Introduction
Endometrial carcinoma (EC) is the most common
gynecological malignancy, and its prevalence is increasing annually
(1). EC is often diagnosed at an
early stage to the presence of various symptoms, including vaginal
bleeding, pain with urination, pain during sexual intercourse or
pelvic pain (2). However, a smaller
proportion of patients with EC are diagnosed at advanced stage
accompanied by malignant proliferation, extensive invasion, and
lymphatic metastasis or some patients develop disease recurrence
(3). The prognosis for advanced stage
or recurrent patients is still poor and the mortality is high
(4). Therefore, improved
understanding of the pathogenesis and identification of the
molecular alterations is urgent for the identification of novel
therapeutic targets that assist novel effective therapies for
endometrial cancer.
Enhancer of zeste homolog 2 (EZH2) is a member of
the polycomb group of genes (PcG) and serves as a histone
methyltransferase that mediates target genes silencing by
trimethylating the lysine 27 of histone H3 (5). EZH2 has been found to be associated with
multiple biological processes, including tumor proliferation, cell
cycle regulation, cell fate determination, cell differentiation,
senescence, metastasis and angiogenesis (6). Mounting evidence demonstrates that EZH2
is overexpressed or mutant in aggressive forms of prostate cancer
(7), breast cancer (8), bladder cancer (9), non-small cell lung carcinoma (10) and ovarian cancer (11) and is associated with poor prognosis in
multiple types of cancer.
EZH2 expression is associated with a high
proliferation rate and aggressive tumor subgroups of endometrial
cancer (12). EZH2 expression has
been demonstrated to be positively associated with the expression
of lipocalin 2 (13) and focal
adhesion kinase (FAK) (14), which is
associated with aggressive features of endometrial cancer. EZH2
expression is associated with tumor cell proliferation, migration,
and invasion in endometrial cancer cell lines as well as with
increased stage, grade, depth of invasion, and nodal metastasis in
human cancer tissue specimens, which is parallel to an increased
expression of Wnt pathway inhibitors and a concomitant decrease in
β-catenin levels (15).
Although EZH2 expression is associated with the
proliferation and aggressive feature of endometrial cancer
(12,15,16), its
function in EC is poorly elucidated, especially for the underlying
mechanism. In this article, we examined the potential role of EZH2
on EC cell line proliferation, apoptosis, and invasion.
Materials and methods
Cell culture
Human endometrial cancer, RL95-2, HEC1-A, Ishikawa
cells and the normal human endometrial cell line ESC, as negative
control cells, were purchased from American Type Culture Collection
(Manassas, VA, USA). HEC-1 and Ishikawa cells were maintained in
Mcoy's 5A media supplemented with 10% fetal bovine serum (FBS;
Hyclone, Logan, UT, USA); ESC cells were cultured in a phenol-free
DMEM-F12 1:1 mixture supplemented with 1% ITS liquid media
supplement (Sigma-Aldrich, St. Louis, MO, USA) + premix, and 10%
charcoal treated FBS; RL95-2 cells were grown in DMEM-F12medium
supplemented with 10% FBS and 0.005 mg/ml insulin (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). All cells were supplemented with
1% penicillin and streptomycin (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in humidified air at 37°C with 5% CO2.
All cell lines were tested and authenticated by DNA (short tandem
repeat genotyping) profiling before use.
Tissue samples
A total of 40 endometrial tissues (including 20
endometrial cancer samples and 20 adjacent normal endometrial
tissues as negative controls) were obtained from patients who
underwent surgery and for immunohistochemical analysis at the Sixth
People's Hospital of Shanghai Jiao Tong University. All specimens
were frozen immediately in liquid nitrogen and stored at −80°C
until RNA extraction. All specimens were reviewed by two
pathologists from the Department of Obstetrics and Gynecology, The
Sixth People's Hospital, Shanghai Jiao Tong University (Shanghai,
China). None of the patients had received preoperative
radiotherapy, chemotherapy, or hormonal therapy. All patients
provided written informed consent permitting the use of their
tissue for research. The present study has received Scientific and
Ethical Committee approval from the institutional review board of
the Sixth People's Hospital of Shanghai Jiao Tong University.
