Introduction
Nasopharyngeal carcinoma (NPC) is a pernicious
epithelial tumor originating from the nasopharynx, and is one of
the most common types of cancer in the southern region of the
People's Republic of China (1).
Although anticancer therapy has progressed substantially over the
last few years, the survival rate of individuals with NPC remains
unsatisfactory. Due to the complex etiology of NPC (2), the factors contributing to the
tumorigenesis and metastasis of NPC cells require elucidation in
order to benefit the identification of novel therapeutic targets
involved in the progression of NPC.
MicroRNAs (miRNAs) represent a type of
short-sequence RNA, which regulate gene or protein levels via
combining directly with the target mRNA (3). They are involved in the cell growth,
differentiation, apoptosis and metastasis of cells (4,5). Several
studies have identified that functional disorder of miRNAs is
involved in the abnormal growth of several types of malignant tumor
(6–8),
and microRNAs may serve as important regulators in NPC treatment
(9–11). As an important functional microRNA,
microRNA (miR)-371-5p exerts pro- and anti-tumor effects in
different types of tumor. For example, the upregulation of
miR-371-5p has been identified to inhibit the malignant behavior of
colorectal cancer via regulating superoxide dismutase 2 (12), however, others have demonstrated that
the overexpression of miR-371-5p enhances pancreatic tumor growth
via inhibitor of growth protein 1 (13) and human hepatocellular carcinoma cell
proliferation by inhibiting the expression of pre-mRNA processing
factor 4 homolog B (14). It is
possible that miR-371-5p has different roles in diverse tumor
cells. To date, the function of miR-371-5p in the development of
NPC remains to be fully elucidated.
The mechanism involved in the avoidance of apoptosis
is complex (15), and the
overexpression of genes and proteins, which inhibit apoptosis, may
be vital in this mechanism. B-cell lymphoma 2 (BCL2) acts as an
influential factor in favoring cell survival by inhibiting cell
apoptosis (16,17). The upregulation of BCL2 in malignant
tumors has been confirmed previously (18). The function of miR-371-5p in the
development of NPC via regulating the level of BCL2 was
investigated in the present study.
Materials and methods
Clinical tissues and cell culture
A total of 78 NPC samples and 30 nasopharyngeal
epithelium samples were obtained from the Department of
Otorhinolaryngology, Hezhou Renmin Hospital (Hezhou, China) from
March 2013 until November 2016. None of the patients had received
radiation therapy or chemotherapy prior to resection. Written
informed consent was obtained from the individuals with NPC. The
clinicopathological features of the patients are presented in
Table I. The Ethics Committee of
Hezhou Renmin Hospital ratified all written agreement according to
the Declaration of Helsinki (2004). The 5-8F cell line, 6-10B cell
line and nasopharyngeal-derived NP69 cell line were obtained from
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China) and were incubated in RPMI-1640 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% FBS, 100
U/ml streptomycin and 100 U/ml penicillin at 37°C in 5%
CO2.
 | Table I.Association between the
clinicopathological features of patients and level of miR-371-5p in
nasopharyngeal cancer. |
Table I.
Association between the
clinicopathological features of patients and level of miR-371-5p in
nasopharyngeal cancer.
|
|
| miR-371-5p
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Patients (n)
N=78 | High | Low | P-value |
|---|
| Age (years) |
|
|
|
|
| ≤40 | 43 | 24 | 19 | 0.255 |
|
>40 | 35 | 15 | 20 |
|
| Sex |
|
|
|
|
| Male | 40 | 19 | 21 | 0.453 |
|
Female | 38 | 20 | 18 |
|
| TNM stage |
|
|
|
|
| I+II | 23 | 18 | 5 | 0.001a |
|
III+IV | 55 | 21 | 34 |
|
| Distant
metastasis |
|
|
|
|
| No | 51 | 31 | 20 | 0.009a |
| Yes | 27 | 8 | 19 |
|
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
The RNA was extracted from the patient samples and
all cell lines using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RT-qPCR analysis was utilized to detect the
levels of miR-371-5p and the levels of U6 using the TaqMan reverse
transcription kit and TaqMan miRNA assay kit according to the
manufacturer's instructions. U6 was used as a control for
normalization; primers used are listed in Table II. A total of 1 µg RNA was
reverse-transcribed into cDNA. PCR was performed with the following
thermocycling conditions: Initial denaturation at 95°C for 5 min,
followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing at 60°C for 30 sec. The products were identified by
melting curve analysis. Fold changes were calculated through
relative quantification using the 2−ΔΔCq method
(19). Experiments were performed in
triplicate.
 | Table II.Primers used in the present study. |
Table II.
