Introduction
The crucial prognostic factor for breast carcinoma
is still axillary lymph nodes (ALNs) metastasis (1,2), and the
status of ALNs can influence the effect of adjuvant therapy and
surgery. Because of increased consciousness and advanced imaging
modalities, breast cancer can be diagnosed earlier, and many
patients have clinically negative axillae (3).
Earlier axillary lymph node dissection (ALND) was
the criterion for detecting axillary metastasis. But with the
advent of sentinel lymph node biopsy (SLNB), which was regarded as
a better imaging and minimal biopsy method, ALND was no longer
considered as the only way to detect axillary metastasis. Now SLNB
is the gold standard for histopathological staging of early breast
cancer, because information on ALNs status can be obtained at a
lower complication rate (4). However,
the location of the sentinel lymph node can be identified by
preoperative lymphoscintigraphy or using blue-dye, which will
increase surgical costs. Furthermore, clinical complications may
occur, including allergic reactions, lower sensitivity and strength
of the ipsilateral upper limb and even the uncommon occurrence of
lymphedema (5,6).
Axillary ultrasound (US) is a major non-surgical
approach to assess ALNs (7).
Particularly when using morphological criteria to detect axillary
metastasis, it is moderately sensitive (8). However, axillary US is
operator-dependent and machine-dependent. When only US is used to
assess ALNs, the sensitivity and specificity vary greatly (9,10).
Therefore, in order to solve this problem, US-guided fine needle
aspiration biopsy (FNAB) is required. With the application of FNAB
in suspicious lymph nodes, the specificity of detecting metastatic
lymph nodes can be increased (11,12).
Recent studies show that 7.8–16.2% of patients with axillary
metastasis have been successfully diagnosed preoperatively via
US-guided FNAB (12,13).
Some clinical trials such as the BOOG 2013-08
(14), NCT 01821768 (15), and the SOUND (16) have been conducted recently, and breast
cancer patients with negative US/FNAB findings were randomly
assigned to SLNB and non-SLNB groups. These clinical trials
demonstrated that it was necessary to perform SLNB in patients with
negative US-guided FNAB of suspicious lymph nodes. A number of
diagnostic tools were used to determine the status of negative ALNs
in these trials, such as axillary palpation, axillary US, computed
tomography (CT), or intervening suspicious lymph nodes with FNAB.
Therefore, an accurate evaluation of ALNs status before surgery was
an important condition to omit SLNB or ALND. The aim of the present
study was to evaluate the efficiency of preoperative diagnostic
techniques for ALNs status.
Materials and methods
Patient selection criteria
All the patients with stage I and II early breast
cancer presenting to the Affiliated Tumor Hospital of Guangxi
Medical University from June 2015 to January 2017 were
prospectively included in the present study. Patients who had a
previous axillary-breast surgery or radiotherapy, inflammatory
breast cancer or neo-adjuvant chemotherapy were excluded from the
present study. Patient demographics, clinicopathological features,
axillary US ± FNAB findings, intraoperative SLNB findings and final
axillary histopathology results were recorded.
Ethical approval was obtained from the Ethics
Committee for Clinical Research of The Affiliated Tumor Hospital of
Guangxi Medical University (Guangxi, China), and the study itself
was performed in accordance with the Declaration of Helsinki. All
patients provided written informed consent.
