Introduction
As one of the leading causes of cancer-associated
mortality, pancreatic carcinoma affects ~1/10,000 people and
results in ~200,000 mortalities each year worldwide (1). The prognosis of pancreatic carcinoma is
usually poor, and the mortality rate of this disease in China
within 5 years is >90% (2). It has
been demonstrated that the incidence of pancreatic carcinoma is
closely associated with chronic pancreatitis, smoking, aging, male
gender, obesity, diabetes mellitus and imbalanced diet among other
factors (3). Although various
treatment strategies have been developed to treat pancreatic
carcinoma, surgical resection remains the most effective approach
to treat this disease. However, surgical treatment following
diagnosis is only suitable in <20% of patients, and the overall
5-year survival rate of patients with pancreatic carcinoma remains
at <5% (4). Therefore, early
diagnosis and treatment are crucial for improving the survival of
patients affected by pancreatic carcinoma.
Long non-coding RNAs (lncRNA) is a group of
non-protein coding RNAs with a length of >200 nucleotides
(5). Numerous studies have indicated
that lncRNAs serve pivotal roles in the onset, development and
progression of a variety of human diseases, including different
types of cancer (6–8). The lncRNA HOXA distal transcript
antisense RNA (HOTTIP) has been proven to be able to participate in
the progression of pancreatic carcinoma through different pathways
(9,10). Although the direct involvement of
metabotropic glutamate receptor 1 (mGluR1) in pancreatic carcinoma
has not been reported thus far, the pivotal functions of glutamine
in the growth of pancreatic carcinoma indicate the possible
participation of mGluR1 in this disease (11). In an osteosarcoma study, Li et
al (12) observed that the lncRNA
HOTTIP, which is closely correlated with the poor prognosis of
patients with osteosarcoma, may potentially serve as a prognostic
marker of this disease. In view of these observations, it will be
reasonable to hypothesize that the lncRNA HOTTIP and mGluR1 may
also have prognostic values in pancreatic carcinoma.
In the present study, the expression levels of
HOTTIP and mGluR1 in pancreatic carcinoma and adjacent normal
healthy tissues were detected and compared, while the correlation
between these expression levels was analyzed. The prognostic value
of HOTTIP and mGluR1 in pancreatic carcinoma was also
discussed.
Materials and methods
Patients
A total of 211 patients with pancreatic carcinoma
admitted at the Daqing Longnan Hospital hospital (Daqing, China)
between January 2008 and January 2013 were selected. All patients
were diagnosed by histological evaluation according to the
‘guidelines for the diagnosis and treatment of pancreatic cancer’
established by the Chinese Medical Association, Branch of Pancreas
Surgery (2014 edition). Among the 211 patients, 40 patients were
subjected to surgery, whilst patients who were not appropriate for
surgical operation were exluced from the study. The 40 patients
received surgical operations included 19 males and 21 females, and
their ages ranged between 21 and 78 years with a mean age of 44±8.1
years. Distant metastasis was observed in 23 patients. A primary
tumor diameter of >2 cm was observed in 28 patients, while the
primary tumor diameter was <2 cm in 12 patients. Cancer tissues
and adjacent normal tissue at 2 cm around the tumor were collected
during the surgical procedures. The present study was approved by
the Ethics Committee of the Daqing Longnan Hospital, and all
patients signed informed consent. Patients were followed up for 8
months to monitor the survival conditions.
Cell lines and cell culture
The normal pancreatic cell line CRL-2279™ and
pancreatic cancer cell line CRL-2549™ were purchased from the
American Type Culture Collection (Manassas, VA, USA). CRL-2279™
cells were cultured with ATCC-formulated Dulbecco's modified
Eagle's medium (cat. no. 30-2002) containing 5% fetal bovine serum,
while CRL-2549™ cells were cultured with ATCC-formulated RPMI-1640
medium (cat. no. 30-2001) containing 10 U/ml human recombinant
insulin and 15% fetal bovine serum. The two cell lines were
cultured in an incubator at 37°C with 5% CO2. Cells were
harvest during the logarithmic growth phase for subsequent
experiments.
Establishment of HOTTIP overexpression
and silencing cell lines
HOTTIP small interfering RNA (siRNA; cat. no.