Primers used in the present study.
| Gene | Sequence (5′-3′) |
|---|
| miR-371-5p | RT:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGTGCC |
|
| Forward:
GTCGTATCCAGTGCAGCCG-CCACTCAAACTGTGGGG |
| U6 | RT:
GGGTCCGAGGTGCACTGGATACGACAAAAT-ATGG |
|
| Forward:
TGCGGGTGCTCGCTTCGGCAGC |
Cell transfection
The transfection of 5-8F cells with miR-371-5p
mimics, miR-control or miR-371-5p mimics/pcDNA3.1 vector encoding
BCL2 were performed using Lipofectamine 2000 (Thermo Fisher
Scientific, Inc.).
MTT assay
At 48 h post-transfection, the 5-8F cells were
washed with PBS, resuspended in serum-free medium and seeded into
96-well plates (5×104 cells/well). Following incubation
for 1, 2, 3 and 4 days at 37°C, 20 µl MTT (5 mg/ml) was added to
the culture for 4 h. Subsequently, the cells were treated with 150
µl dimethyl sulfoxide. Cell proliferation was determined by
calculating the relative optical densities at 570 nm for each
well.
Analysis of apoptosis
At 48 h post-transfection, the 5-8F cells were
washed, resuspended, and harvested. Subsequently, staining of the
cells was performed with propidium iodide and anti-Annexin V
antibody (BD Biosciences, Franklin Lakes, NJ, USA). Cell apoptosis
was identified using flow cytometry.
Transwell assay
A Transwell membrane coated with Matrigel (BD
Biosciences) was applied to measure cell invasion. At 48 h
post-transfection, 1×105 cells were added into the upper
compartment, and medium containing 20% FBS was added to the lower
compartment. After 48 h at 37°C, the cells on the lower side of the
membranes were fixed and stained with 0.5% crystal violet, and
counted under a light microscope (Olympus Corporation, Tokyo,
Japan).
Wound healing assays
Following transfection, 2×105 of the
transfected cells were seeded in 12-well plates (2×105
cells/well) and cultured at 37°C. On reaching 100% confluence, a
wound was created by scratching the cell surface with a pipette
tip, following by washing three times in medium and incubation in
RPMI-1640 with 10% FBS. The wounds were observed under a light
microscope at 0 and 48 h (Olympus Corporation). The cell migration
capability was determined by subtracting the final wound width from
the initial wound width.
Western blot analysis
At 48 h post-transfection, the cells were washed and
lysed with M-PER protein extraction reagent (Pierce; Thermo Fisher
Scientific, Inc.) Protease inhibitors were added to the lysates and
the cell lysate centrifuged for 15 min at 12,000 × g, and 4°C. A
bicinchoninic acid protein assay was performed to measure the
protein concentration. Proteins (80 µg/lane) were separated by 12%
SDS-PAGE and transferred to polyvinylidene fluoride membranes.
Membranes were blocked with 5% dry skim milk for 1 h at room
temperature and probed with the following primary antibodies
overnight at 4°C: BCL2 antibody (1:500; cat. no. 2872; Cell
Signaling Technology, Inc., Danvers, MA, USA), cleaved caspase-3
antibody (1:500; cat. no. ab32042; Abcam, Cambridge, UK), cleaved
caspase-9 antibody (1:500; cat. no. ab2324; Abcam), β-actin
antibody (1:1,000; cat. no. sc-47778; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). Horseradish peroxidase-conjugated secondary
antibodies (1:5,000; cat. nos. sc-2357 and sc-2005; Santa Cruz
Biotechnology, Inc.) were subsequently incubated with the membranes
at room temperature for 1 h. An ECL chemiluminescent kit (EMD
Millipore, Billerica, MA, USA) was used to detect the protein
bands. Band density was analyzed with FluorChem FC3 gel imaging
software (version 3.4; ProteinSimple; Bio-Techne, Minneapolis, MN,
USA).
Luciferase reporter assay
A luciferase reporter plasmid was established
through cloning the 3′-UTR of BCL2 or the mutated sequence into the
pMIR-Report construct (Ambion; Thermo Fisher Scientific, Inc.). The
miR-371-5p mimics, or the corresponding control were transfected
together with a reporter plasmid and pRL-SV40 (Promega Corporation,
Madison, WI, USA) into 5-8F cells. After 48 h, the luciferase
activity was measured using the Luciferase Reporter Assay system
(Promega Corporation) in accordance with the manufacturer's
protocol.