Radiologic technique and criteria
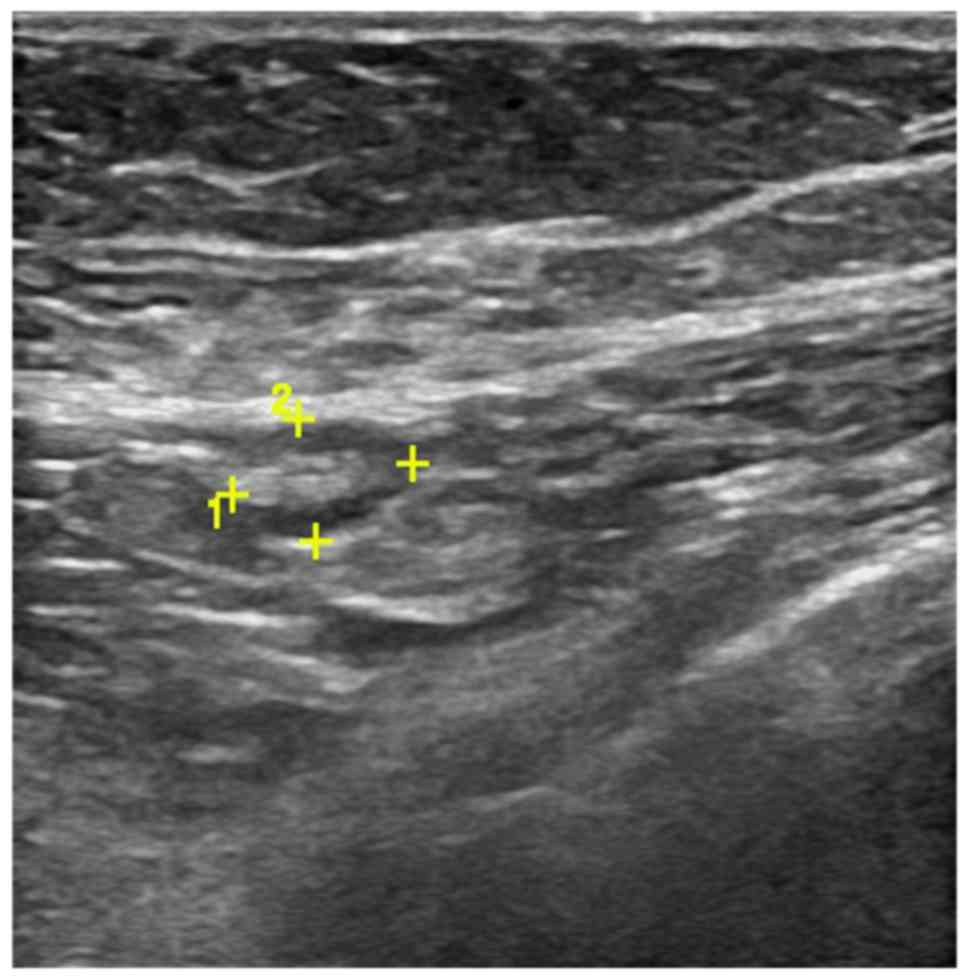
Axilla was scanned by three experienced radiologists
using a high frequency linear 12 MHz transducer. The criteria of US
to define abnormal lymph nodes included: diffuse cortical
thickening, complete or partial effacement of the fatty hilum,
focal cortical bulge, round or hypoechoic nodes with short axis
>5 mm (Figs. 1 and 2) show the sonographic images for suspicious
lymph nodes.
After evaluating the axillary lymph node status by
US, a US-guided FNAB was performed by the specific radiologist
using a fine-needle (22-gauge) toward the most representative
abnormal node. 10 ml of 1% xylocaine was used to achieve local
anesthesia. Under US guidance, the needle was inserted into the
cortex of the lymph node in a way parallel to the long axis of the
probe. A minimum of three aspirations were performed when the
needle tip was confirmed to be inserted into the target area. The
aspirate was then sent to a cytopathologist for subsequent
analysis.
Pathologic technique
The aspirated material was smeared and fixed with
95% alcohol, then smears were stained with Papanicolaou and Giemsa
procedures. The samples were examined by an experienced pathologist
and were divided into four groups: Benign, suspicious for
malignancy, malignant, and inadequate for evaluation. In the
assessment of US-guided FNAB accuracy, malignant sampling on FNAB
was regarded as positive. Benign, suspicious for malignancy and
insufficient sampling were regarded as negative results in the
present study.
Surgical technique
The patients who were diagnosed with positive FNAB
underwent directly ALND after they decided to have an operation.
For a negative axillary US or a negative FNAB, a SLNB procedure was
performed using blue-dye or radiocolloid injection. Similarly, if a
positive node was found by SLNB or if there were no sentinel lymph
nodes detected intraoperatively, then ALND was performed. All lymph
node tissues, together with breast samples, were sent to the
laboratory for final histopathological examination. (Fig. 3) depicts the procedure for the
perioperative diagnosis of axillary metastasis.
Statistical analysis
Axillary US results and the cytological findings of
FNAB were further identified by the final histological findings.
The sensitivity, specificity, positive predictive value (PPV),
negative predictive value (NPV) and accuracy of axillary US and
FNAB were computed. An exact 95% confidence interval (CI) was
calculated on the basis of the binomial distribution. The P-values
below 0.05 were considered to be statistically significant.
Statistical analyses were performed using Statistical Product and
Service Solutions version 17.0 Windows (SPSS, Inc., Chicago, IL,
USA) and MedCalc software (MedCalc Software, Mariakerke, Belgium),
and Fisher's exact or a Chi-square test were performed.
