Introduction
Gastric cancer is one of the most common types of
cancer in China and worldwide (1–3).
Epidemiological evidence indicates that the incidence rate of
gastric cancer is higher in males than that in females (4). The onset of gastric cancer in males
occurs 10–15 years earlier compared with females; however,
following menopause, the incidence rate of gastric cancer increases
and becomes equally prevalent between men and women (4). It has been reported that estrogen of
ovarian or exogenous origin, may suppress gastric cancer (5). The results of a Swedish cohort study of
male patients with prostate cancer reveled that estrogen exposure
reduced the risk of gastric cancer (6). It has therefore been postulated that
estrogen may serve a protective role in development of gastric
cancer.
There are two estrogen receptors (ERs); ER-α and
ER-β. Several studies have investigated the role of estrogen
signaling in gastric cancer, as well as its potential mechanisms
and clinical relevance (7). However,
reports on the function of ERs in the development of gastric cancer
have been inconsistent (8).
ER-α36, a truncated 36-kDa form of ER-α, has been
reported to be upregulated in gastric cancer and, furthermore,
ER-α36 expression is associated with lymph node metastasis in
gastric cancer (9–13). Compared with ER-α66, ER-α36 is
primarily expressed in the cytoplasm and at the plasma membrane,
mediating rapid, non-genomic estrogen signaling (11–13). A
previous study revealed that gastric cancer cells with an increased
expression of ER-α36 are more sensitive to estrogen compared with
cells expressing decreased levels of ER-α36 (14). ER-α36 mediates rapid estrogen
signaling to promote cell growth via the phosphoinositide
3-kinase/RAC serine/threonine-protein kinase and the
mitogen-activated protein kinase/extracellular signaling-related
kinase signaling pathways (15).
However, the exact molecular mechanisms by which ER-α36 functions
in the carcinogenesis of gastric cancer remain unclear.
The 78 kDa glucose-regulated protein (GRP78) is an
endoplasmic reticulum chaperone that functions as a regulator of
intracellular calcium, protein folding and the unfolded-protein
response (16,17). Additionally, GRP78 is a
stress-response protein that is upregulated disturbances to
cellular homeostasis, including reduced levels of oxygen or
glucose, and is translocated from the endoplasmic reticulum to
other cellular locations, including the nucleus or the plasma
membrane, where it functions as a membrane receptor to transduce
proliferation, invasion, apoptosis, inflammation and immunity
signals (16,17). GRP78 overexpression has been
documented in a number of cancer cell lines and tissues and is
associated with aggressive growth and invasive properties (18–20).
Upregulation of GRP78 expression is associated with increased lymph
node metastasis and poor prognosis in patients with gastric cancer
(21). Knockdown of GRP78 expression
in established gastric cancer cells attenuates invasion in
vitro and tumor metastasis in vivo (22). These results indicate that GRP78
serves an important role in the progression of gastric carcinoma
and has the potential to be used as an effective marker for
aggressive disease and poor prognosis in patients with gastric
carcinoma. It has been reported that estrogens also regulate GRP78
expression in endometrial cancer cells (23). GRP78 overexpression was detected in
samples from aggressive ER-negative breast cancer, but not in those
from benign human breast lesions (24). Elevated GRP94 expression was also
reported to be associated with cancer progression and ER-α36
expression in gastric and breast cancer (25,26).
Crosstalk between GRP94, ER-α36 and HER2 forms positive feedback
loops in breast cancer, which may affect tumor growth, metastasis
and drug resistance (26). Inhibition
of GRP94 expression with siRNA or monoclonal antibody (mAb) blocked
the GRP94-ER-α36 interaction and inhibited breast cancer growth and
invasion in vitro and in vivo (26). HER2 signaling activated ER-α36
transcription, which mediates non-genomic estrogen and
anti-estrogen (tamoxifen) signaling and stimulated cell
proliferation (9–13,27).