Quantitative reverse
transcription-polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from cancerous/noncancerous
specimens or cell lines with TRIzol reagents (Invitrogen; Thermo
Fisher Scientific, Inc.). Total RNA (1 µg) was reverse transcribed
into cDNA using the PrimeScript RT reagent kit (TaKaRa
Biotechnology, Shiga, Japan) under the following conditions; 37°C
for 15 min and 85°C for 5 sec. RT-qPCR was performed according to
the manufacturer's protocol of the SYBR Premix Ex Taq kit (Takara
Biotechnology Co., Ltd., Dalian, China) on an Mx3005P thermal
cycler (Stratagene; Agilent Technologies, Inc., Santa Clara, CA,
USA). The PCR conditions were 25–30 cycles at 95°C for 30 sec, 56°C
for 30 sec, and 72°C for 1 min. All reactions were performed in
triplicate. The 2−ΔΔCq method was used to calculate the
relative expression of each gene relative to the amount of GAPDH
(17). The PCR primers used are as
follows: EZH2 forward, 5′-TTGTTGGCGGAAGCGTGTAAAATC-3′ and reverse,
5′-TCCCTAGTCCCGCGCAATGAGC-3′; GAPDH forward,
5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′. All primers were obtained from
Invitrogen (Thermo Fisher Scientific, Inc).
Western blot analysis
Cells were lysed in RIPA buffer (150 mM NaCl, 50 mM
Tris-base, 5 mM EDTA, 1% NP-40, 0.25% deoxycholate, pH 7.4) with
protease and phosphatase inhibitors (Roche Applied Science,
Penzberg, Germany). Protein concentrations were measured using the
BCA protein assay (cat. no. 23227; Thermo Fisher Scientific,
Waltham, MA, USA). Equal amounts (30–50 µg) of the protein were
electrophoresed using SDS-PAGE (10% gel), transferred to
nitrocellulose membranes and incubated with the following primary
antibodies overnight at 4°C: Anti-EZH2 antibody, (cat. no. 5246S,
Cell Signaling Technology); anti-β-actin antibody, (Cell Signaling
Technology); rabbit polyclonal anti-E-cadherin (cat. no. A01589,
GenScript, Edison, NJ), mouse monoclonal anti-N-cadherin (BD,
Transduction, San Jose, CA), rabbit polyclonal anti-Vimentin (cat.
no. A01189, GenScript, Edison, NJ). Anti-Bax (cat. no. ab32503),
anti-Bcl-2 (cat. no. ab117115), anti-caspase-3 (cat. no. ab2302),
anti-caspase-9 (cat. no. ab52298) were purchased from Abcam. The
primary antibody incubation was followed by incubation with goat
anti-rabbit immunoglobulin (Ig)G-horseradish peroxidase (HRP; cat.
no. sc-2004; 1:2,000, dilution), goat anti-mouse IgG-HRP (cat. no.
sc-2005; 1:2,000, dilution) or rabbit anti-goat IgG-HRP (cat. no.
sc-2922; 1:2,000, dilution) from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA) for 1 h at room temperature. The bound antibodies
were detected using the Enhanced Chemiluminscence western blotting
substrate kit (cat. no. K820-50; BioVision, Inc., Milpitas, CA,
USA).
EZH2 small interfering (si)RNA
transfection
HEC-1A and RL95-2 cells were plated on 6-well plates
at a density of 2×105 cells/well and cultured in
corresponding mediums previously described overnight at 37°C until
30–40% confluency. The cells were transfected with three validated
siRNA for EZH2 (si-EZH2-1: 5′-CCTGGTCTGGCTTTATGCTAAGTTT-3′;
si-EZH2-2: 5′-TCGAGCTGCTCTGCTCTCTATTGAT-3′; si-EZH2-3:
5′-GCTCTCTATTGATTGTGTTTCTGGA-3′; si-Control:
5′-CCTGTCTTTCGGTATAATCGGGTTT-3′) and a scramble siRNA-FAM (negative
control; Invitrogen) at a concentration of 100 nM. Using
Lipofectamine 2000 transfection reagent (Invitrogen) according to
the manufacturer's protocol. The medium was replaced with standard
culture medium 6 h post-transfection. Transfection was repeated 72
h after the initial transfection.