Results
Patients and tumour
characteristics
There were 476 patients with invasive breast cancer
included in this continuous study between June 2015 and January
2017. Out of which 214 patients were stage I and II early breast
cancer and underwent axillary US prior to definitive surgery. The
histopathologic and demographic features of these patients were
compared with final axillary histopathology reports (Table I).
 | Table I.Comparison of the patients
demographic and tumor characteristics with final axillary
histopathology results (n=214). |
Table I.
Comparison of the patients
demographic and tumor characteristics with final axillary
histopathology results (n=214).
| Characteristic | Axilla (+) (%) | Axilla (−) (%) | P-value |
|---|
| Age (years) |
|
| 0.189 |
|
≤50 | 25 (32.9) | 58 (42.0) |
|
|
>50 | 51 (67.1) | 80 (58.0) |
|
| Axillary side |
|
| 0.271 |
|
Left | 37 (48.7) | 78 (56.5) |
|
|
Right | 39 (51.3) | 60 (43.5) |
|
| cT stage |
|
| 0.898 |
| T1 | 34 (44.7) | 63 (45.7) |
|
| T2 | 42 (55.3) | 75 (54.3) |
|
| cN stage |
|
| 0.074 |
| N0 | 57 (75.0) | 87 (63.0) |
|
| N+ | 19 (25.0) | 51 (37.0) |
|
| Clinical stage |
|
| 0.559 |
| I | 31 (40.8) | 62 (44.9) |
|
| IIA +
IIB | 45 (59.2) | 76 (55.1) |
|
| Primar tumor
histology |
|
|
<0.001a |
|
Invasive ductal carcinoma | 76 (100.0) | 95 (68.8) |
|
|
Invasive lobular
carcinoma | 0 (0.0) | 24 (17.4) |
|
|
Other | 0 (0.0) | 19 (13.8) |
|
| Surgery |
|
| 0.143 |
|
BCS | 29 (38.2) | 67 (48.6) |
|
|
Mastectomy | 47 (61.8) | 71 (51.4) |
|
| ER status |
|
| 0.761 |
|
Negative (<1%) | 20 (26.3) | 39 (28.3) |
|
|
Positive (≥1%) | 56 (73.7) | 99 (71.7) |
|
| PR status |
|
| 0.677 |
|
Negative (<1%) | 20 (26.3) | 40 (29.0) |
|
|
Positive (≥1%) | 56 (73.7) | 98 (71.0) |
|
| Her2/neu
status |
|
|
<0.001a |
|
Negative (0, 1+, 2+ FISH not
amplified) | 33 (43.4) | 114 (82.6) |
|
|
Positive (3+, 2+ FISH
amplified) | 43 (56.6) | 24 (17.4) |
|
| Proliferative
index |
|
| 0.950 |
| Ki-67
(<15%) | 24 (31.6) | 43 (31.2) |
|
| Ki-67
(≥15%) | 52 (68.4) | 95 (68.8) |
|
The median patient age was 52 years (range 28–72) in
the present study. There were 171 invasive ductal carcinomas, 24
invasive lobular carcinomas and 19 other histological types
(invasive mucinous, medullary, tubular, and mixed carcinoma)
(17). The right ALNs were detected
by US in 99 patients, and the left ALNs were detected by US in 115
patients. Of the 214 primary breast cancer patients, ninety-three
cases were of grade I, and 121 cases were of grade II. In the final
histopathology reports, 44.4% patients (76/171) had positive lymph
nodes in patients with invasive ductal carcinoma. However, no
positive lymph nodes were found in other histological types. The
present study showed that there was a statistically significant
difference in the histopathological ALNs involvement rate between
different histological types (P<0.001). In addition, the final
histopathology reports differed significantly with respect to
Her-2/neu status (P<0.001).
Axillary US results
As shown in (Table
II), there were 75 patients with suspicious ALNs diagnosed by
pre-operative axillary US, while the remaining 139 patients were
sonographically benign. Seventy-six patients (35.5%) were diagnosed
with metastatic disease in the final histopathology reports. Of
these 76 positive cases, the result of axillary US was suspicious
for malignancy in 45 cases (59.2%). Furthermore, the comparison
between the accuracy of axillary US and the final histopathology
reports of ALNs by SLNB or ALND was shown in (Table III). The sensitivity, specificity,
PPV, and NPV of axillary US alone were 59.2% (45/76), 78.3%
(108/138), 60.0% (45/75), and 77.7% (108/139) respectively. False
negative rate for axillary US was 22.3% (31/139). The whole
diagnostic accuracy for axillary US was 71.5% (153/214).
 | Table II.Association between axillary
ultrasound, ultrasound-guided fine needle aspiration biopsy with
final histopathology. |
Table II.