Although the induction of GRP protein expression by estrogen
signaling has been documented, the functions and underlying
mechanisms of the induction of GRP78 expression by estrogen in
gastric cancer have not been elucidated (28).
The present study examined the expression of GRP78
and ER-α36 in tumor samples from gastric cancer patients and their
association with sex and lymph node metastasis. GRP78 expression
and glycogen synthase kinase 3β (GSK-3β) phosphorylation were also
examined in gastric cancer cells with different levels of ER-α36
expression.
Materials and methods
Reagents
17β-estradiol (E2) was obtained from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Rabbit anti-ER-α36 antibody was
provided by the Shenogen Pharma Group Ltd. (Beijing, China). The
antibody was generated using the custom service provided by the
Pacific Immunology (Ramona, CA, USA) using the final 20 amino acids
of ER-α36 encoded by exon 9. The produced antibody was purified
using an affinity column consisting of immunogen peptides (11–13). The
rabbit anti-GRP78 antibody (cat. no. ab21685) was obtained from
Abcam (Cambridge, UK). Rabbit anti-phospho-GSK-3β at Ser9
(Ser9-GSK-3β; cat. no. 9323) was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). Mouse anti-β-actin antibody
(cat. no. sc-47778), goat anti-mouse horseradish
peroxidase-conjugated secondary antibody (cat. no. sc-2005) and
goat anti-rabbit horseradish peroxidase-conjugated secondary
antibody (cat. no. sc-2004) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Bicinchoninic acid protein
detection kit, the chemiluminescent substrate kit and
polyvinylidene difluoride membranes were obtained from Pierce
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). A SuperPicture
3rd Generation Immunohistochemical (IHC) Detection kit was obtained
from Invitrogen (Thermo Fisher Scientific, Inc.).
Radioimmunoprecipitation assay (RIPA) buffer and enhanced
chemiluminescence reagents were obtained from Beyotime Institute of
Biotechnology (Haimen, China).
Cell culture, treatment, and lysate
preparation
The human gastric adenocarcinoma cell line SGC-7901
was obtained from the Department of Immunology of Tongji Medical
College (Wuhan, China). Gastric cancer cells expressing low levels
of ER-α36 (SGC-7901-Low36 cells) were established using small
hairpin RNAs (shRNAs). The ER-α36-specific shRNA expression vector
was constructed by cloning the DNA oligonucleotides
(5′-TTAACCGTACCACTCTGCTGATTGATATCCGTCAGCAGAGTGGTACGGTTA-3′)
targeting the 3′-untranslated region of ER-α36 cDNA into the
pRNAT-U6.1/neo expression vector purchased from GenScript Biotech
Corporation (Piscataway, NJ, USA). A gastric cancer cell line
overexpressing ER-α36 (SGC-7901-High36 cells) was established via
transfection with an ER-α36 expression vector, as previously
described (11,13,25,29).
SGC-7901, SGC-7901-Low36 cells and SGC-7901-High36 were all
maintained in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C in an atmosphere containing 5%
CO2. Prior to E2 treatment, cells were cultured at 37°C
in phenol-red-free medium (Gibco; Thermo Fisher Scientific, Inc.)
with 5% charcoal-stripped FBS (Biological Industries, Kibbutz
Beit-Haemek, Israel) for 6 h, and then in 2% charcoal-stripped FBS
for 24 h, then cells were treated with 10−10 M E2 for 24
h.