4390771; Thermo Fisher Scientific, Inc.) and Silencer™
Select Negative Control No. 1 siRNA (cat. no. 4390843; Thermo
Fisher Scientific, Inc.) were used for silencing of the HOTTIP
expression in cells. Due to the commerical nature of these
products, sequence information wasnot available. In addition, a
HOTTIP overexpression vector was established by inserting an
EcoRI-EcoRI fragment containing full length HOTTIP
into pIRSE2-EGFP (Clontech Laboratories, Inc., Palo Alto, CA, USA).
Prior to transfection, the pancreatic cancer CRL-2549™ cells were
cultured at 37°C overnight to reach 80–90% confluent. Lipofectamine
2000 transfection reagent (cat. no. 11668-019; Invitrogen; Thermo
Fisher Scientific, Inc.) was used for transfection with the siRNA
or overexpression vector according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cancer and adjacent normal tissues were ground in
liquid nitrogen, and total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Total RNA from in vitro cultured cells was also extracted
using TRIzol reagent. The concentration and quality of the RNA
samples were examined by a NanoDrop™ 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.), and only the RNA samples with a ratio of
A260/A280 between 1.8 and 2.0 were used. RT was then conducted with
SuperScript III Reverse Transcriptase (Thermo Fisher Scientific,
Inc.) to synthesize cDNA from total RNA. A qPCR reaction system was
subsequently prepared using SYBR® Green Real-Time PCR
Master Mix (Thermo Fisher Scientific, Inc.). The following primers
were used in the qPCR reactions: HOTTIP,
5′-AACGATGTGTGTGTGCCTTGAT-3′ (forward) and
5′-TGGTCCGACAGGGTGAATT-3′ (reverse); mGluR1,
5′-AGCGCCTCCAGTTGGCCT-3′ (forward) and 5′-TGCGTGCAATACGATTGGTT-3′
(reverse); β-actin, 5′-GACCTCTATGCCAACACAGT-3′ (forward) and
5′-AGTACTTGCGCTCAGGAGGA-3′ (reverse). The CFX96 Touch™ Real-Time
PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) was used to conduct the qPCR reactions under the following
conditions: 95°C for 50 sec, followed by 40 cycles of 95°C for 12
sec and 60°C for 35 sec. Cq values were processed using the
2−ΔΔCq method (13). The
relative expression level of each gene was normalized to that of
the endogenous control, β-actin.
Western blot analysis
Subsequent to total protein extraction using
Radioimmunoprecipitation Assay Lysis and Extraction Buffer (Thermo
Fisher Scientific, Inc.), the protein concentration was measured
using a bicinchoninic acid protein assay kit (catalog number,
23225; Thermo Fisher Scientific, Inc.) Protein samples (30 µg) were
then subjected to 15% SDS-PAGE gel electrophoresis, followed by
transfer to a polyvinylidene difluoride membrane. Following
blocking with 5% skimmed milk at room temperature for 1.5 h, the
membranes were washed with Tris-buffered saline/Tween 20 (TBST) and
incubated with primary antibodies, including the rabbit anti-mGluR1
(1:2,000; ab82211; Abcam, Cambridge, MA, USA) and rabbit
anti-β-actin (1:1,000; ab8226; Abcam) antibodies, overnight at 4°C.
Membranes were then washed with TBST and incubated with anti-rabbit
horseradish peroxidase-conjugated IgG secondary antibody (1:1,000;
MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room
temperature for 1 h. Subsequent to washing with TBST, an enhanced
chemiluminescence detection reagent (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to detect the signals, and signals
were scanned using MyECL imager (Thermo Fisher Scientific). ImageJ
software (version 1.8.0; National Institutes of Health, Bethesda,
MD, USA) was then used to calculate the relative expression level
of mGluR1 according to that of the endogenous control, β-actin.
Cell migration and invasion assay
A Transwell cell migration assay (BD Biosciences,
San Jose, CA, USA) was performed in order to examine the migration
of cells. Briefly, cells were transferred to the upper chamber of
6-well plate at 5×104 cells per well, while
ATCC-formulated RPMI-1640 medium containing 20% fetal bovine serum
(Sigma-Aldrich; Merck KGaA) was used to fill the lower chamber.