Nude mouse models
Female nude mice (n=6; 25–30 g; 6 week old) were
obtained from and housed in the Laboratory Animal Center of Guangxi
Medical University (Nanning, China). Nude mice were maintained in a
12 h light/dark cycle at 22–25°C with 50–60% humidity, with free
access to food and water in a specific pathogen-free laboratory
animal facility. Stably transfected 5-8F cells (1×107)
were subcutaneously injected into the flank of female nude mice.
The mice were sacrificed on day 5, 10, 15, 20 and 25, and the
weight and volume of the tumors were measured.
Statistical analysis
SPSS software 15.0 (SPSS, Inc., Chicago, IL, USA)
was used for statistical analyses. Student's t-test and one-way
analysis of variance were performed to analyze the difference
between groups and multiple comparisons. Pearson's χ2
test was performed to analyze the correlation between the level of
miR-371-5p and the protein level of BCL2. A Kaplan-Meier plot was
performed to analyze the overall survival of patients with NPC. All
data are expressed as he mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Downregulation of miR-371-5p in
nasopharyngeal carcinoma samples and cells
The level of miR-371-5p in nasopharyngeal carcinoma
samples was significantly reduced, compared with that in normal
tissue (P<0.001; Fig. 1A). The
level of miR-371-5p was significantly decreased in the two NPC cell
lines, compared with that in the NP69 cell line (5-8F, P<0.001;
6-10B, P<0.001; Fig. 1B).
Levels of miR-371-5p are associated
with an unfavorable prognosis in patients with NPC
According to the average level of miR-371-5p, all
cases were divided into a low level group (n=39) and a high level
group (n=39). Low expression levels of miR-371-5p were
significantly associated with tumor-node-metastasis phase (P=0.001;
Table I) and metastasis (P=0.009;
Table II). The patient survival
rates were significantly reduced in patients with NPC with a low
level of miR-371-5p (P<0.001; Fig.
1C). Pearson's correlation test was applied to detect the
association between the level of miR-371-5p and protein level of
Bcl-2 in NPC samples. An inverse association was confirmed between
the level of miR-219-5p and protein level of Bcl-2 in the NPC
tissues (Fig. 1D). The data showed
that miR-371-5p may exert an important function in NPC
tumorigenesis.
Restoration of miR-371-5p suppresses
the growth of 5-8F cells
The relative level of miR-371-5p was markedly
increased in the miR-371-5p mimics group, compared with that in the
miR-control group in 5-8F cells (Fig.
1E). The data also showed that the absorbance of 5-8F cells was
decreased in the miR-371-5p mimics group, compared with that in the
miR-control group (Fig. 1F).
Restoration of miR-371-5p promotes the
apoptosis of 5-8F cells
FACS was performed to quantify apoptosis. The 5-8F
cells were selected for experiments, and it was concluded that cell
apoptosis was promoted by the upregulation of miR-371-5p (Fig. 2A). The present study also detected the
levels of apoptosis-related proteins. The upregulation of
miR-371-5p decreased the level of BCL2, and increased the levels of
cleaved caspase-9 and cleaved caspase-3 (Fig. 2B). The restoration of BCL-2 partially
inhibited the cell apoptosis induced by miR-371-5p.