Gastric tumor samples
Frozen tumor tissues of 20 patients with gastric
cancer collected between January 2009 and December 2010 and the
paraffin-embedded tissues of 86 patients with gastric cancer
collected between January 2006 and December 2010 were obtained from
the Jiangda Pathology Institute (Wuhan, China). Written informed
consent was obtained from all patients and the study protocols were
approved by The Ethics Committee of the School of Medicine,
Jianghan University (Wuhan, China). The histological type was
adenocarcinoma in all cases. Tumor tissues used for IHC staining
were fixed in 10% neutral formalin at room temperature for 24 h,
embedded in paraffin, and stained with hematoxylin and eosin
(H&E). The tissues for western blotting were snap-frozen in
liquid nitrogen and stored at −150°C. Frozen samples were obtained
from 13 men and 7 women aged 34–78 years (mean, 60.1 years), and
the formalin-fixed paraffin-embedded samples for IHC were obtained
from 56 men and 30 women aged 34–82 years (mean, 57.13 years). No
patient received any anticancer treatment prior to surgery. Tumor
size, histological differentiation, size and location of the
primary tumor (T stage) and lymph node involvement (N stage) were
all evaluated according to the clinicopathological classification
outlined by the World Health Organization (2013) (25).
Tissue microarray
Representative areas of solid tumors were identified
in H&E-stained sections. A 0.6-mm diameter tissue core was
punched out from each block and transferred to a recipient block
with 86 samples using an MTA-1 tissue microarray (Beecher
Instruments, Inc., Sun Prairie, WI, USA). Sections 4 µm thick were
consecutively sliced from the recipient block and transferred to
polylysine-coated glass slides. H&E staining with Mayer's
hematoxylin for 2 min and 1% eosin for 30 sec at room temperature
was performed on the tissue microarray to check the quality, such
as integrity of tumor tissues, thickness and dye uniformity, of the
sections prior to experiments.
Western blot analysis
Western blot analysis was conducted as previously
described (25). Briefly, cells were
washed with cold PBS and lysed with RIPA buffer. The tumor tissues
were dissected and homogenized using RIPA buffer. The protein
concentration was determined using the bicinchoninic acid kit.
Proteins (20 µg GRP78, 20 µg ER-α36 and 30 µg Ser9-GSK-3β) were
separated using SDS-PAGE (10% gel) and transferred to
polyvinylidene fluoride membranes. Membranes were incubated with
the aforementioned primary antibodies at 4°C overnight. Blots were
subsequently washed, incubated with the appropriate secondary
antibodies for 1 h at 37°C and visualized using enhanced
chemiluminescence. The Total Lab analysis software (version TL120;
Total Lab Ltd., Newcastle upon Tyne, UK) was used to perform
densitometry analysis of protein bands.
Immunohistochemistry assays
The tissue slides were de-paraffinized with xylene
for 15 min, and gradually rehydrated in an ethanol series (100,
100, 95, 95 and then 80% alcohol; 3 min each). Endogenous
peroxidase activity was blocked by incubating with 3% hydrogen
peroxide/methanol buffer for 10 min at room temperature. Antigen
retrieval was performed by immersing slides in an ethylenediamine
tetraacetic acid buffer (pH 8.0) followed by boiling in a water
bath at 100°C for 25 min. Slides were rinsed with PBS and then
incubated with a rabbit anti-GRP78 antibody (dilution, 1:400) or
with a rabbit anti-ER-α36 antibody (dilution, 1:400) overnight at
4°C. Slides were washed and incubated with the secondary antibody,
horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin
(dilution, 1:100; cat. no. A16096; Invitrogen; Thermo Fisher
Scientific, Inc.), for 1 h at 37°C. Diaminobenzidine was used as a
chromogen, and the slides were counterstained with hematoxylin at
room temperature for 5 min.
GRP78 and ER-α36 staining was scored as follows: 0,
no staining or staining observed in <10% of tumor cells; 1+,
faint/barely perceptible staining detected in ≥10% of tumor cells;
2+/3+, moderate or strong staining, respectively, observed in ≥10%
of tumor cells. A score of 0 or 1+ was considered negative, and a
score of 2+ or 3+ was considered positive. The immunostained slides
were evaluated independently by two pathologists in a
double-blinded manner. In the majority of cases, the evaluations of
the two pathologists were identical; discrepancies were resolved by
re-examination and consensus (25).
Statistical analysis
Data are presented as the mean ± standard deviation.