In vitro cell proliferation assay
Cell proliferation was assayed using a cell
proliferation kit, Cell Counting kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kyushu, Japan) according to the manufacturer's
protocol. HEC-1A and RL95-2 cells were seeded into 96-well tissue
culture plates at a density of 2×103 cells/well the day
before EZH2 siRNA transfection. Cell proliferation was analyzed at
a wavelength of 450 nm at 24, 48, 72, 96 and 120 h after
transfection using Envision (PerkinElmer, Inc., Waltham, MA, USA).
Experiments were performed in triplicate. Cells transfected with
siRNA-FAM were used as a negative control.
Annexin V/propidium iodide (PI)
staining for detecting apoptotic cells
Cell apoptosis was determined by an Annexin V/PI
assay according to the protocol of the Annexin V-FITC apoptosis kit
(BD, Carlsbad, CA, USA). The Annexin V-FITC and PI fluorescence
levels were measured by flow cytometry (BD Biosciences, FACS
Calibur). The Annexin V-positive cells (PI-negative and -positive)
were defined as apoptotic cells. Cells transfected with siRNA-FAM
were used as a negative control.
Matrigel invasion assay
BD BioCoat™ Matrigel™ Invasion Chamber was used to
measure cell invasion according to the manufacturer's protocol.
Cells (1×105 cells/well) suspended in 0.5 ml medium were
added to the upper compartment of 24-well Matrigel-coated or
non-coated 8 micron membrane and medium supplemented with 10% FBS
was applied to the lower compartment. After incubation for 22 h at
37°C and 5% CO2, the cells were fixed with 4%
formaldehyde at 37°C for 15 min and stained with 1% crystal violet
in PBS at 37°C for 30 min. The number of cells that migrated across
the control membrane or invaded through the Matrigel-coated
membrane was determined in 9 fields across the center and the
periphery of the membrane. Cells transfected with siRNA-FAM were
used as negative controls.
Statistical analysis
All assays were conducted on triplicate samples in
triplicate. The data are presented as the mean ± standard
deviation. Statistical analyses were conducted using one-way
analysis of variance with Tukey's post-hoc testing or unpaired
student t-test using Prism 5.0 software (GraphPad Software, Inc.,
La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