Association between axillary
ultrasound, ultrasound-guided fine needle aspiration biopsy with
final histopathology.
|
|
| Final
histopathology |
|
|---|
|
|
|
|
|
|---|
| Investigation | Category | Positive | Negative | Total (n) |
|---|
| Axillary US | Suspicious | 45 (60.0%) | 30 (40.0%) | 75 |
|
| Benign | 31 (22.3%) | 108 (77.7%) | 139 |
|
| Total (n) | 76 | 138 | 214 |
| US-guided FNAB | Positive | 32 (100.0%) | 0 (0.0%) | 32 |
|
| Negative | 13 (30.2%) | 30 (69.8%) | 43 |
|
| Total (n) | 45 | 30 | 75 |
 | Table III.Accuracy of axillary ultrasound and
ultrasound-guided fine needle aspiration biopsy. |
Table III.
Accuracy of axillary ultrasound and
ultrasound-guided fine needle aspiration biopsy.
|
| Axillary US | US-guided FNAB |
|---|
|
|
|
|
|---|
| Variable | % | 95% CI | % | 95% CI |
|---|
| Sensitivity | 59.2 | 47.3–70.4 | 71.1 | 55.7–83.6 |
| Specificity | 78.3 | 70.4–84.8 | 100.0 | 88.4–100.0 |
| PPV | 60.0 | 48.0–71.2 | 100.0 | 89.1–100.0 |
| NPV | 77.7 | 69.9–84.3 | 69.8 | 53.9–82.8 |
| Accuracy | 71.5 | 64.7–79.1 | 82.7 | 71.7–90.8 |
| Total (n) | 214 | 75 |
US-guided FNAB results
In the present study, all the patients with
suspicious ALNs on axillary US were performed FNAB. Of these 75
patients, 32 (42.7%) were confirmed to have malignant cytology on
FNAB. 42.7% of the patients underwent ALND directly instead of the
SLNB procedure.
Table II showed the
axillary findings of cytology and histopathology. All 32 patients
with positive FNABs had axillary metastases in the final ALNs
histopathological report. Negative FNABs were found in 43 patients,
13 of which (30.2%) had positive results after SLNB or axillary
dissection.
The diagnostic power of FNAB which was used to
identify metastatic ALNs was statistically analyzed. In predicting
ALNs status, the sensitivity, specificity, PPV, and NPV of
US-guided FNAB were 71.1% (32/45), 100.0% (30/30), 100.0% (32/32),
and 69.8% (30/43), respectively. False negative rate was 30.2%
(13/43). The overall diagnostic accuracy of US-guided FNAB was
82.7% (62/75) (Table III).
SLNB results
SLNB was performed in 182 patients. A mean of 2.21
nodes were excised per patient (range 1–5 nodes). Sentinel lymph
nodes were positive in 44 patients. A total of 64 sentinel lymph
nodes were identified as metastases. In these patients, isolated
tumor cells (ITC) were identified in 6 lymph nodes, micrometastases
in 11 lymph nodes, and macrometastases in 47 lymph nodes.
Discussion
The following procedure is applied to the
conventional staging of breast carcinoma: Breast and axillary US,
physical examination, history, chest X-ray, abdominal CT, and bone
survey. The most important parameter which can affect surgery and
medical treatment in breast cancer patients is the status of the
axillary lymph node. In addition, the mode of ALND depends on
preoperative detection of axillary metastases.
Due to both the increased awareness of breast
carcinoma and comprehensive breast screening programs, the disease
is detected at an early stage at present. In this case, the
possibility of patients with microscopic or clinical ALNs
involvement on admission is low. Breast-conservative surgery can be
performed, if there are no axillary lymph node involvement in T1
and T2 breast cancers. Preoperative neoadjuvant chemotherapy is
used if distant metastases or lymph nodes metastases are detected
in locally advanced breast cancer (18).
Axillary US combined with FNAB was implemented in
1997 (19). US-guided FNAB, which was
an alternative technique, appeared nearly at the same time with
SLNB. It has been shown that ultrasound might be used to assess
lymph nodes quite accurately (20).