After 24 h, membranes were collected and stained with 0.5% crystal
violet (Sigma-Aldrich; Merck KGaA) for 25 min. The number of
stained cells was calculated under an optical microscope (Olympus
Corp., Tokyo, Japan). An invasion assay was also performed using
the same method, with the exception that the upper chamber was
pre-coated with Matrigel (cat. no. 356234; EMD Millipore,
Billerica, MA, USA).
Statistical analysis
SPSS version 19.0 software (IBM Corp., Armonk, NY,
USA) was used for all statistical analyses. Data are expressed as
the mean ± standard deviation. Comparison of data between two
groups was performed using Student's t-test. The Kaplan-Meier
method was used to draw the survival curves, followed by comparison
of survival curves using the log-rank test. The correlation between
the expression levels of HOTTIP and mGluR1 was analyzed by Spearman
analyses. P<0.05 was considered to indicate a difference that
was statistically significant.
Results
Expression of HOTTIP and mGluR1 in
cancer and adjacent healthy tissues
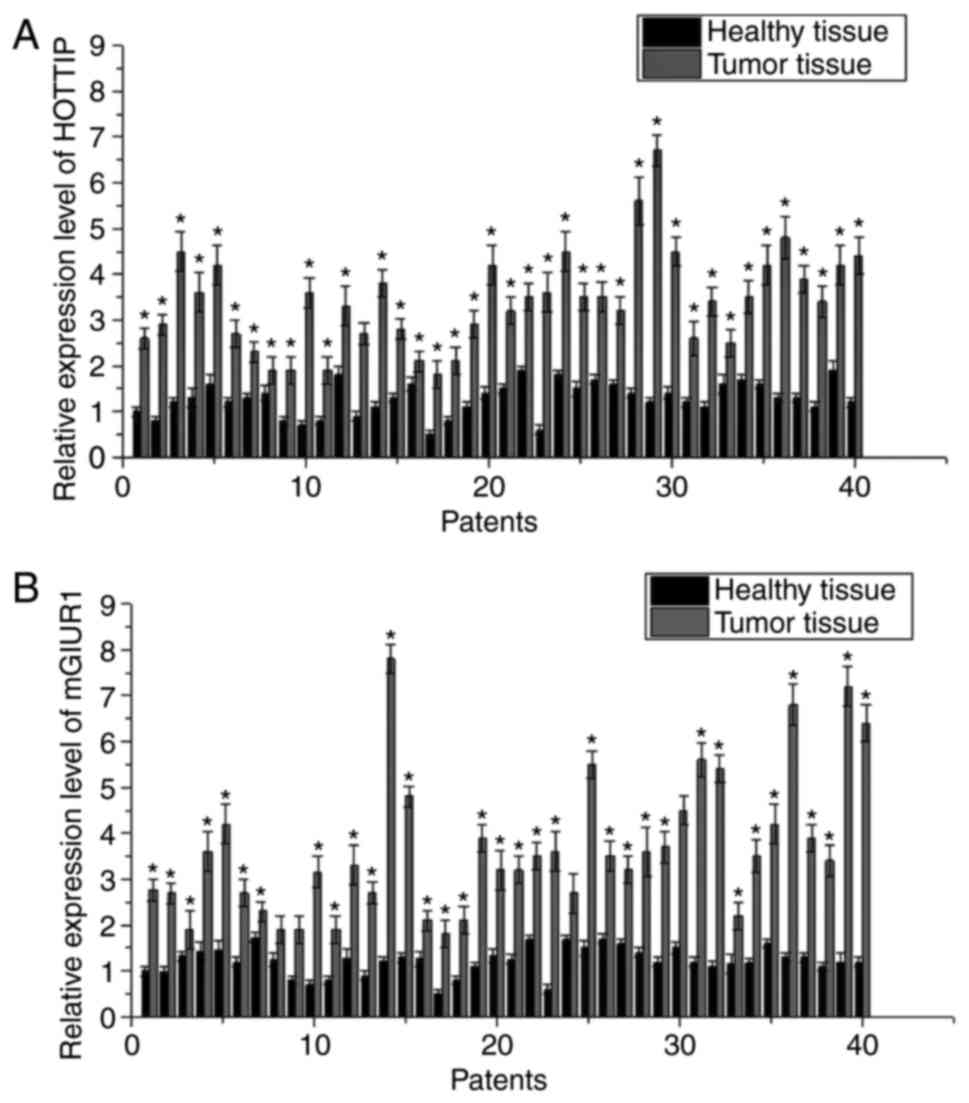
The expression levels of HOTTIP and mGluR1 mRNA in
cancer and adjacent healthy tissues of 40 patients with pancreatic
carcinoma were detected by RT-qPCR. As shown in Fig. 1, the expression levels of HOTTIP and
mGluR1 mRNA were significantly higher in cancer tissues as compared
with those in adjacent healthy tissues, indicating the possible
involvement of HOTTIP and mGluR1 in the development of pancreatic
carcinoma. Furthermore, Spearman analyses demonstrated that the
expression of HOTTIP mRNA in cancer tissues was positively
correlated with the expression of mGluR1 (r=0.59789; P<0.01;
data not shown).
