Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer type, and it is also the third highest cause of
cancer-associated mortalities globally (1). Of all cases of CRC, ~20–25% are
metastatic and 50–60% of the remainder eventually develop
metastases as per statistical estimation (2,3).
Therefore, it is important to identify novel CRC biomarkers to
enhance the efficiency of early diagnosis and improve therapeutic
strategies.
Nucleobindin 2 (NUCB2), a precursor of the
hypothalamic neuropeptide nesfatin-1, is mainly expressed in the
hypothalamic nuclei, and it has specific roles in energy
homeostasis (4). It is reportedly
distributed in the central nervous system, gastrointestinal system,
reproductive organs and adipose tissue (5). Additionally, NUCB2 has been indicated to
serve an important role in cancer progression and metastasis
(6–10). High levels of NUCB2 mRNA and protein
are associated with shorter recurrence-free survival time in
prostate cancer (6,7) and increased migration of prostate cancer
cells compared with low expression of NUCB2 (8). High expression of NUCB2 is also
associated with metastasis and reduced overall survival in clear
cell renal cell carcinoma (9). In
addition, NUCB2 has been reported to be a potent prognostic factor
for primary breast carcinoma as it is associated with its
metastasis (10).
These findings strongly indicate that NUCB2 could
serve a vital role in inducing metastasis in various types of
cancer. However, a number of studies have also demonstrated that
NUCB2 inhibited the proliferation of human adrenocortical carcinoma
and ovarian epithelial carcinoma cells (11,12). These
conflicting results point towards a possible tissue-specific
regulatory function of NUCB2. Kan et al (8) indicated that NUCB2 promoted the
migration, invasion and epithelial-mesenchymal transition (EMT) CRC
cells in vitro and in vivo. Nevertheless, the exact
underlying mechanism of NUCB2's action in CRC remains unclear. In
the present study, the expression levels of NUCB2 mRNA and protein
were analyzed in CRC tissues and adjacent non-cancerous tissues via
reverse transcription-quantitation polymerase chain reaction
(RT-qPCR) and immunohistochemistry (IHC), respectively. The
association between NUCB2 expression and the clinicopathological
parameters of CRC was also evaluated to determine its clinical
significance.
It was predicted that utilizing a larger sample size
would generate more consistent data that would help improve the
understanding of the role of NUCB2 in the pathological progression
of CRC.
Materials and methods
Patients and tissue samples
The project was approved by the Ethics Committee of
the First Affiliated Hospital, Zhejiang University School of
Medicine (Hangzhou, China), and each patient was required to
provide written informed consent. Samples of cancerous and adjacent
non-cancerous colorectal tissue were collected between January 2010
and December 2010 from 34 patients (age range 45–68 years, mean age
55.7 years), comprising of 19 males and 15 females) with CRC
admitted to the First Affiliated Hospital, Zhejiang University
School of Medicine.
Tissue microarrays (TMA) with 251 CRC
paraffin-embedded specimens were purchased from Shanghai Biochip
Co. Ltd. (Shanghai, China), and were used for detection of NUCB2
expression by IHC staining. The TMAs included samples from 138
males and 112 females, and 1 of unknown sex, with a median age of
66 years (range, 27–91) at the time of operation. Tumor node
metastasis (TNM) stage was classified using AJCC cancer staging
manual (13). All patients were
followed-up for >5 years following surgery, and the survival
time was calculated from the date of surgery until the deadline for
follow-up, or until the date of mortality. An additional 30 samples
of normal colorectal tissues were also collected from patients with
CRC between January 2014 and December 2014 (16 males and 14
females, age range 38–70 years, mean age 58.6 years) at the First
Affiliated Hospital, Zhejiang University School of Medicine
(Zhejiang, China).
RT-qPCR
Total RNA was extracted from fresh tissues using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and the concentration was determined using
Nanodrop™ 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). The mRNA samples were stored at −80°C. RT-qPCR
was performed with iTaq™ Universal One-Step RT-qPCR kit
(Bio-Rad Laboratories Inc., Hercules, CA, USA) according to the
manufacturer's protocol. The NUCB2 primers used were: Forward,
5′-TCTTGGAGCCAGATAGCTGG-3′ and reverse, 5′-AGCTTCTGAGCCTCCAGTTG-3′.