Many studies have shown that ALNs FNAB-guided preoperative staging
was more accurate. Chang et al (21) indicated that the PPV and NPV of
US-guided FNAB were quite high as 98.7 and 81.8%, respectively.
At present, the sentinel node dissection procedure
can be skipped, and ALND is performed even in patients who have a
single positive axillary lymph node (22,23).
Preoperative axillary US or FNAB on suspicious lymph nodes can
reduce the demand for SLNB by 21–65%. In the present study,
US-guided FNAB was used to detect axillary metastases, and the
demand for SLNB was eliminated in 42.7% of the cases. If
unnecessary SLNB step can be avoided, the duration of the surgery
will be shortened and the costs of the procedure will be decreased
markedly, resulting in a reduction in healthcare expenses by nearly
20% (22,24,25).
Leenders et al (26) reported sensitivity, specificity, PPV
and NPV of axillary US alone as 60.8, 80.7, 67.5 and 70.7%,
respectively. When combining axillary US with FNAB of suspicious
lymph nodes, sensitivity was 73.5%, specificity was 99.9%, PPV was
99.1% and NPV was 69.0% (26).
Similarly García Fernández et al (27) demonstrated that US-guided FNAB showed
PPV of 87%, NPV of 82%, sensitivity of 70% and specificity of 100%,
when compared with final axillary histology (27). The sensitivity, specificity, PPV, and
NPV of axillary US alone were 59.2% (45/76), 78.3% (108/138), 60.0%
(45/75) and 77.7% (108/139), respectively, in the present study.
This result is similar to above studies concerning the role of
axillary US alone in preoperative detection of positive ALNs.
However, the sensitivity and specificity of US-guided FNAB in
evaluating the axillary lymph node grew to 71.1 and 100.0%,
respectively, after combining the axillary US with FNAB. The PPV
and NPV of this method were evaluated to be 100.0 and 69.8%,
respectively, which was again consistent with previous studies.
Inadequate sampling was the most important cause of
the false negative results found in recent studies. The proportion
of insufficient sampling can be reduced when the number of
aspirations was increased (23). The
inadequate sampling rate was 8.5% in the present study, which was
similar to the rate reported in the literature. The small size of
metastatic lymph nodes and the deficient imaging of ALNs detected
by US caused an inadequate aspiration (23,28).
The sensitivity of US-guided FNAB is related to the
number of involved ALNs. The sensitivity will increase from 47.1 to
80%, if two or more lymph nodes are involved (29). Deurloo et al (30) showed that when ALNs with a cortical
thickness of >2 mm were selected for puncture, the sensitivity
and specificity of US-guided FNAB would be increased. Studies have
shown that as the tumor size increased, the number of involved
lymph nodes also increased. The prognosis of small tumors with
axillary involvement was superior to that of large ones (31).
In the present study, 30.2% of cases had false
negative results for the axillary lymph node FNAB. The reason for
the false negative results may be due to inappropriate sampling,
micro-metastasis, the deficient imaging of all the lymph nodes, and
mistakes in radiologic and pathologic evaluation (22,24). In
the case of non-diagnostic/inadequate cytology result, the biopsy
should be repeated, because the proportion of positive nodes in
these patients is extremely high (32).
It has been reported that the causes of false
positive results are mainly due to the insufficient sampling of
ALNs or to an inaccurate assessment of cell types obtained from the
puncture (22,33). The enlargement of reactive lymph node
is an important cause of clinically false positive assessment. To
distinguish between normal and abnormal ALNs, the size of lymph
nodes is not the standard to differentiate them. Fatty or reactive
lymph nodes can be mistaken for metastatic disease, because they
may be large enough in size (34). In
the present study, the US-guided FNAB did not produce false
positive results. Some studies showed that there were no false
positive results for the US-guided FNAB, while another of them
reported false positive results ranging from 1.4 to 1.6% (22,23,33,35).
We do not have enough evidence to suggest that the
node submitted to FNAB was exactly the lymph node which was
detected in subsequent axillary dissection. Abe et al
(36) used thick-needle biopsy to
detect axillary metastases. They reported that the positive lymph
node was the only sentinel node found after SLNB or ALND, in
>15% of positive US-guided core needle biopsy cases. This
indicated that the lymph node which underwent US-guided core needle
biopsy was the same node detected to be histologically positive on
the basis of axillary dissection. When performing FNAB, the largest
node which has the most pathological morphology is usually chosen
as the target. The closest lymph node from the breast is very
likely to be the sentinel node. It has been reported that the
sensitivity of the procedure would be increased, if the lymph node
closest to the breast was performed FNAB (36,37).