The association between GRP78 expression, clinicopathological
characteristics, and ER-α36 expression was determined using
Pearson's χ2 test. Statistical analysis was performed
using one-way analysis of variance, followed by least significant
difference tests. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS 12.0 (SPSS, Inc., Chicago, IL, USA).
Results
Association between GRP78 and ER-α36
expression and clinicopathological characteristics of gastric
adenocarcinomas
The expression of GRP78 and ER-α36 was assessed in
gastric tumor tissues, adjacent non-tumor tissues and corresponding
normal tissues using western blot analysis. Compared with the
paired normal tissues, higher levels of GRP78 and ER-α36 were
observed in 15/20 (75%) and 13/20 (65%) tumor specimens,
respectively, whereas 11/20 (55%) cases co-expressed the two
proteins (Fig. 1).
The expression pattern of GRP78 was also examined in
86 specimens using IHC. High levels of GRP78 expression (2+ or 3+)
were observed in 55 cases of gastric carcinoma patients (55/86;
63.95%). The IHC assay also revealed that ER-α36 was expressed in
76 specimens (76/86; 88.37%; Table
I).
 | Table I.Associations between GRP78
expression, clinicopathological features of gastric carcinomas and
ER-α36 expression. |
Table I.
Associations between GRP78
expression, clinicopathological features of gastric carcinomas and
ER-α36 expression.
|
| GRP78
expression |
|
|---|
|
|
|
|
|---|
| Factors | Positive | Negative | P-value |
|---|
| Age, years |
|
| >0.05 |
|
≤60 | 34 | 14 |
|
|
>60 | 21 | 17 |
|
| Sex |
|
| 0.01 |
|
Male | 36 | 20 |
|
|
Female | 19 | 11 |
|
| Tumor size, cm |
|
| >0.05 |
| ≤5 | 25 | 7 |
|
|
>5 | 30 | 24 |
|
| Histological
differentiation |
|
| >0.05 |
|
High | 45 | 24 |
|
|
Low | 10 | 7 |
|
| T stage |
|
| >0.05 |
|
T2-3 | 46 | 21 |
|
| T4 | 9 | 10 |
|
| N stage |
|
| 0.01 |
| N0 | 11 | 6 |
|
|
N1-3 | 44 | 25 |
|
| ER-36 |
|
| 0.01 |
|
Positive | 49 | 27 |
|
|
Negative | 6 | 4 |
|
Analysis of the associations between GRP78
expression and clinicopathological properties of gastric
adenocarcinoma was then performed. GRP78 expression was
significantly associated with male and lymph node metastasis
(P<0.05), but not with other features including age, tumor size,
tumor stage and histological differentiation (P>0.05). GRP78 was
expressed at greater levels in males compared with females, at a
ratio of 1.87:1 (P<0.05; Table I).
GRP78 expression was positively associated with increased lymph
node metastasis (P<0.05, Table I).
A significant association was also detected between GRP78 and
ER-α36 expressions (P<0.05; Table
I).