GAPDH was used as an internal control with the following primers:
Forward, 5′-TGAAGGTCGGAGTCAACGG-3′ and reverse,
5′-CTGGAAGATGGTGATGGGATT-3′. All primers were purchased from Sangon
Biotech Co., Ltd. (Shanghai, China). The following cycling
conditions were used: 10 min at 50°C for cDNA synthesis plus 1 min
at 95°C for reverse transcription inactivation and Taq polymerase
activation, followed by 35 cycles of 10 sec at 95°C denaturation
and 30 sec at 58°C for extension and data collection. The NUCB2
mRNA level was calculated relative to the internal GAPDH control
with the ΔΔCq method (14). For each sample, the PCR was run in
duplicates every time and was repeated for a total of three
times.
Haematoxylin and eosin staining
Sections were stained with haematoxylin and eosin
(H&E) prior to IHC staining. First, sections were
de-paraffinized with xylene, rehydrated in a graded alcohol series
(100, 95 and 75%), and then washed briefly in distilled water and
stained with hematoxylin for 8 min at room temperature. Following
this, the sections were washed in running tap water for 5 min and
differentiated in 1% acid alcohol for 30 sec at room temperature.
The sections were washed in running tap water for 5 min and
counterstained in eosin-phloxine solution for 30 sec to 1 min at
room temperature. Then the sections were dehydrated and
mounted.
IHC staining
Tissue were fixed by 10% formaldehyde for 24 h at
room temperature, and TMA sections was cut to 3–4 µm thicknesses
and then used for IHC. Briefly, TMA sections were first
de-paraffinized with xylene, rehydrated in graded alcohol (100, 95,
85 and 75% at room temperature) and autoclaved for 3 min in 0.01 M
citrate buffer (pH 6.0) for antigen retrieval. The sections were
then incubated with 3% (v/v) H2O2 for 10 min
at room temperature in order to block endogenous peroxidase. This
was followed by incubation with 10% (v/v) normal goat serum (Thermo
Fisher Scientific, Inc.) for 15 min at room temperature to reduce
nonspecific binding. Subsequently, the slides were incubated
overnight with rabbit polyclonal antibody against human NUCB2
(dilution, 1:1,000; catalog no. HPA008395; Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany) at 4°C. Following rinsing with
phosphate-buffered saline (PBS) three times, the sections were
incubated with biotin-labeled secondary antibody contained within
the Histostain-Plus kit (HRP, Broad Spectrum) for IHC staining
(ready-to-use, 859043; Thermo Fisher Scientific, Inc.) at room
temperature for 20 min, followed by horseradish
peroxidase-conjugated goat anti-rat antibody (ready-to-use, 859043;
Thermo Fisher Scientific, Inc.) for an additional 20 min at room
temperature. Finally, the sections were stained with
3,3-diaminobenzidine for 3 min at room temperature, counterstained
with hematoxylin, dehydrated with graded alcohol (75, 85, 95 and
100%) and then mounted. For the negative control, PBS was used
instead of the primary antibody.
Evaluation of IHC staining
The degree of immunostaining was semi-quantitatively
evaluated by two independent expert pathologists who were blinded
to the clinical data. The pathologists scored number of positively
stained cells per field under a light microscope and viewed five
fields under ×200 magnification. The level of NUCB2 expression was
calculated on the basis of the intensity of staining and the
percentage of positively stained cells. The staining intensity was
graded according to the following criteria: 0, no staining; 1, weak
staining; 2, moderate staining; and 3, strong staining. To
determine the percentage of stained cells, the number of stained
and unstained cells was counted per field under ×200 magnification,
and the average of five fields was calculated. Based on the
percentage of positive cells, the tumor score was graded as
follows: 0, ≤5% positively stained tumor cells; 1, 6–25% positive
tumor cells; 2, 26–50% positive tumor cells; and 3, ≥51% positive
tumor cells. The staining index was calculated by multiplying the
staining intensity score with the percentage of positive cells. For
the final evaluation, a staining index score of ≤3 was defined as
low NUCB2 expression, and a staining index score of ≥4 was defined
as high NUCB2 expression.