Correlation of the expression levels
of HOTTIP and mGluR1 with the clinicopathological features and
prognostic values
Using the median HOTTIP and mGluR1 mRNA expression
levels in cancer tissues as the cutoff value, the 40 patients with
pancreatic carcinoma were divided into the high (n=20) and low
(n=20) HOTTIP expression groups, as well as the high (n=20) and low
(n=20) mGluR1 expression groups. As shown in Table I, the mRNA expression levels of HOTTIP
and mGluR1 were significantly positively correlated with the tumor
size (P=0.01 and P=0.02, respectively) and distant metastasis
(P=0.01 and P=0.000, respectively), but were not correlated with
the gender (P=0.73 and P=0.94, respectively) and age of patients
(P=0.73 and P=0.59, respectively). Furthermore, survival curves
were calculated using Kaplan-Meier plots to evaluate the prognostic
value of HOTTIP and mGluR1 mRNA in pancreatic carcinoma, and these
curves were compared by log-rank test. The result revealed that the
overall survival of pancreatic carcinoma patients with a high
expression level of HOTTIP or mGluR1 mRNA was significantly reduced
in comparison with that of patients with a low expression level of
HOTTIP or mGluR1 mRNA (P=0.01 and P=0.00, respectively; Fig. 2).
 | Table I.Correlation of the expression levels
of HOTTIP and mGluR1 with the clinicopathological features of
patients. |
Table I.