Involvement of GRP78 and GSK-3β in
ER-α36-mediated rapid estrogen signaling
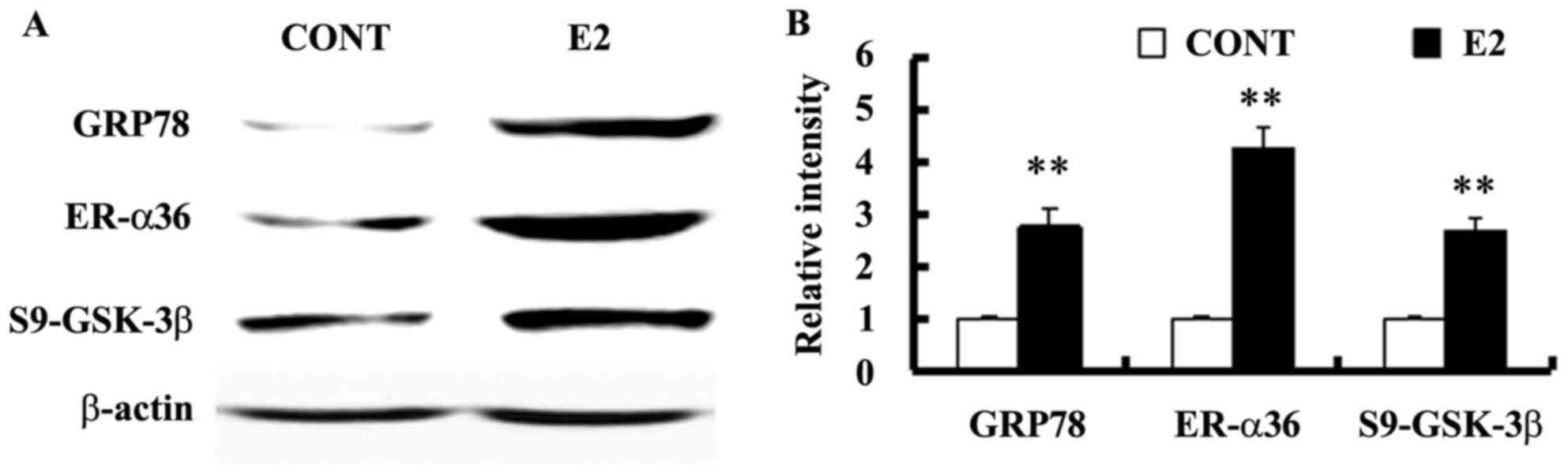
Estrogen-deprived SGC-7901 cells were treated with
10−10 M E2 for 24 h to assess whether estrogen regulates
GRP78 expression. Levels of GRP78 expression and GSK-3β
phosphorylation at Ser9 were also assessed using western blotting.
E2 treatment increased the expression of GRP78 and ER-α36, as well
as the level of GSK-3β phosphorylation at Ser9 in SGC-7901 cells
(Fig. 2).
GRP78 expression and GSK-3β
phosphorylation in gastric cancer cells expressing different levels
of ER-α36
To confirm the involvement of ER-α36 in regulation
of GRP78 expression, GRP78 expression and GSK-3β phosphorylation
was assessed in SGC-7901-Low36 cells and in SGC-7901-High36 cells.
Fig. 3 demonstrates the decrease of
the levels of GRP78 expression and the phosphorylation of GSK-3β at
Ser9 in the SGC-7901-Low36 cells compared with the control cells
transfected with an empty vector. In cells overexpressing ER-α36,
GRP78 expression and Ser9-phosphorylated GSK-3β were significantly
increased (Fig. 3). The results of
the present study therefore indicate that GRP78 expression is
positively regulated by ER-α36-mediated signaling, presumably via
the GSK-3β pathway.
Discussion
Epidemiological evidence indicates a consistent
predominance of gastric cancer in men worldwide (4). It has been postulated that estrogen may
serve a protective role in gastric tumorigenesis (4–6). Estrogen
receptors are nuclear receptors that mediate estrogen signaling in
different tissues (11–15). Dysregulation of ER-mediated estrogen
signaling is reported to be associated with development of gastric
cancer (9,10,14).
However, ER-α66 expression in gastric cancer is inconsistent, with
variable patterns (0–62.5%) (30,31). ER-β
expression, however, is reported to be associated with lower tumor
stage, negative perineural invasion and lower risk of recurrence
(7). A previous study indicated that
estrogen receptors are present at low levels in gastric tumors and
normal tissues (32). Thus, their
roles in gastric carcinogenesis may be limited.