NUCB2 staining in the tumor cells was also
quantified as integrated optical density (IOD) by estimating the
area of the objects and medium pixel intensity per object using the
Image-Pro Plus software version 6 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Statistical analysis
SPSS (version 13.0; SPSS, Inc., Chicago, IL, USA)
was used to perform all statistical analyses. For quantitative
values with normal distribution, the paired-sample Student's t-test
was used for comparison, and if the values were not distributed
normally, the Wilcoxon Sign Rank test was used to compare two
groups of paired values. χ2 or Fisher's exact test was
used to analyze categorical data and evaluate the associations
between the expression of NUCB2 and the clinicopathological
parameters of CRC. The Kaplan-Meier method was used to perform
univariate survival analysis, and the log-rank test was used to
calculate differences between the survival curves. Multivariate
survival analysis and Cox proportional hazards regression model
were used to assess predictors associated with prognosis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Detection of NUCB2 mRNA expression
level
To detect NUCB2 mRNA expression level, a total of 34
paired fresh CRC specimens and their surrounding normal mucosal
tissues were analyzed using RT-qPCR. NUCB2 upregulation was defined
when the NUCB2 mRNA level was higher in the cancer tissue compared
with the non-cancerous tissue from the same patient. Conversely,
downregulation of NUCB2 was defined when a lower NUCB2 expression
was detected in the cancer tissue compared with the non-cancerous
tissue.
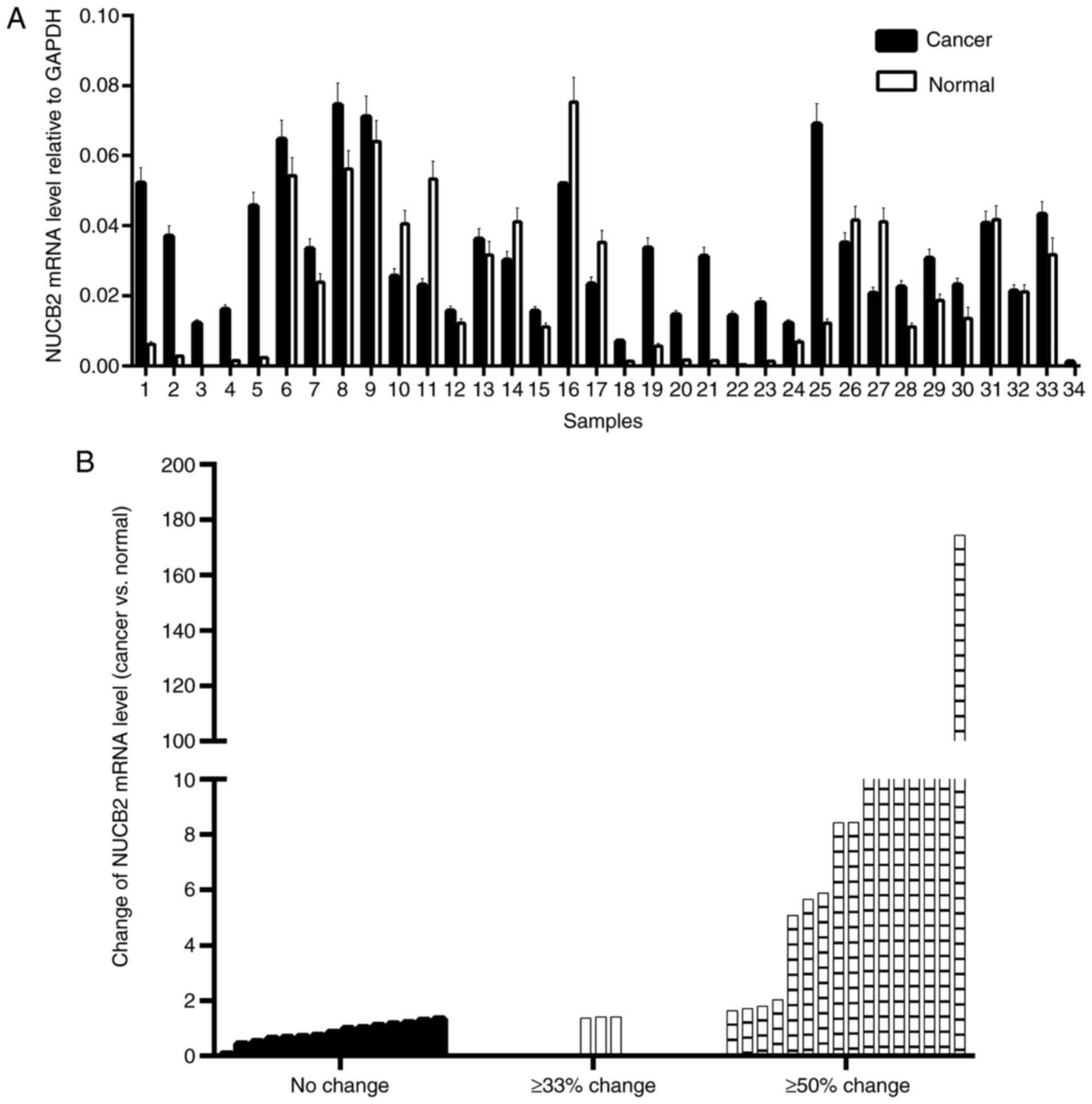
NUCB2 mRNA levels were upregulated in 73.5% of CRC
(25/34) samples and downregulated in the remaining 26.5% of samples
(9/34) (Fig. 1A). Paired Student's
t-test demonstrated that the mean level of NUCB2 mRNA was
upregulated in CRC tissues compared with the normal tissues
(P=0.018). Depending on the extent of the relative change in NUCB2
mRNA level in the cancer tissues, the samples were divided into
three groups (no change, ≥33% change and ≥50% change) and nearly
half of the samples indicated a ≥50% change (Fig. 1B).
Association of NUCB2 expression with
clinicopathological features of CRC
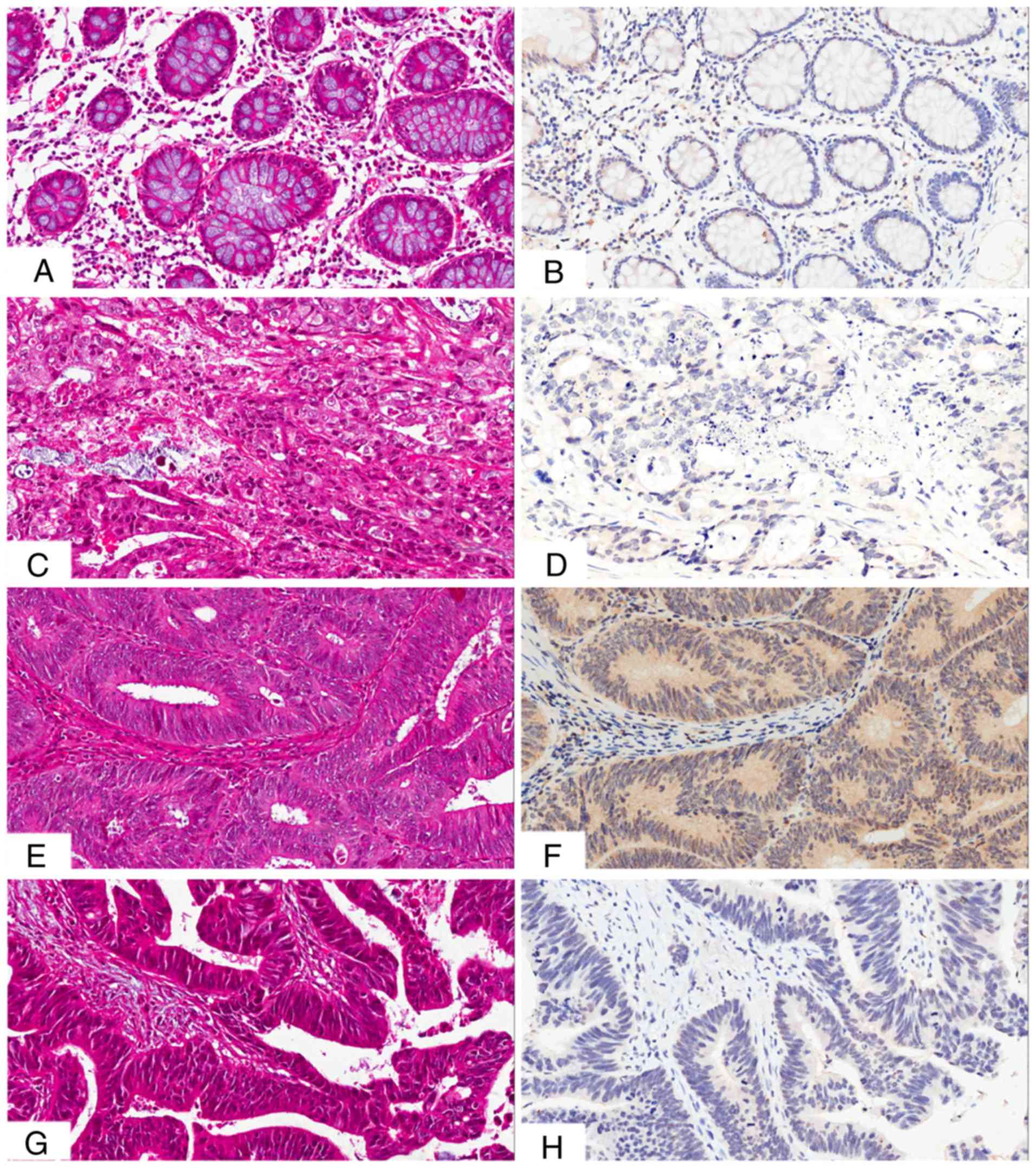
The presence and distribution of NUCB2 in the
tissues was assessed via IHC staining. The NUCB2 protein was
primarily localized in the cytoplasm and, to a lesser extent, in
the membrane of the cancer cells (Fig.
2). High expression of NUCB2 was detected in 5/30 (16.7%)
normal tissue samples, and in 105/251 (41.8%) CRC tissue samples,
indicating a significant difference (χ2=7.124,
P=0.008).
In order to assess the association between NUCB2
expression and colon cancer progression, the clinicopathological
parameters of colon cancer was analyzed in samples that exhibited
high levels of NUCB2. The results indicated a positive association
between NUCB2 expression and lymph node metastasis and TNM stage
(13) (Table I). Patients with lymph node metastasis
had a higher expression of NUCB2 (49.5%, 50/101) compared with
those without lymph node metastasis (36.7%, 55/150; P=0.043). In
addition, patients with TNM stage III–IV exhibited significantly
higher NUCB2 expression compared with those with TNM stage I–II
(50.9% vs. 35.0%; P=0.011). The quantification of NUCB2 staining
also indicated that the IOD values in tumors with lymph node
metastasis and TNM stage III–IV were higher compared with those in
tumors without lymph node metastasis and TNM stage I–II (Fig. 3; P<0.05).
 | Table I.Association between NUCB2 expression
and clinicopathological features of colorectal cancer. |
Table I.
Association between NUCB2 expression
and clinicopathological features of colorectal cancer.