It is obvious that FNAB has more advantages than
SLNB in case of skipped metastasis. Previous study has shown that
metastatic disease may not first metastasize to level I nodes, but
skip to level II nodes directly (38). FNAB can be used not only for sentinel
lymph nodes, but for any lymph nodes which have sonographic
malignancy standard.
An advantage of the present study is its prospective
design with a consecutive series of patients treated at our
hospital. Another advantage is that 3 radiologists with 5–10 years
of work experience performed axillary US and FNAB. However, this is
a single-center study that may have a bias related to external
validity, which is a limitation of the present study. Another
limitation of the present study is that it is difficult for us to
perform a direct correlation between ALNs submitted to US-guided
FNAB and those obtained in axillary surgery.
In conclusion, axillary US can be complementary to
physical examination in axillary assessment. In addition, combining
axillary US and FNAB can detect axillary metastases timely and help
us plan our first surgery accordingly. Axillary US-alone or
combined US/FNAB had a high accuracy rate and a satisfactory result
because they cost less and are easy to assess the status of ALNs.
Thanks to the excellent PPV of US-guided FNAB, ALND can be
performed in patients with positive FNAB, which can avoid SLNB and
intraoperative frozen procedure. As a minimally invasive technique,
comprehensive clinical trials should be conducted to assess the
value of US-guided FNAB.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Guangxi
Scientific Research and Technology Development Project (grant no.
14124004-1-12), The National Natural Science Foundation of China
(grant no. 30960427) and The Natural Science Foundation of Guangxi
(grant no. 2013GXNSFAA019235).
Availability of data and materials
The dataset supporting the conclusions of the
present study is included in this article.
Authors' contributions
XH and JL conceived and designed the study. HY, WW,
and YJ performed the surgery. XH collected the data and wrote this
manuscript, which HY and YJ revised. XZ analyzed and interpreted
the data. HY, WW, YJ and JL reviewed the article for important
intellectual content. All authors read and approved the submitted
manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the Ethics
Committee for Clinical Research of The Affiliated Tumor Hospital of
Guangxi Medical University (Guangxi, China), and the study itself
was performed in accordance with the Declaration of Helsinki. All
patients provided written informed consent.
Consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Department of Breast Surgery, The Affiliated Tumor
Hospital of Guangxi Medical University, Nanning, Guangxi 530021,
China.
References
|
1
|
Cianfrocca M and Goldstein LJ: Prognostic
and predictive factors in early-stage breast cancer. Oncologist.
9:606–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher ER, Anderson S, Redmond C and
Fisher B: Pathologic findings from the national surgical adjuvant
breast project protocol B-06. 10-year pathologic and clinical
prognostic discriminants. Cancer. 71:2507–2514. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Swinson C, Ravichandran D, Nayagam M and
Allen S: Ultrasound and fine needle aspiration cytology of the
axilla in the pre-operative identification of axillary nodal
involvement in breast cancer. Eur J Surg Oncol. 35:1152–1157. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giuliano AE, Hunt KK, Ballman KV, Beitsch
PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM and
Morrow M: Axillary dissection vs no axillary dissection in women
with invasive breast cancer and sentinel node metastasis: A
randomized clinical trial. JAMA. 305:569–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boland MR, Prichard RS, Daskalova I,
Lowery AJ, Evoy D, Geraghty J, Rothwell J, Quinn CM, O'Doherty A
and McDermott EW: Axillary nodal burden in primary breast cancer
patients with positive pre-operative ultrasound guided fine needle
aspiration cytology: Management in the era of ACOSOG Z011. Eur J
Surg Oncol. 41:559–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moorman AM, Bourez RL, de Leeuw DM and
Kouwenhoven EA: Pre-operative ultrasonographic evaluation of
axillary lymph nodes in breast cancer patients: For which group
still of additional value and in which group cause for special
attention? Ultrasound Med Biol. 41:2842–2848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bedi DG, Krishnamurthy R, Krishnamurthy S,
Edeiken BS, Le-Petross H, Fornage BD, Bassett RL Jr and Hunt KK:
Cortical morphologic features of axillary lymph nodes as a
predictor of metastasis in breast cancer: In vitro sonographic
study. AJR Am J Roentgenol. 191:646–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abe H, Schmidt RA, Kulkarni K, Sennett CA,
Mueller JS and Newstead GM: Axillary lymph nodes suspicious for
breast cancer metastasis: Sampling with US-guided 14-gauge
core-needle biopsy-clinical experience in 100 patients. Radiology.