Correlation of the expression levels
of HOTTIP and mGluR1 with the clinicopathological features of
patients.
|
|
|
| HOTTIP
expression |
| mGluR1
expression |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Feature | Groups | Total, n | High, n | Low, n | P-value | High | Low | P-value |
|---|
| Gender | Male | 19 | 9 | 10 | 0.78276 | 11 | 8 | 0.937030 |
|
| Female | 21 | 12 | 9 |
| 13 | 8 |
|
| Age (years) | >35 | 27 | 12 | 15 | 0.73144 | 10 | 17 | 0.593478 |
|
| ≤35 | 13 | 5 | 8 |
| 6 | 7 |
|
| Tumor size (cm) | >2 | 28 | 22 | 6 | <0.01 | 19 | 9 | 0.024032 |
|
| ≤2 | 12 | 3 | 9 |
| 4 | 8 |
|
| Distant
metastasis | Yes | 23 | 19 | 4 | <0.01 | 17 | 6 | 0.004277 |
|
| No | 17 | 4 | 13 |
| 5 | 12 |
|
Expression levels of HOTTIP and mGluR1
in normal pancreatic and pancreatic cancer cell lines
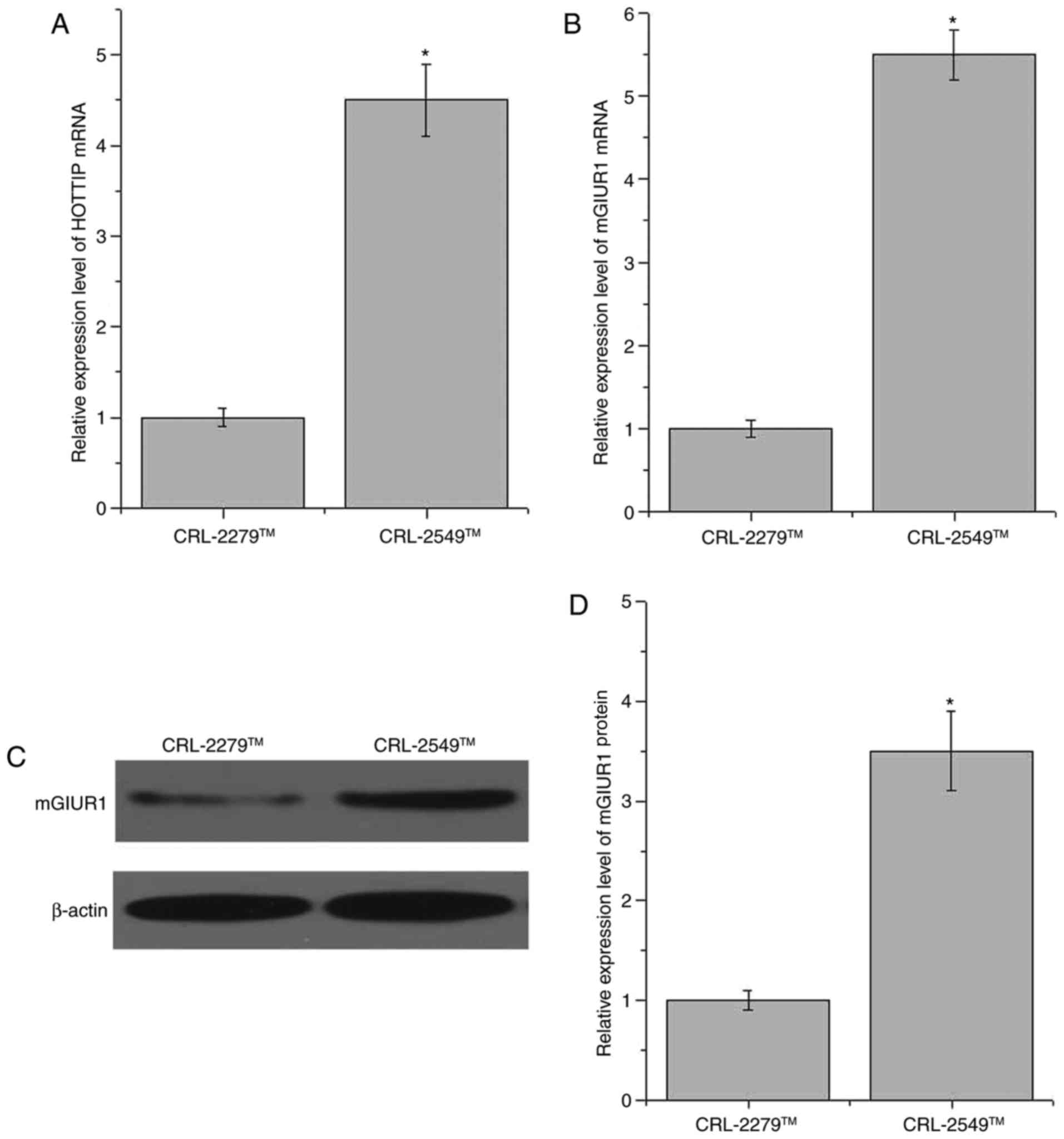
The expression levels of HOTTIP and mGluR1 in the
normal pancreatic cell line CRL-2279™ and the pancreatic cancer
cell line CRL-2549™ were detected by RT-qPCR and western blot
analysis. As shown in Fig. 3A, the
expression of HOTTIP was significantly increased in the CRL-2549™
cell line as compared with that in CRL-2279™ cells (P=0.02). In
addition, compared with the normal pancreatic CRL-2279™ cell line,
the expression of mGluR1 was significantly increased in the
pancreatic cancer CRL-2549™ cell line at the mRNA and protein
levels (P<0.05; Fig. 3B-D).