|
| NUCB2 expression |
|
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Negative (%) | Positive (%) | χ2 | P-value |
|---|
| Sex |
|
| 0.011 | 0.917 |
| Male | 81 (58.3) | 58 (41.7) |
|
|
|
Female | 66 (58.9) | 46 (41.1) |
|
|
| Age, years |
|
| 0.077 | 0.781 |
|
<60 | 37 (59.7) | 25 (40.3) |
|
|
| ≥60 | 109 (57.7) | 80 (42.3) |
|
|
| Tumor diameter,
cm |
|
| 2.522 | 0.112 |
|
<20 | 76 (63.3) | 44 (36.7) |
|
|
| ≥20 | 70 (53.4) | 61 (46.6) |
|
|
| Differentiation |
|
| 0.918 | 0.632 |
| High | 32 (64.0) | 18 (36.0) |
|
|
|
Moderate | 85 (56.3) | 66 (43.7) |
|
|
|
Poor | 29 (58.0) | 21 (42.0) |
|
|
| TNM stage |
|
| 6.442 | 0.011 |
| I +
II | 93 (65.0) | 50 (35.0) |
|
|
| III +
IV | 53 (49.1) | 55 (50.9) |
|
|
| Lymph node
metastasis |
|
| 4.088 | 0.043 |
| No | 95 (63.3) | 55 (36.7) |
|
|
|
Yes | 51 (50.5) | 50 (49.5) |
|
|
| Distant
metastasis |
|
| 2.366 | 0.171 |
| No | 143 (59.1) | 99 (40.9) |
|
|
|
Yes | 3 (33.3) | 6 (66.7) |
|
|
| Liver
metastasis |
|
| 1.558 | 0.212 |
|
Negative | 144 (58.8) | 101 (41.2) |
|
|
|
Positive | 2 (33.3) | 4 (66.7) |
|
|
Clinical significance of NUCB2
expression in prognosis of CRC
Univariate survival analysis demonstrated that the
3- and 5-year cumulative survival rates of patients with high
expression of NUCB2 were 64.3 and 52.0%, and the 3- and 5-year
cumulative survival rates were 71.9 and 61.6% in those with no
NUCB2 expression, respectively. The mean survival duration of
patients with CRC with a high NUCB2 expression was 56.27±3.42
months, and the mean survival duration of patients with low NUCB2
expression was 62.59±2.79 months (Fig.
4). In addition, the analysis indicated no significant
association of NUCB2 expression with overall survival (Fig. 4; χ2=2.044, P=0.153).
Discussion
CRC is one of the most frequently occurring cancer
types globally (15). Metastasis in
patients with colon cancer is associated with poor prognosis at
early stages of the disease and subsequent mortality (16). Therefore, it is vital to investigate
the underlying molecular mechanisms of metastasis and develop
therapeutic strategies specifically targeting this process
(17). NUCB2 has a widespread
expression pattern in the body and mainly participates in
physiological processes, including nocturnal feeding and regulation
of body weight (18). Recent reports
have demonstrated the diverse functions of NUCB2 in various tissues
and in different cancer types (11–13,19).
Takagi et al (19) reported
that NUCB2 expression was positively correlated with Ki67
expression, and the knockdown of NUCB2 significantly inhibited the
proliferation and migration of tumor cells in endometrial
carcinoma. By contrast, NUCB2 also inhibited cell proliferation in
adrenocortical and ovarian epithelial carcinoma (11,12).
Notably, Kan et al (8)
determined that NUCB2 enhanced migration, invasion and EMT of
cancer cells in colon cancer. Nevertheless, the clinical
significance of NUCB2 in colon cancer remains unclear.
In the present study, RT-qPCR indicated an
upregulation of NUCB2 mRNA levels in CRC tissues compared with the
adjacent non-cancerous tissues, which was consistent with previous
reports on prostate cancer (6,20). The
association of NUCB2 protein expression with CRC progression was
further investigated on the basis of a large clinical sample
cohort. The results demonstrated an upregulation of NUCB2 in a
large proportion of patients with CRC, and elevated NUCB2 protein
level was associated with the TNM stage and lymph node metastasis.
These results support the hypothesis that NUCB2 may act as an
oncogene in CRC with a key role in metastasis and progression.
A number of studies have also indicated an
interaction between NUCB2 and the mechanistic target of rapamycin
(mTOR) or AMP-activated protein kinase (AMPK) pathways (8,21). For
example, treatment with Nestafin-1/NUCB2 enhanced the
phosphorylation of AMPK and TORC2 in the rat brain (21). Kan et al (8) also indicated that NUCB2 enhanced cell
migration and invasion via the liver kinase B1/AMPK/transducer of
CREB protein 1/ZEB1 pathways in colon cancer. Therefore, the AMPK
and mTOR pathways may serve an important role in metastasis.