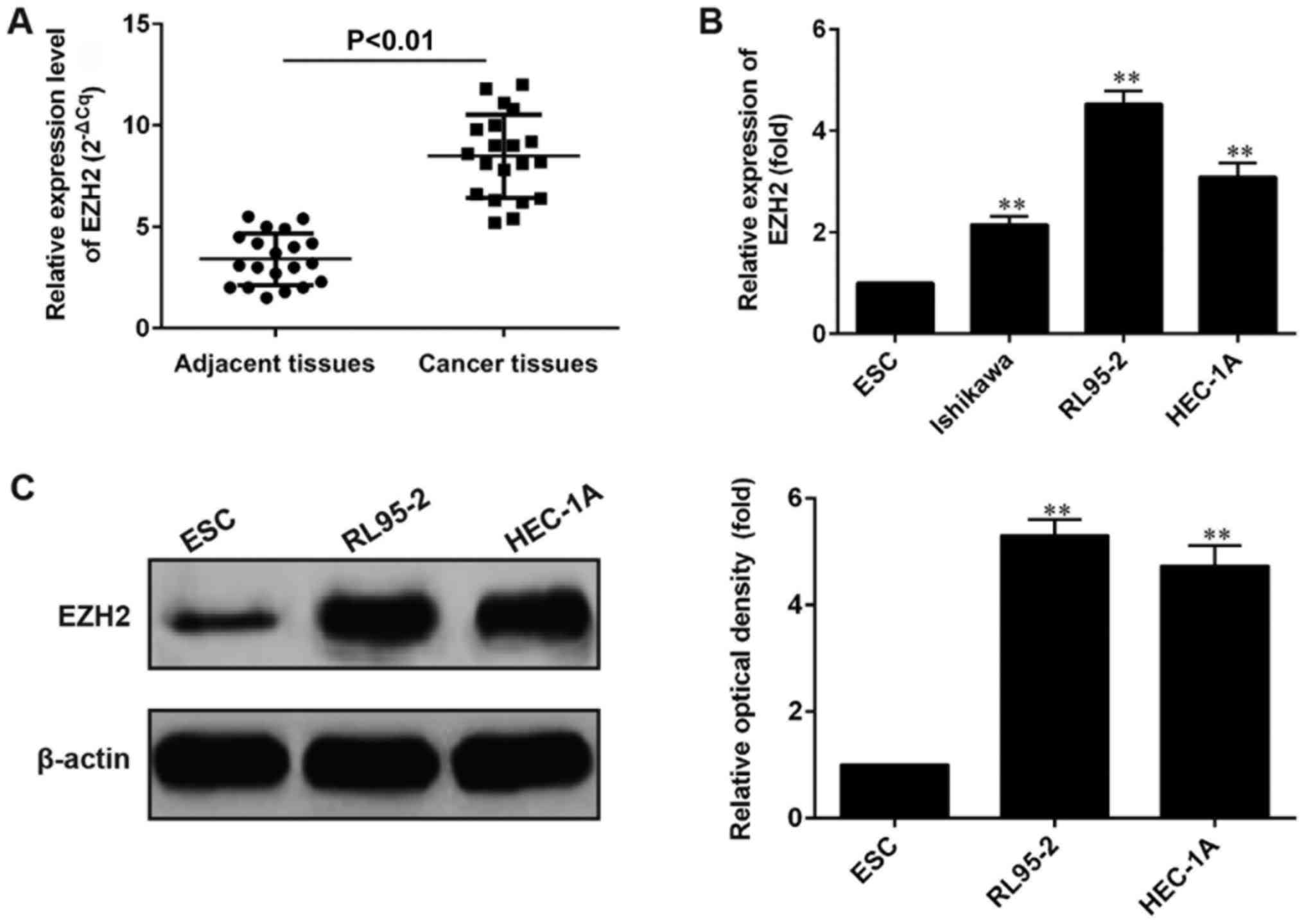
EZH2 is overexpressed in endometrial
cancer
The level of EZH2 was detected in 20 paired
endometrial cancer samples and adjacent histological normal tissues
by RT-qPCR and normalized to GAPDH. In cancerous tissues, EZH2
expression was at a level significantly increased compared with the
mean level of normal specimens (P<0.01; Fig. 1A). Expression of EZH2 was further
examined by RT-qPCR in 3 separate endometrial cancer cell lines
(Ishikawa, RL95-2 and HEC1-A) as well as the normal endometrial
cell line ESC. When compared with ESC cells, EZH2 was expressed at
increased levels (2–5 folds) in all cancer cell lines (Fig. 1B). We further confirmed the expression
of EZH2 by Western Blot in HEC-1A and RL95-2 cancer cells (Fig. 1C). The expression of EZH2 was also at
increased levels (4–6 folds), consistent with Fig. 1A and Fig.
1B.
EZH2 knockdown inhibits the growth of
human EC cells in vitro
There was a significant correlation between EZH2
expression and Ki67 expression (12,18).
Therefore, the present study sought to examine the effects of EZH2
knockdown on proliferation of human EC cells. To knockdown EZH2
expression in EC cell lines (HEC-1A and RL95-2), we developed 3
individual siEZH2. The knockdown efficacy of siEZH2 in EC cell
lines was confirmed by RT-qPCR (Fig.
2A). Compared with controls, EZH2 knockdown significantly
decreased cell proliferation in the 2, 3, 4, 5 days as in indicated
by CCK8 assays (Fig. 2B).
EZH2 knockdown increases the apoptosis
of human EC cells in vitro
The present study sought to determine the mechanisms
by which EZH2 knockdown inhibits the growth of human EC cells.
Annexin V positively stained cells determined by FACS showed that
there was statistical difference between HEC-1A and RL95-2 cells
transfected with siEZH2 and respective control (Fig. 3A). We next examined the markers of
apoptosis in HEC-1A and RL95-2 cells transfected with siEZH2
compared with respective control. When compared with controls,
markers of pro-apoptosis were significantly induced and
anti-apoptosis Bcl2 protein was significantly decreased by siEZH2
(Fig. 3B). Taken together, it was
concluded that EZH2 knockdown induces apoptosis of human EC
cells.