Effect of HOTTIP knockdown and
overexpression on the expression of mGluR1
CRL-2549™ cell lines with HOTTIP siRNA silencing or
overexpression were constructed to investigate the effects of
HOTTIP on the expression of mGluR1. As shown in Fig. 4A, the expression of HOTTIP was
significantly decreased in siRNA-treated cells and significantly
increased in the HOTTIP overexpression cells (P<0.05). However,
no significant differences were identified between the control cell
line and the siRNA negative control or overexpression negative
control cells. These observations indicated that the HOTTIP
knockdown and overexpression CRL-2549™ cells were successfully
established. As shown in Fig. 4B,
compared with the control cell line, the expression of mGluR1 mRNA
was significantly reduced in HOTTIP knockdown cells and
significantly increased in HOTTIP overexpression cells (P<0.05).
In addition, as shown in Fig. 4C and
D, the expression of mGluR1 protein was also significantly
reduced in HOTTIP knockdown cells and significantly increased in
HOTTIP overexpression cells (P<0.05). These results suggested
that the expression of mGluR1 was positively regulated by HOTTIP at
the mRNA and protein levels.
Effect of HOTTIP knockdown and
overexpression on pancreatic cancer cell migration and
invasion
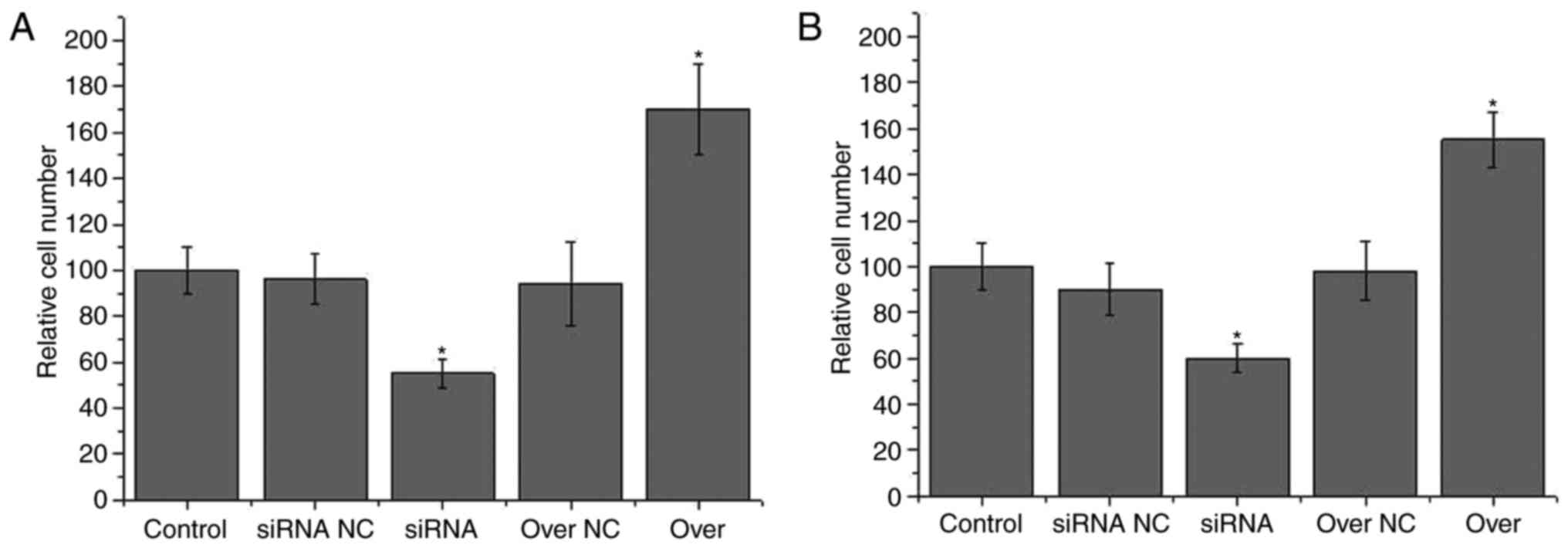
As shown in Fig. 5A,
the migration ability of HOTTIP knockdown pancreatic cancer
CRL-2549™ cells was significantly lower in comparison with that of
control CRL-2549™ cells (P<0.05). In addition, the migration
ability of the HOTTIP overexpression pancreatic cancer CRL-2549™
cells was significantly higher compared with that of control
CRL-2549™ cells (P<0.05). However, no significant differences in
migration ability were detected between the control and two
negative control groups. Similar results were observed for the
invasion ability of cells. A significantly lower invasion ability
was identified in the HOTTIP knockdown CRL-2549™ cells, while a
significantly higher invasion ability was observed in the HOTTIP
overexpression CRL-2549™ cells, as compared with the control cell
line (P<0.05). These data suggested that HOTTIP expression was
positively correlated with the migration and invasion abilities of
pancreatic cancer cells.
