranged non-small-cell lung cancer and brain metastasis. J
Clin Oncol. 33:1881–1888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Camidge DR: Taking aim at ALK across the
blood-brain barrier. J Thorac Oncol. 8:389–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Metro G, Lunardi G, Floridi P, Pascali JP,
Marcomigni L, Chiari R, Ludovini V, Crinò L and Gori S: CSF
concentration of crizotinib in two ALK-positive non-small-cell lung
cancer patients with CNS metastases deriving clinical benefit from
treatment. J Thorac Oncol. 10:e26–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ou SH, Ahn JS, De Petris L, Govindan R,
Yang JC, Hughes B, Lena H, Moro-Sibilot D, Bearz A, Ramirez SV, et
al: Alectinib in crizotinib-refractory ALK-rearranged
non-small-cell lung cancer: A phase II global study. J Clin Oncol.
34:661–668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shaw AT, Gandhi L, Gadgeel S, Riely GJ,
Cetnar J, West H, Camidge DR, Socinski MA, Chiappori A, Mekhail T,
et al: Alectinib in ALK-positive, crizotinib-resistant,
non-small-cell lung cancer: A single-group, multicentre, phase 2
trial. Lancet Oncol. 17:234–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Costa DB, Kobayashi S, Pandya SS, Yeo WL,
Shen Z, Tan W and Wilner KD: CSF concentration of the anaplastic
lymphoma kinase inhibitor crizotinib. J Clin Oncol. 29:e443–445.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Metro G, Lunardi G, Floridi P, Pascali JP,
Marcomigni L, Chiari R, Ludovini V, Crinò L and Gori S: CSF
concentration of crizotinib in two ALK-positive non-small-cell lung
cancer patients with CNS metastases deriving clinical benefit from
treatment. J Thorac Oncol. 10:e26–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ou SH, Jänne PA, Bartlett CH, Tang Y, Kim
DW, Otterson GA, Crinò L, Selaru P, Cohen DP, Clark JW and Riely
GJ: Clinical benefit of continuing ALK inhibition with crizotinib
beyond initial disease progression in patients with advanced
ALK-positive NSCLC. Ann Oncol. 25:415–422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hong X, Chen Q, Ding L, Liang Y, Zhou N,
Fang W, Chen X and Wu H: Clinical benefit of continuing crizotinib
therapy after initial disease progression in Chiniese pateints with
advanced ALK-rearranged non-small-cell lung cancer. Oncotarget.
8:41631–41640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takeda M, Okamoto I and Nakagawa K:
Clinical impact of continued crizotinib administration after
isolated central nervous system progresssion in patients with lung
cancer positive for ALK rearrangement. J Thorac Oncol. 8:654–657.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weickhardt AJ, Scheier B, Burke JM, Gan G,
Lu X, Bunn PA Jr, Aisner DL, Gaspar LE, Kavanagh BD, Doebele RC and
Camidge DR: Local ablative therapy of oligoprogressive disease
prolongs disease control by tyrosine kinase inhibitors in
oncogene-addicted non-small-cell lung cacner. J Thorac Oncol.
7:1807–1814. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bendaly E, Dalal AA, Culver K, Galebach P,
Bocharova I, Foster R, Sasane M, Macalalad AR and Guérin A:
Monitoring for and characterizing crizotinib progression: A chart
review of ALK-positive non-small cell lung cancer pateints. Adv
Ther. 34:1673–1685. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu JJ, Savooji J and Liu DL: Second- and
third-generation ALK inhibitors for non-small cell lung cancer. J
Hematol Oncol. 9:192016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Su S and Wu YL: Clinical trials of
tyrosine kinase inhibitors for lung cancer in China: A review. J
Hematol Oncol. 10:1472017. View Article : Google Scholar : PubMed/NCBI
|