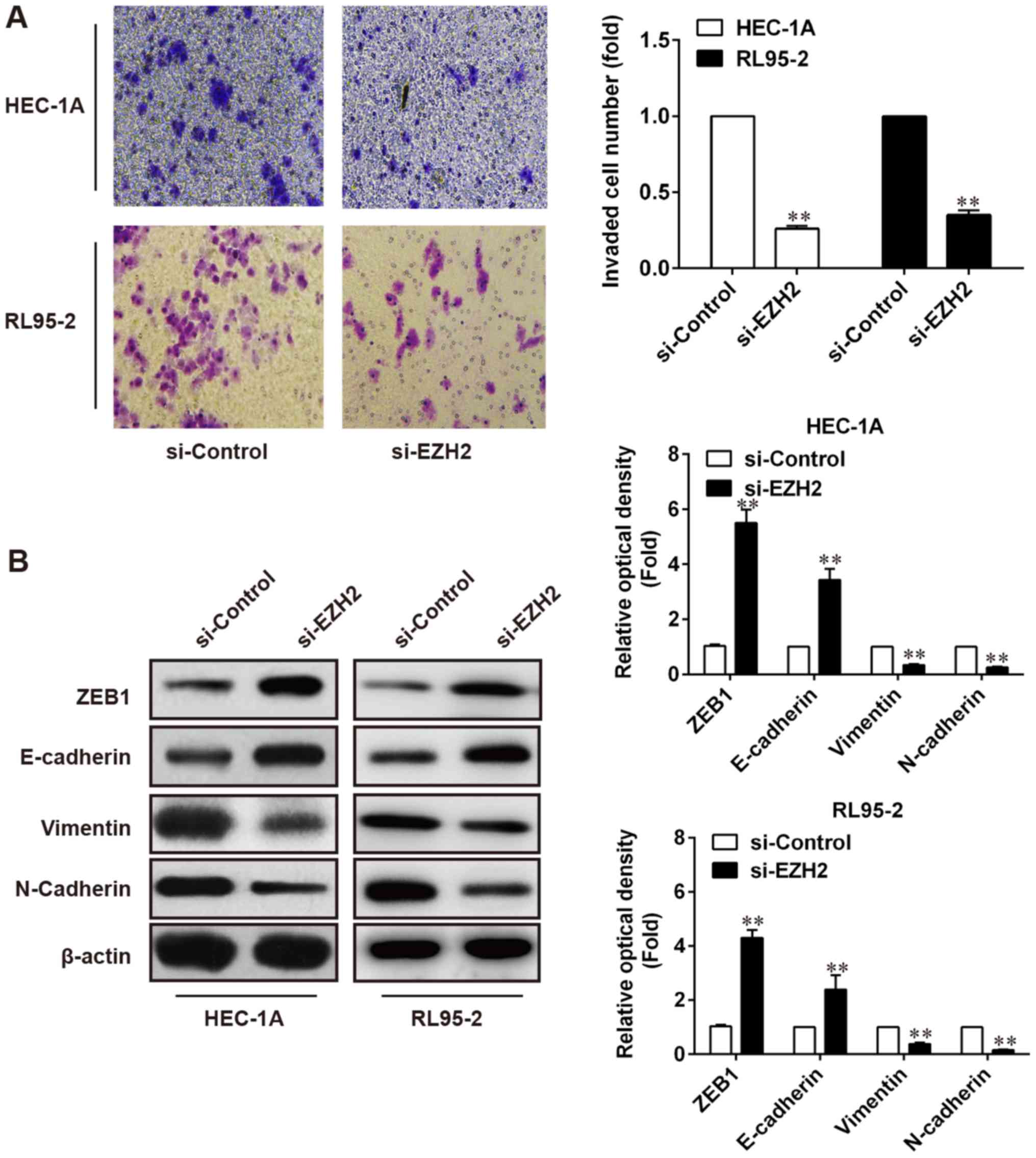
EZH2 knockdown inhibits the invasion
of endometrial cancer cells
EZH2 has been associated with regulating cell
invasion and migration in numerous cancer tissues and cell lines,
such as ovarian carcinoma cell (19),
glioblastoma cells (20), and bladder
cancer cells (21). Thus, we strived
to examine the effects of EZH2 knockdown on invasion of human EC
cells (HEC-1A and RL95-2). Towards this goal, HEC-1A and RL95-2
cells transfected with siEZH2 and respective control were tested
for their ability to migrate through uncoated control membrane or
invade through Matrigel-coated membrane. Compared with controls,
EZH2 knockdown significantly inhibited the invasion of HEC-1A and
RL95-2 cells as revealed by a decreased invasion index that is
calculated as the ratio between the number of cells invaded through
Matrigel-coated membrane and the number of cells migrated through
control membrane (Fig. 4A). The
epithelial-mesenchymal transition (EMT) has been implicated in cell
invasion in multiple types of cancer cell lines, including breast
cancer (22), colorectal cancer
(23), and gastric cancer (24). Therefore, the present study further
explored the association between the expression of EMT markers and
EZH2 in the endometrial cancer cells by western blot analysis and
RT-qPCR. As shown in Fig. 4B, EZH2
knockdown was associated with decreased vimentin and N-cadherin and
increased E-cadherin, suggesting EZH2 may influence the invasion of
EC cells through promoting EMT.