Discussion
Despite the advances in understanding the underlying
mechanism and improving the diagnosis and treatment of pancreatic
carcinoma, this disease remains one of the malignant tumors with
the highest mortality, severely affecting patient survival
(4). The lncRNA HOTTIP has been
proven to be involved in the progression of various types of human
cancer. In a gastric cancer study, Ye et al (14) reported that the expression of the
lncRNA HOTTIP was positively correlated with the invasion ability
of tumor cells, while increased expression of HOTTIP contributed to
the poor prognosis of patients. Furthermore, the expression level
of HOTTIP was frequently upregulated in hepatocellular carcinoma
tissues (15), and increased
expression of this lncRNA was observed to be closely associated
with the progression of hepatocellular carcinoma and the treatment
outcomes of patients affected by this disease (16). A recent study reported that HOTTIP was
also able to promote the proliferation, migration and survival of
pancreatic carcinoma cells (10).
Consistent with this previous study, the expression level of HOTTIP
was reported to be significantly increased in pancreatic carcinoma
tissues as compared with adjacent healthy tissues in the present
study. In addition, HOTTIP was proven to be positively correlated
with the migration and invasion ability of in vitro cultured
pancreatic carcinoma cells. Therefore, the current study further
confirmed the involvement of HOTTIP in pancreatic carcinoma.
The expression of mGluRs is mainly detected in the
central nervous system, with these receptors mediating
neurotransmitter release and neuronal excitability (17). Subtypes of mGluRs are typically
selectively expressed in tissues of different cancer types,
indicating the involvement of mGluRs in tumorigenesis (18). As a member of the mGluR family, the
functionality of mGluR1 has been well studied in breast cancer.
During the development of breast cancer, mGluR1 has been
demonstrated to promote angiogenesis, which in turn contributes to
the progression of the tumor (19).
Another study conducted by Speyer et al (20) reported that mGluR1 may serve as a
potential target for the treatment of patients with breast cancer.
As a receptor of glutamate, the functionality of mGluR in the
development of pancreatic carcinoma remains known. However, the
functions of glutamine in the growth of pancreatic carcinoma
indicate the involvement of mGluR1 in this disease (11). In the present study, the expression
level of mGluR1 was observed to be significantly higher in
pancreatic carcinoma tissues and in vitro cultured
pancreatic carcinoma cells as compared with that in the
corresponding normal controls, indicating the participation of
mGluR1 in this disease.
The present study also demonstrated that the
expression level of mGluR1 was positively correlated with the
expression level of the lncRNA HOTTIP. In addition, HOTTIP
knockdown was able to downregulate the expression of mGluR1,
whereas the expression of mGluR1 was significantly upregulated by
HOTTIP overexpression. To the best of our knowledge, no studies on
the regulation of mGluR expression by lncRNA HOTTIP have been
reported. However, glutamine metabolism, which is important for the
development of various types of tumors, such as bladder cancer, has
been proven to be regulated by lncRNAs (21), indicating the possible interactions
between lncRNAs and mGluRs. Furthermore, the results of the present
study revealed that increased expression level of HOTTIP and mGluR1
were significantly correlated with the tumor size and distant
metastasis, indicating that HOTTIP and mGluR1 may potentially serve
as prognostic markers for pancreatic carcinoma.
In conclusion, the expression levels of HOTTIP and
mGluR1 were significantly higher in pancreatic carcinoma tissues
and cells as compared with the corresponding controls, while HOTTIP
was able to positively regulate the expression of mGluR1. In
addition, increased expression levels of HOTTIP and mGluR1 were
significantly correlated with the tumor size and distant
metastasis. These data suggested that HOTTIP and mGluR1 may
potentially serve as prognostic markers in pancreatic carcinoma
patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XW, LX and HY designed experiments, XW and LX
performed experiments, HY analyzed data and wrote the manuscript.