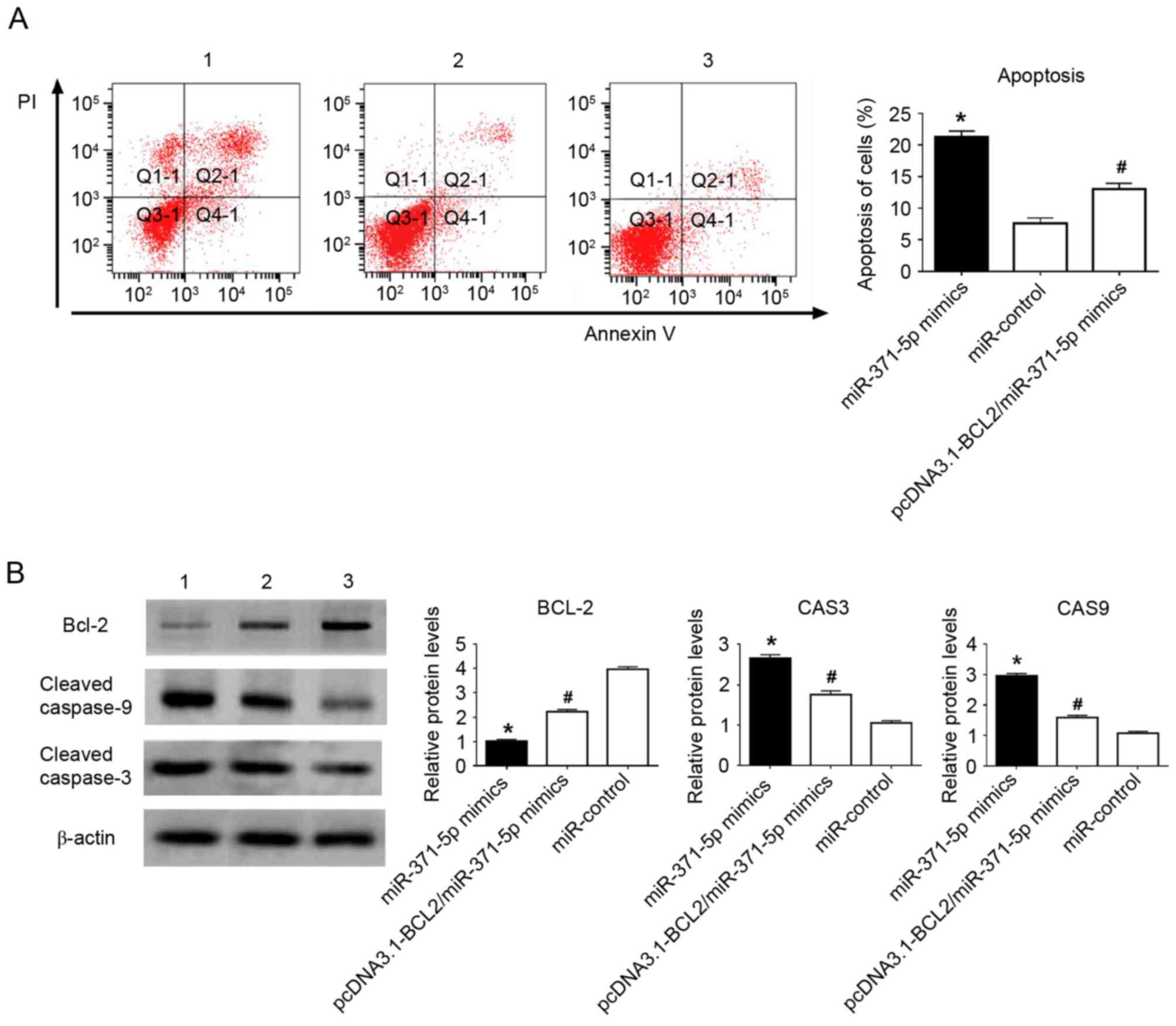 | Figure 2.Flow cytometric analyses and western
blot analysis of the effects of miR-371-5p on 5-8F cells. (A)
miR-371-5p promoted 5-8F cell apoptosis. (B) miR-371-5p decreased
the expression of BCL2, and increased the expression of cleaved
caspase 3 and cleaved caspase 9 in 5-8F cells. Restoration of BCL-2
partially reversed the pro-apoptotic function of miR-371-5p. 1,
miR-371-5p mimics; 2, miR-371-5p mimics/pcDNA3.1 vector containing
BCL2; 3, miR-control. *P<0.01, vs. miR-control;
#P<0.01, vs. miR-371-5p mimics. miR, microRNA; BCL2,
B-cell lymphoma 2; CAS3, caspase 3; CAS9, caspase 9; PI, propidium
iodide. |
Restoration of miR-371-5p inhibits
5-8F cell metastasis
Transwell assays and wound healing assays showed
that the upregulation of miR-371-5p alleviated the migratory and
invasive ability of the 5-8F cells. The cotransfection of
pcDNA3.1-BCL2/miR-371-5p mimics partially recovered the metastatic
function of 5-8F cells, compared with the miR-371-5p group
(Fig. 3A and B). The data confirmed
that the overexpression of miR-371-5p functioned in the inhibition
of tumor metastasis.