250:41–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boughey JC, Moriarty JP, Degnim AC, Gregg
MS, Egginton JS and Long KH: Cost modeling of preoperative axillary
ultrasound and fine-needle aspiration to guide surgery for invasive
breast cancer. Ann Surg Oncol. 17:953–958. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pessoa EC, Rodrigues JR, Pessoa CP,
Vespoli HM and Uemura G: Axillary lymph node aspiration guided by
ultrasound is effective as a method of predicting lymph node
involvement in patients with breast cancer? Rev Bras Ginecol
Obstet. 36:118–123. 2014.(In Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee MC, Eatrides J, Chau A, Han G, Kiluk
JV, Khakpour N, Cox CE, Carter WB and Laronga C: Consequences of
axillary ultrasound in patients with T2 or greater invasive breast
cancers. Ann Surg Oncol. 18:72–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SH, Kim MJ, Park BW, Moon HJ, Kwak JY
and Kim EK: Impact of preoperative ultrasonography and fine-needle
aspiration of axillary lymph nodes on surgical management of
primary breast cancer. Ann Surg Oncol. 18:738–744. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Baruah BP, Goyal A, Young P, Douglas-Jones
AG and Mansel RE: Axillary node staging by ultrasonography and
fine-needle aspiration cytology in patients with breast cancer. Br
J Surg. 97:680–683. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Roozendaal LM, Vane MLG, van Dalen T,
van der Hage JA, Strobbe LJA, Boersma LJ, Linn SC, Lobbes MBI,
Poortmans PMP, Tjan-Heijnen VCG, et al: Clinically node negative
breast cancer patients undergoing breast conserving therapy,
sentinel lymph node procedure versus follow-up: A Dutch randomized
controlled multicentre trial (BOOG 2013–08). BMC Cancer.
17:4592017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cyr AE, Tucker N, Ademuyiwa F,
Margenthaler JA, Aft RL, Eberlein TJ, Appleton CM, Zoberi I, Thomas
MA, Gao F and Gillanders WE: Successful completion of the pilot
phase of a randomized controlled trial comparing sentinel lymph
node biopsy to no further axillary staging in patients with
clinical T1-T2 N0 breast cancer and normal axillary ultrasound. J
Am Coll Surg. 223:399–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gentilini O and Veronesi U: Abandoning
sentinel lymph node biopsy in early breast cancer? A new trial in
progress at the European Institute of Oncology of Milan (SOUND:
Sentinel node vs. observation after axillary ultrasound. Breast.
21:678–681. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nenutil R: A revolution postponed
indefinitely. WHO classification of tumors of the breast 2012: The
main changes compared to the 3rd edition (2003). Cesk Patol.
51:23–25. 2015.PubMed/NCBI
|
|
18
|
Murray AD, Staff RT, Redpath TW, Gilbert
FJ, Ah-See AK, Brookes JA, Miller ID and Payne S: Dynamic contrast
enhanced MRI of the axilla in women with breast cancer: Comparison
with pathology of excised nodes. Br J Radiol. 75:220–228. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonnema J, van Geel AN, van Ooijen B, Mali
SP, Tjiam SL, Henzen-Logmans SC, Schmitz PI and Wiggers T:
Ultrasound-guided aspiration biopsy for detection of nonpalpable
axillary node metastases in breast cancer patients: New diagnostic
method. World J Surg. 21:270–274. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oruwari JU, Chung MA, Koelliker S,
Steinhoff MM and Cady B: Axillary staging using ultrasound-guided
fine needle aspiration biopsy in locally advanced breast cancer. Am
J Surg. 184:307–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang MC, Crystal P and Colgan TJ: The
evolving role of axillary lymph node fine-needle aspiration in the
management of carcinoma of the breast. Cancer Cytopathol.
119:328–334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuenen-Boumeester V, Menke-Pluymers M, de
Kanter AY, Obdeijn IM, Urich D and Van Der Kwast TH:
Ultrasound-guided fine needle aspiration cytology of axillary lymph
nodes in breast cancer patients. A preoperative staging procedure.