All authors read the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Daqing Longnan Hospital. All participants provided written
informed consent.
Consent for publication
All participants provided written informed consent
for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zuo T, Zeng H, Zhang S
and He J: National cancer incidence and mortality in China, 2012.
Chin J Cancer Res. 28:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma C, Eltawil KM, Renfrew PD, Walsh MJ
and Molinari M: Advances in diagnosis, treatment and palliation of
pancreatic carcinoma: 1990–2010. World J Gastroenterol. 17:867–897.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perkel JM: Visiting ‘noncodarnia’.
Biotechniques. 54(301): 303–304. 2013.
|
|
6
|
Wang K, Liu F, Zhou LY, Long B, Yuan SM,
Wang Y, Liu CY, Sun T, Zhang XJ and Li PF: The long noncoding RNA
CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res.
114:1377–1388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi K, Yan I, Haga H and Patel T:
Long noncoding RNA in liver diseases. Hepatology. 60:744–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Zhao X, Zhou Y, Liu Y, Zhou Q, Ye H,
Wang Y, Zeng J, Song Y, Gao W, et al: The long non-coding RNA
HOTTIP promotes progression and gemcitabine resistance by
regulating HOXA13 in pancreatic cancer. J Transl Med. 13:842015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng Y, Jutooru I, Chadalapaka G, Corton
JC and Safe S: The long non-coding RNA HOTTIP enhances pancreatic
cancer cell proliferation, survival and migration. Oncotarget.
6:10840–10852. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Son J, Lyssiotis CA, Ying H, Wang X, Hua
S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, et
al: Glutamine supports pancreatic cancer growth through a
KRAS-regulated metabolic pathway. Nature. 496:101–105. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li F, Cao L, Hang D, Wang F and Wang Q:
Long non-coding RNA HOTTIP is up-regulated and associated with poor
prognosis in patients with osteosarcoma. Int J Clin Exp Pathol.
8:11414–11420. 2015.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye H, Liu K and Qian K: Overexpression of
long noncoding RNA HOTTIP promotes tumor invasion and predicts poor
prognosis in gastric cancer. Onco Targets Ther. 9:2081–2088.
2016.PubMed/NCBI
|
|
15
|
Tsang FHC, Au SLK, Wei L, Fan DN, Lee JM,
Wong CC, Ng IO and Wong CM: Long non-coding RNA HOTTIP is
frequently up-regulated in hepatocellular carcinoma and is targeted
by tumour suppressive miR-125b. Liver Int. 35:1597–1606. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quagliata L, Matter MS, Piscuoglio S,
Arabi L, Ruiz C, Procino A, Kovac M, Moretti F, Makowska Z,
Boldanova T, et al: Long noncoding RNA HOTTIP/HOXA13 expression is
associated with disease progression and predicts outcome in
hepatocellular carcinoma patients. Hepatology. 59:911–923. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Cilz NI, Yang C, Hu B, Dong H and
Lei S: Depression of neuronal excitability and epileptic activities
by group II metabotropic glutamate receptors in the medial
entorhinal cortex. Hippocampus. 25:1299–1313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu LJ, Wall BA, Wangari-Talbot J and Chen
S: Metabotropic glutamate receptors in cancer. Neuropharmacology.
115:193–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Speyer CL, Hachem AH, Assi AA, Johnson JS,
DeVries JA and Gorski DH: Metabotropic glutamate receptor-1 as a
novel target for the antiangiogenic treatment of breast cancer.
PLoS One. 9:e888302014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Speyer CL, Smith JS, Banda M, DeVries JA,
Mekani T and Gorski DH: Metabotropic glutamate receptor-1: A
potential therapeutic target for the treatment of breast cancer.
Breast Cancer Res Treat. 132:565–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li H J, Li X, Pang H, Pan JJ, Xie XJ and
Chen W: Long non-coding RNA UCA1 promotes glutamine metabolism by
targeting miR-16 in human bladder cancer. Jpn J Clin Oncol.
45:1055–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|