BCL2 mRNA is a binding target of
miR-371-5p
A luciferase reporter assay was utilized, as
described above. The results showed that, in the wild-type,
miR-371-5p inhibited luciferase reporter activity, compared with
that in the miR-control group. However, no significant differences
in activity were found between these two groups in the mutated type
(Fig. 4A and B).
miR-371-5p inhibits tumor growth in
animal experiments
The antitumor function of miR-371-5p was confirmed
via animal experiments in NPC (Fig. 4C
and D). The volume of tumors was reduced in the miR-371-5p
mimics group, compared with that in the miR-control group. The
final weight of tumors was decreased in the miR-371-5p mimics
group, compared with that in the miR-control group.
Discussion
As shown in previous studies, a number of genetic
factors affect the evolution and growth of NPC, and miRs are an
important factor (20). The BCL2
gene, an important anti-apoptotic gene, affects the intrinsic
apoptotic pathway (21,22). Bcl-2 can promote tumor invasion and it
has been shown to enhance tumor growth and metastasis (23). Inhibition of the expression of BCL2
has been considered as an important treatment strategy for several
types of cancer (24). However, the
specific effects of miR-371-5p via modulation of the expression of
BCL2 in the pathogenesis of NPC remain to be fully elucidated.
The present study revealed important novel evidence
that miR-371-5p has an important regulatory function on the
expression of BCL2 in NPC cells. Firstly, the level of miR-371-5p
was decreased in NPC patient samples and NPC cell lines. The
downregulation of miR-371-5p was associated with the unfavorable
clinicopathological features of patients. Secondly, a high
expression level of miR-371-5p inhibited the growth and metastasis
of 5-8F cells. The upregulation of BCL-2 partially reversed the
characteristics of 5-8F cells induced by miR-371-5p with respect to
proliferation and metastasis, as observed in previous studies
(25,26). Thirdly, the important function of
miR-371-5p on 5-8F cells via regulating the expression of BCL2 was
confirmed using the luciferase reporter assay system and western
blot analysis. Finally, these functions were identified using
animal experiments, revealing the potential function of miR-371-5p
on inhibiting NPC growth by targeting BCL2. miR-371-5p suppressed
the expression of BCL2 by binding to the targeting sequence of
BCL2. Cell apoptosis was significantly enhanced, and the growth and
metastasis of NPC were inhibited by the upregulation of
miR-371-5p.
In conclusion, the data obtained demonstrated that
miR-371-5p decreased the level of BCL2, inhibited the development
and metastasis of NPC, and provided a novel clue for understanding
the underlying function of miR-371-5p in the pathogenesis of NPC.
However, larger investigations assess the association between
miR-371-5p and BCL2 in different types of tumor are required to
further elucidate the precise molecular mechanisms.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Project of Guangxi Zhuang Autonomous Region (grant no.
14279006).
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
BD contributed to study design, data acquisition,
statistical analysis, data interpretation, manuscript preparation,
literature search and funding collection. FS contributed to data
acquisition, literature search and manuscript preparation. RX
contributed to data acquisition, statistical analysis and
manuscript preparation. WT contributed to study design, data
interpretation, manuscript preparation and funding collection.
Ethics approval and consent to
participate
The Ethics Committee of Hezhou Renmin Hospital
ratified all written agreement (approval no. 130201; Hezhou, China)
according to the Declaration of Helsinki (2004). All patients
expressed their full intentions to participate in the present study
and a written consent form was obtained from each patient.
Consent for publication
Patient's information, including names, initials,
date of birth or hospital numbers, images or statements have not
been included in the manuscript. Meanwhile, the patient, or parent,
guardian or next of kin have provided written informed consent for
the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou
ZX and Chen WQ: Nasopharyngeal carcinoma incidence and mortality in
China in 2010. Chin J Cancer. 33:381–387. 2014.PubMed/NCBI
|
|
2
|
Liu MT, Hsieh CY, Chang TH, Lin JP, Huang
CC and Wang AY: Prognostic factors affecting the outcome of
nasopharyngeal carcinoma. Jpn J Clin Oncol. 33:501–508. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang Z and Xi Y: MicroRNAs mediate
therapeutic and preventive effects of natural agents in breast
cancer. Chin J Nat Med. 14:881–887. 2016.PubMed/NCBI
|
|
4
|
Xing Z, Li D, Yang L, Xi Y and Su X:
MicroRNAs and anticancer drugs. Acta Biochim Biophys Sin
(Shanghai). 46:233–239. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin X, Chen J, Wu L and Liu Z: miR-30b-5p
acts as a tumor suppressor, repressing cell proliferation and cell
cycle in human hepatocellular carcinoma. Biomed Pharmacother.