Eur J Cancer. 39:170–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ciatto S, Brancato B, Risso G, Ambrogetti
D, Bulgaresi P, Maddau C, Turco P and Houssami N: Accuracy of fine
needle aspiration cytology (FNAC) of axillary lymph nodes as a
triage test in breast cancer staging. Breast Cancer Res Treat.
103:85–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krishnamurthy S, Sneige N, Bedi DG,
Edieken BS, Fornage BD, Kuerer HM, Singletary SE and Hunt KK: Role
of ultrasound-guided fine-needle aspiration of indeterminate and
suspicious axillary lymph nodes in the initial staging of breast
carcinoma. Cancer. 95:982–988. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davis JT, Brill YM, Simmons S, Sachleben
BC, Cibull ML, McGrath P, Wright H, Romond E, Hester M, Moore A and
Samayoa LM: Ultrasound-guided fine-needle aspiration of clinically
negative lymph nodes vs. sentinel node mapping in patients at high
risk for axillary metastasis. Ann Surg Oncol. 13:1545–1552. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leenders MW, Broeders M, Croese C, Richir
MC, Go HL, Langenhorst BL, Meijer S and Schreurs WH: Ultrasound and
fine needle aspiration cytology of axillary lymph nodes in breast
cancer. To do or not to do? Breast. 21:578–583. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
García Fernández A, Fraile M, Giménez N,
Reñe A, Torras M, Canales L, Torres J, Barco I, González S, Veloso
E, et al: Use of axillary ultrasound, ultrasound-fine needle
aspiration biopsy and magnetic resonance imaging in the
preoperative triage of breast cancer patients considered for
sentinel node biopsy. Ultrasound Med Biol. 37:16–22. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Popli MB, Sahoo M, Mehrotra N, Choudhury
M, Kumar A, Pathania OP and Thomas S: Preoperative
ultrasound-guided fine-needle aspiration cytology for axillary
staging in breast carcinoma. Australas Radiol. 50:122–126. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tahir M, Osman KA, Shabbir J, Rogers C,
Suarez R, Reynolds T and Bucknall T: Preoperative axillary staging
in breast cancer-saving time and resources. Breast J. 14:369–371.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deurloo EE, Tanis PJ, Gilhuijs KG, Muller
SH, Kröger R, Peterse JL, Rutgers EJ, Olmos Valdés R and Kool
Schultze LJ: Reduction in the number of sentinel lymph node
procedures by preoperative ultrasonography of the axilla in breast
cancer. Eur J Cancer. 39:1068–1073. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gilissen F, Oostenbroek R, Storm R,
Westenend P and Plaisier P: Prevention of futile sentinel node
procedures in breast cancer: Ultrasonography of the axilla and
fine-needle aspiration cytology are obligatory. Eur J Surg Oncol.
34:497–500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
MacNeill M, Arnott I and Thomas J: Fine
needle aspiration cytology is a valuable adjunct to axillary
ultrasound in the preoperative staging of breast cancer. J Clin
Pathol. 64:42–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Rijk MC, Deurloo EE, Nieweg OE,
Gilhuijs KG, Peterse JL, Rutgers EJ, Kröger R and Kroon BB:
Ultrasonography and fine-needle aspiration cytology can spare
breast cancer patients unnecessary sentinel lymph node biopsy. Ann
Surg Oncol. 13:31–35. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mainiero MB: Regional lymph node staging
in breast cancer: The increasing role of imaging and
ultrasound-guided axillary lymph node fine needle aspiration.
Radiol Clin North Am. 48:989–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sapino A, Cassoni P, Zanon E, Fraire F,
Croce S, Coluccia C, Donadio M and Bussolati G:
Ultrasonographically-guided fine-needle aspiration of axillary
lymph nodes: Role in breast cancer management. Br J Cancer.
88:702–706. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abe H, Schmidt RA, Sennett CA, Shimauchi A
and Newstead GM: US-guided core needle biopsy of axillary lymph
nodes in patients with breast cancer: Why and how to do it.
Radiographics. 27 Suppl 1:S91–S99. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Koelliker SL, Chung MA, Mainiero MB,
Steinhoff MM and Cady B: Axillary lymph nodes: US-guided
fine-needle aspiration for initial staging of breast
cancer-correlation with primary tumor size. Radiology. 246:81–89.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pigott J, Nichols R, Maddox WA and Balch
CM: Metastases to the upper levels of the axillary nodes in
carcinoma of the breast and its implications for nodal sampling
procedures. Surg Gynecol Obstet. 158:255–259. 1984.PubMed/NCBI
|