Discussion
As a transcriptional repressor, EZH2 plays a
critical role in the control of cell proliferation, determination
of stem cell fate and carcinogenesis (25). In addition, EZH2 has been identified
as a prognostic marker for breast, ovarian, colon, gastric, oral,
prostate cancer and melanoma (26).
The present study demonstrated upregulated EZH2 expression in EC
tissues and cell lines. The present study assessed the role of EZH2
in the biological behavior of EC through silencing EZH2. Taken
together, these data indicated a significant role for targeting
EZH2 in the influences of the development and progression in
EC.
Multiple genes and microRNA (miR) have been
associated with EZH2 inhibition-induced apoptosis (27,28). For
instance, EZH2 knockdown induces apoptosis through the
overexpression of FBX032 in breast cancer cells (29), abnormal expression of Bim and
proapoptotic miR-31 as well as miR205 in prostate cancer cells and
hepatocellular carcinoma (30–32). In
the present study, it was found that EZH2 knockdown significantly
increased the apoptosis in EC cells. The present study further
examined the mechanism of EZH2 inhibition induced apoptosis and
found markers of pro-apoptosis were significantly induced and
anti-apoptosis Bcl2 protein was significantly reduced by
siEZH2.
The tumor suppressor p53 and the transcriptional
repressor EZH2 have been associated with the regulation of
epithelial-mesenchymal transition (EMT) and tumor metastasis
(33). Inhibition of EZH2 expression
and upregulation of E-cadherin expression decrease the EMT process
in vitro and in vivo (34). The EZH2 polycomb group protein drives
an aggressive phenotype in melanoma cancer stem cells and is a
target of diet derived sulforaphane (35). EZH2 is associated with poor prognosis
in head-and-neck squamous cell carcinoma via regulating the
epithelial-to-mesenchymal transition (36). Consistently, the results of the
present study further confirmed the effect of EZH2 inhibition on
the EMT by upregulation of E-cadherin and downregulation of
N-cadherin as well as vimentin, with an associated decrease in
invasion.
There are limitations to the present study. First,
the regulation effect of silencing EZH2 in vivo should be
further explored. Second, the precise molecular mechanisms of EZH2
on apoptosis and EMT in EC have not been clearly clarified to date.
Finally, future studies are required to further elucidate the
comprehensive mechanisms for the influence of EZH2 in the
development of EC and it was considered important for the forming
of targeted treatment strategy in patients with EC.
To conclude, the data reported here revealed that
EZH2 is overexpressed in ECs and its overexpression is associated
with proliferation, apoptosis, invasion and EMT of EC cells in
vitro. A comprehensive study of epigenetic mechanisms and the
relevance of EZH2 in EC are important for fully understanding this
disease and as a basis for developing novel treatment options in
patients with EC.
References
|
1
|
Arnold M, Pandeya N, Byrnes G, Renehan
PAG, Stevens GA, Ezzati PM, Ferlay J, Miranda JJ, Romieu I, Dikshit
R, et al: Global burden of cancer attributable to high body-mass
index in 2012: A population-based study. Lancet Oncol. 16:36–46.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arenas M, Gascón M, Rovirosa À, Hernández
V, Riu F, López I, Montero A and Sabater S: The effect of
lymphadenectomy and radiotherapy on recurrence and survival in
endometrial carcinoma. Experience in a population reference centre.