A previous study by Zhang et al (7), which focused on the role of NUCB2 in
cancer prognosis, demonstrated that high levels of the NUCB2
protein in prostate cancer was significantly associated with the
biochemical recurrence-free survival rate, and multivariate
analysis also indicated that high NUCB2 levels could be an
independent prognostic factor in patients with prostate cancer.
NUCB2 expression level was also reported as an independent
prognostic predictor in patients with renal cell carcinoma
(9,22). However, in the present study,
Kaplan-Meier analysis indicated no significant association between
disease-free survival of patients and NUCB2 expression. Several
studies have demonstrated poorer prognosis in younger patients with
CRC compared with older patients (23,24),
whilst other studies have indicated the opposite (25,26). This
may be due to certain characteristics of the younger patients,
which vary across different regions (27) and ethnicities (28). For instance, a higher frequency of
colorectal neoplasia was observed among 40–49 year-old African
Americans when compared with Hispanic Americans suggesting an
increased susceptibility to CRC risk in this population (28). In the present study, it was determined
that there was no significant association between NUCB2 expression
and the age of patients with CRC as well as clinical prognosis. It
is possible that the clinicopathological features of the tissue
samples are responsible for this finding. Further studies are
required to continuously collect more clinical data, and larger
samples are also needed in order to yield more accurate and
consistent results in the future.
Furthermore, in the present study, multivariate
analysis demonstrated that the upregulation of NUCB2 was also not
an independent prognostic predictor in patients with CRC. In view
of the limitations of the present study, caused by the sample size
and collection, more clinical studies are being considered for
future studies, which would include a larger cohort to investigate
the role of NUCB2 in CRC prognosis.
In conclusion, a significant association was
detected between high NUCB2 expression level and metastasis or poor
clinical outcome of CRC. This strongly indicated that NUCB2 is a
cancer-associated oncogene, which is associated with aggressive
progression in CRC. Additionally, NUCB2 may be useful a novel
biomarker for the diagnosis, prognosis and as a potential
therapeutic target for CRC. However, these results are based on a
single Chinese cohort, and therefore further studies are required
to validate the results.
Acknowledgements
Not applicable.
Funding
No funding received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WC was responsible for study conception and design
and revised the manuscript, JX performed experiments and drafted
the manuscript, LC analyzed the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Approval for the present study was obtained by the
Ethics Committee of the First Affiliated Hospital, Zhejiang
University School of Medicine (Hangzhou, China).
Consent for publication
All patients admitted to the study provided informed
consent for their participation of the present study and the
publication of this data.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E and Oliveira J: ESMO
Guidelines Working Group: Advanced colorectal cancer: ESMO clinical
recommendations for diagnosis, treatment and follow-up. Ann Oncol.
20:61–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoo PS, Lopez-Soler RI, Longo WE and Cha
CH: Liver resection for metastatic colorectal cancer in the age of
neoadjuvant chemotherapy and bevacizumab. Clin Colorectal Cancer.
6:202–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oh-I S, Shimizu H, Satoh T, Okada S,
Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, et
al: Identification of nesfatin-1 as a satiety molecule in the
hypothalamus. Nature. 443:709–712. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garcia-Galiano D, Navarro VM, Gaytan F and
Tena-Sempere M: Expanding roles of NUCB2/nesfatin-1 in
neuroendocrine regulation. J Mol Endocrinol. 45:281–290. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang H, Qi C, Li L, Luo F and Xu Y:
Clinical significance of NUCB2 mRNA expression in prostate cancer.
J Exp Clin Cancer Res. 32:562013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Qi C, Wang A, Yao B, Li L, Wang Y
and Xu Y: Prognostication of prostate cancer based on NUCB2 protein
assessment: NUCB2 in prostate cancer. J Exp Clin Cancer Res.
32:772013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ,
Ho YW and Kuo PL: Nesfatin-1/Nucleobindin-2 enhances cell
migration, invasion, and epithelial-mesenchymal transition via
LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget.