89:742–750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang L, Wu L and Wu J: Downregulation of
miR-154 in human glioma and its clinicopathological and prognostic
significance. J Int Med Res. 44:994–1001. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian F, Chen J, Zheng S, Li D, Zhao X,
Jiang P, Li J and Wang S: miR-124 targets GATA6 to suppress
cholangiocarcinoma cell invasion and metastasis. BMC Cancer.
17:1752017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He B, Xu Z, Chen J, Zheng D, Li A and
Zhang LS: Upregulated microRNA-143 inhibits cell proliferation in
human nasopharyngeal carcinoma. Oncol Lett. 12:5023–5028. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao W, Lam JW, Li JZ, Chen SQ, Tsang RK,
Chan JY and Wong TS: MicroRNA-138-5p controls sensitivity of
nasopharyngeal carcinoma to radiation by targeting EIF4EBP1. Oncol
Rep. 37:913–920. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang T, Yin L, Wu J, Gu JJ, Wu JZ, Chen
D, Yu HL, Ding K, Zhang N, Du MY, et al: MicroRNA-19b-3p regulates
nasopharyngeal carcinoma radiosensitivity by targeting
TNFAIP3/NF-kB axis. J Exp Clin Cancer Res. 35:1882016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Y, Lv Z, He G, Wang J, Zhang X, Lu G,
Ren X, Wang F, Zhu X, Ding Y, et al: The SOX17/miR-371-5p/SOX2 axis
inhibits EMT, stem cell properties and metastasis in colorectal
cancer. Oncotarget. 6:9099–9112. 2015.PubMed/NCBI
|
|
13
|
He D, Miao H, Xu Y, Xiong L, Wang Y, Xiang
H, Zhang H and Zhang Z: miR-371-5p facilitates pancreatic cancer
cell proliferation and decreases patient survival. PloS One.
9:e1129302014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu RY, Diao CF, Zhang Y, Wu N, Wan HY,
Nong XY, Liu M and Tang H: miR-371-5p down-regulates pre mRNA
processing factor 4 homolog B (PRPF4B) and facilitates the G1/S
transition in human hepatocellular carcinoma cells. Cancer Lett.
335:351–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Igney FH and Krammer PH: Immune escape of
tumors: Apoptosis resistance and tumor counterattack. J Leukoc
Boil. 71:907–920. 2002.
|
|
16
|
Lessene G, Czabotar PE and Colman PM:
BCL-2 family antagonists for cancer therapy. Nat Rev Drug Discov.
7:989–1000. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bajwa N, Liao C and Nikolovska-Coleska Z:
Inhibitors of the anti-apoptotic Bcl-2 proteins: A patent review.
Expert Opin Ther Pat. 22:37–55. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fendri A, Kontos CK, Khabir A,
Mokdad-Gargouri R, Ardavanis A and Scorilas A: Quantitative
analysis of BCL2 mRNA expression in nasopharyngeal carcinoma: An
unfavorable and independent prognostic factor. Tumour Boil.
31:391–399. 2010. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gomes BC, Rueff J and Rodrigues AS:
MicroRNAs and cancer drug resistance. Methods Mol Biol.
1395:137–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cory S, Huang DC and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hockenbery D, Nunez G, Milliman C,
Schreiber RD and Korsmeyer SJ: Bcl-2 is an inner mitochondrial
membrane protein that blocks programmed cell death. Nature.
348:334–336. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi J, Choi K, Benveniste EN, Rho SB,
Hong YS, Lee JH, Kim J and Park K: Bcl-2 promotes invasion and lung
metastasis by inducing matrix metalloproteinase-2. Cancer Res.
65:5554–5560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheng H, Shao J, Morrow JD, Beauchamp RD
and DuBois RN: Modulation of apoptosis and Bcl-2 expression by
prostaglandin E2 in human colon cancer cells. Cancer Res.
58:362–366. 1998.PubMed/NCBI
|
|
25
|
He CY and Yang J: miR-187 induces
apoptosis of SiHa cervical carcinoma cells by downregulating Bcl-2.
Genet Mol Res. 16:gmr160189692017. View Article : Google Scholar
|
|
26
|
Ma Z, Luo Y and Qiu M: miR-143 Induces the
apoptosis of prostate cancer LNCap cells by suppressing Bcl-2
expression. Med Sci Monit. 23:359–65. 2017. View Article : Google Scholar : PubMed/NCBI
|


