Rep Pract Oncol Radiother. 20:50–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iavazzo C, Gkegkes ID and Vrachnis N:
Early recurrence of early stage endometrioid endometrial carcinoma:
Possible etiologic pathways and management options. Maturitas.
78:155–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoo KH and Hennighausen L: EZH2
methyltransferase and H3K27 methylation in breast cancer. Int J
Biol Sci. 8:59–65. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li LY: EZH2: Novel therapeutic target for
human cancer. Biomedicine (Taipei). 4:12014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis
RT, Wu X, Stack EC, Loda M, Liu T, et al: EZH2 oncogenic activity
in castration-resistant prostate cancer cells is
Polycomb-independent. Science. 338:1465–1469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Curry E, Green I, Chapman-Rothe N,
Shamsaei E, Kandil S, Cherblanc FL, Payne L, Bell E, Ganesh T,
Srimongkolpithak N, et al: Dual EZH2 and EHMT2 histone
methyltransferase inhibition increases biological efficacy in
breast cancer cells. Clin Epigenetics. 7:842015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang YB, Niu HT, Chang JW, Dong GL and Ma
XB: EZH2 silencing by RNA interference inhibits proliferation in
bladder cancer cell lines. Eur J Cancer Care (Engl). 20:106–112.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Behrens C, Solis LM, Lin H, Yuan P, Tang
X, Kadara H, Riquelme E, Galindo H, Moran CA, Kalhor N, et al: EZH2
protein expression associates with the early pathogenesis, tumor
progression, and prognosis of non-small cell lung carcinoma. Clin
Cancer Res. 19:6556–6565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H and Zhang R: Role of EZH2 in
epithelial ovarian cancer: From biological insights to therapeutic
target. Front Oncol. 3:472013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mannelqvist M, Stefansson IM, Wik E,
Kusonmano K, Raeder MB, Øyan AM, Kalland KH, Moses MA, Salvesen HB
and Akslen LA: Lipocalin 2 expression is associated with aggressive
features of endometrial cancer. BMC Cancer. 12:1692012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Roh JW, Bandyopadhyay S, Chen Z,
Munkarah AR, Hussein Y, Alosh B, Jazaerly T, Hayek K, Semaan A, et
al: Overexpression of enhancer of zeste homolog 2 (EZH2) and focal
adhesion kinase (FAK) in high grade endometrial carcinoma. Gynecol
Oncol. 128:344–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eskander RN, Ji T, Huynh B, Wardeh R,
Randall LM and Hoang B: Inhibition of enhancer of zeste homolog 2
(EZH2) expression is associated with decreased tumor cell
proliferation, migration, and invasion in endometrial cancer cell
lines. Int J Gynecol Cancer. 23:997–1005. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia N, Li Q, Tao X, Wang J, Hua K and Feng
W: Enhancer of zeste homolog 2 is involved in the proliferation of
endometrial carcinoma. Oncol Lett. 8:2049–2054. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Cai Q, Godwin AK and Zhang R:
Enhancer of zeste homolog 2 promotes the proliferation and invasion
of epithelial ovarian cancer cells. Mol Cancer Res. 8:1610–1618.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rao ZY, Cai MY, Yang GF, He LR, Mai SJ,
Hua WF, Liao YJ, Deng HX, Chen YC, Guan XY, et al: EZH2 supports
ovarian carcinoma cell invasion and/or metastasis via regulation of
TGF-beta1 and is a predictor of outcome in ovarian carcinoma
patients. Carcinogenesis. 31:1576–1583. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Smits M, Nilsson J, Mir SE, van der Stoop
PM, Hulleman E, Niers JM, de Witt Hamer PC, Marquez VE, Cloos J,
Krichevsky AM, et al: miR-101 is down-regulated in glioblastoma
resulting in EZH2-induced proliferation, migration, and
angiogenesis. Oncotarget. 1:710–720. 2010.PubMed/NCBI
|
|
21
|
Luo M, Li Z, Wang W, Zeng Y, Liu Z and Qiu
J: Long non-coding RNA H19 increases bladder cancer metastasis by
associating with EZH2 and inhibiting E-cadherin expression. Cancer
Lett. 333:213–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma L, Teruya-Feldstein J and Weinberg RA:
Tumour invasion and metastasis initiated by microRNA-10b in breast
cancer. Nature. 449:682–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Spaderna S, Schmalhofer O, Hlubek F, Berx
G, Eger A, Merkel S, Jung A, Kirchner T and Brabletz T: A
transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Z, Zhang X, Gang H, Li X, Li Z, Wang
T, Han J, Luo T, Wen F and Wu X: Up-regulation of gastric cancer
cell invasion by twist is accompanied by N-cadherin and fibronectin
expression. Biochem Biophys Res Commun. 358:925–930. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Simon JA and Lange CA: Roles of the EZH2
histone methyltransferase in cancer epigenetics. Mutat Res.
647:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Völkel P, Dupret B, Le Bourhis X and
Angrand PO: Diverse involvement of EZH2 in cancer epigenetics. Am J
Transl Res. 7:175–193. 2015.PubMed/NCBI
|
|
27
|
Zhang B, Liu XX, He JR, Zhou CX, Guo M, He
M, Li MF, Chen GQ and Zhao Q: Pathologically decreased miR-26a
antagonizes apoptosis and facilitates carcinogenesis by targeting
MTDH and EZH2 in breast cancer. Carcinogenesis. 32:2–9. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu ZL, Zheng SS, Li ZM, Qiao YY, Aau MY
and Yu Q: Polycomb protein EZH2 regulates E2F1-dependent apoptosis
through epigenetically modulating Bim expression. Cell Death
Differ. 17:801–810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Salazar H, Godwin AK, Daly MB, Laub PB,
Hogan WM, Rosenblum N, Boente MP, Lynch HT and Hamilton TC:
Microscopic benign and invasive malignant neoplasms and a
cancer-prone phenotype in prophylactic oophorectomies. J Natl
Cancer Inst. 88:1810–1820. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu ZL, Zheng SS, Li ZM, Qiao YY, Aau MY
and Yu Q: Polycomb protein EZH2 regulates E2F1-dependent apoptosis
through epigenetically modulating Bim expression. Cell Death
Differ. 17:801–810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Q, Padi SK, Tindall DJ and Guo B:
Polycomb protein EZH2 suppresses apoptosis by silencing the
proapoptotic miR-31. Cell Death Dis. 5:e14862014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang F, Lv LZ, Cai QC and Jiang Y:
Potential roles of EZH2, Bmi-1 and miR-203 in cell proliferation
and invasion in hepatocellular carcinoma cell line Hep3B. World J
Gastroenterol. 21:13268–13276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang FZ, He YY, Wang HH, Zhang HL, Zhang
J, Yan XF, Wang XJ, Che Q, Ke JQ, Chen Z, et al: Mutant p53 induces
EZH2 expression and promotes epithelial-mesenchymal transition by
disrupting p68-Drosha complex assembly and attenuating miR-26a
processing. Oncotarget. 6:44660–44674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma DN, Chai ZT, Zhu XD, Zhang N, Zhan DH,
Ye BG, Wang CH, Qin CD, Zhao YM, Zhu WP, et al: MicroRNA-26a
suppresses epithelial-mesenchymal transition in human
hepatocellular carcinoma by repressing enhancer of zeste homolog 2.
J Hematol Oncol. 9:12016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fisher ML, Adhikary G, Grun D, Kaetzel DM
and Eckert RL: The Ezh2 polycomb group protein drives an aggressive
phenotype in melanoma cancer stem cells and is a target of diet
derived sulforaphane. Mol Carcinog. 55:2024–2036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang JW, Gwak SY, Shim GA, Liu L, Lim YC,
Kim JM, Jung MG and Koo BS: EZH2 is associated with poor prognosis
in head-and-neck squamous cell carcinoma via regulating the
epithelial-to-mesenchymal transition and chemosensitivity. Oral
Oncol. 52:66–74. 2016. View Article : Google Scholar : PubMed/NCBI
|