7:31336–31349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi C, Ma H, Zhang HT, Gao JD and Xu Y:
Nucleobindin 2 expression is an independent prognostic factor for
clear cell renal cell carcinoma. Histopathology. 66:650–657. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki S, Takagi K, Miki Y, Onodera Y,
Akahira J, Ebata A, Ishida T, Watanabe M, Sasano H and Suzuki T:
Nucleobindin 2 in human breast carcinoma as a potent prognostic
factor. Cancer Sci. 103:136–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramanjaneya M, Tan BK, Rucinski M, Kawan
M, Hu J, Kaur J, Patel VH, Malendowicz LK, Komarowska H, Lehnert H,
et al: Nesfatin-1 inhibits proliferation and enhances apoptosis of
human adrenocortical H295R cells. J Endocrinol. 226:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Pang X, Dong M, Wen F and Zhang Y:
Nesfatin-1 inhibits ovarian epithelial carcinoma cell proliferation
in vitro. Biochem Biophys Res Commun. 440:467–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Edge SB and Compton CC: The American Joint
Committie on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haggar FA and Boushey RP: Colorectal
cancer epidemiology: Incidence, mortality, survival, and risk
factors. Clin Colon Rectal Surg. 22:191–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Field K and Lipton L: Metastatic
colorectal cancer-past, progress and future. World J Gastroenterol.
13:3806–3815. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kraljevic Pavelic S, Sedic M, Bosnjak H,
Spaventi S and Pavelic K: Metastasis: New perspectives on an old
problem. Mol Cancer. 10:222011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao X, Liu XM and Zhou LH: Recent progress
in research on the distribution and function of NUCB2/nesfatin-1 in
peripheral tissues. Endocr J. 60:1021–1027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takagi K, Miki Y, Tanaka S, Hashimoto C,
Watanabe M, Sasano H, Ito K and Suzuki T: Nucleobindin 2 (NUCB2) in
human endometrial carcinoma: A potent prognostic factor associated
with cell proliferation and migration. Endocr J. 63:287–299. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang H, Qi C, Wang A, Li L and Xu Y: High
expression of nucleobindin 2 mRNA: An independent prognostic factor
for overall survival of patients with prostate cancer. Tumour Biol.
35:2025–2028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang M, Zhang Z, Wang C, Li K, Li S, Boden
G, Li L and Yang G: Nesfatin-1 action in the brain increases
insulin sensitivity through Akt/AMPK/TORC2 pathway in diet-induced
insulin resistance. Diabetes. 61:1959–1968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Wang
Z, Xie H, Dai B, Xu J and Ye D: High NUCB2 expression level
represents an independent negative prognostic factor in Chinese
cohorts of non-metastatic clear cell renal cell carcinoma patients.
Oncotarget. 8:35244–35254. 2017.PubMed/NCBI
|
|
23
|
Kaplan MA, Isikdogan A, Gumus M, Arslan
UY, Geredeli C, Ozdemir N, Koca D, Dane F, Suner A, Elkiran ET, et
al: Childhood, adolescents, and young adults (≤25 y) colorectal
cancer: Study of Anatolian Society of Medical Oncology. J Pediatr
Hematol Oncol. 35:83–89. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, Bao F, Yan J, Liu H, Li T, Chen H
and Li G: Poor prognosis of young patients with colorectal cancer:
A retrospective study. Int J Colorectal Dis. 32:1147–1156. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Taggarshe D, Rehil N, Sharma S, Flynn JC
and Damadi A: Colorectal cancer: Are the ‘young’ being overlooked?
Am J Surg. 205:312–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yeo SA, Chew MH, Koh PK and Tang CL: Young
colorectal carcinoma patients do not have a poorer prognosis: A
comparative review of 2,426 cases. Tech Coloproctol. 17:653–661.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
You YN, Xing Y, Feig BW, Chang GJ and
Cormier JN: Young-onset colorectal cancer: Is it time to pay
attention? Arch Intern Med. 172:287–289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ashktorab H, Paydar M, Namin HH, Sanderson
A, Begum R, Brim H, Panchal H, Lee E, Kibreab A, Nouraie M and
Laiyemo AO: Prevalence of colorectal neoplasia among young African
Americans and hispanic Americans. Dig Dis Sci. 59:446–450. 2014.
View Article : Google Scholar : PubMed/NCBI
|