ER-α36, a novel variant of ER-α, was demonstrated to
be highly expressed in specimens of human gastric cancer and its
expression was positively associated with invasion to serosa, lymph
node metastasis and cyclin D1 expression (14). In ER-α36-knockdown SGC-7901 cells,
downregulated ER-α36 expression was associated with smaller tumor
size, decreased nuclear fission and reduced GRP94 expression
(25). In the present study, a high
level of ER-α36 expression was detected in specimens from patients
with gastric carcinoma, which was positively associated with GRP78
expression.
GRP78 is a stress-inducible molecular chaperone that
is upregulated in cancer cells with increasing hypoxia, nutrient
starvation and acidosis (16,17). GRP78 is overexpressed in different
human cancer types, including gastric cancer, and is associated
with the growth, invasion and metastasis of tumors (16–20).
Inhibition of GRP78 activities reduces tumor formation of gastric
cancer cells in xenograft models and suppresses proliferation,
invasion and drug resistance in gastric cancer cells (33). In the present study, high levels of
GRP78 expression were observed in specimens from gastric carcinoma.
GRP78 expression was positively associated with the male sex,
metastasis to the lymph node and ER-α36 expression. Estrogen
treatment increased GRP78 and ER-α36 expression. The steady state
level of GRP78 protein was decreased in SGC-7901-Low36 cells;
however, in SGC-7901-High36 cells, GRP78 expression was
upregulated. These results indicate that ER-α36-mediated rapid
estrogen signaling is associated with the regulation of GRP78
expression in gastric cancer cells, which is consistent with a
previous report that estrogens regulate GRP78 expression (23). It has been reported that the
C-terminal domain of GRP94 interacts directly with ER-α36 at the
cell membrane of breast cancer cells and that this interaction
stabilizes ER-α36, thereby increasing ER-α36 signaling and cell
growth (26). It was reported that
targeting GRP94 with small interfering RNA or a specific monoclonal
antibody blocked the GRP94-ER-α36 interaction, inhibiting breast
cancer growth in vitro and in vivo (26). Taken together, these results suggest
that GRPs are associated with ER-α36 and its signaling, which
serves a role in gastric carcinogenesis.
GSK-3β is a serine/threonine kinase, and its
activity is regulated by the dynamic balance between activating
Tyr216-phosphorylation and inactivating Ser9-phosphorylation.
GSK-3β is associated with the regulation of a number of cellular
processes, including cell growth, stem cell renewal and apoptosis
(34,35). Dysregulation of the GSK-3β signaling
pathway is associated with the onset and progression of various
tumors (34,35). It has previously been reported that
activation of GSK-3β in tumors may be pro- or anti-apoptotic,
depending on the cell type or subcellular localization (34). In certain cases, suppression of GSK-3β
phosphorylation via growth factor-stimulated signaling or
downregulation of GSK-3β expression is associated with cancer
progression (35). In addition, GSK-3
was reported to negatively regulate the activity of proto-oncogenic
proteins and cell cycle regulators via the Hedgehog and
Wnt/β-catenin signaling pathways (36). ERs have also been identified as GSK-3
substrates: Silencing of GSK-3α or GSK-3β resulted in ER-α
downregulation and a resultant reduction in its activity in
ER-positive breast tumor cells (37).
GSK-3β is also activated during endoplasmic reticular stress
(38). The ability of GRP78 to bind
to GSK-3 and tau are increased with elevated GRP78 expression in
vivo and in vitro (39).
In hepatocellular adenoma, novel links between endoplasmic
reticular stress, decreased cyclin D expression and elevated ER-α36
and GSK-3β expressions were detected (40). The present study demonstrates that
GRP78 and ER-α36 expression and GSK-3β phosphorylation at Ser9 are
increased in gastric cancer SGC-7901 cells following estrogen
treatment. A significant decrease in GRP78 expression and GSK-3β
phosphorylation at Ser9 was observed in ER-α36-knockdown cells
compared with the SGC-7901-control cells. In cells overexpressing
ER-α36, levels of GRP78 and GSK-3β phosphorylation at Ser9 were
significantly increased. These results suggest that ER-α36-mediated
estrogen signaling is associated with the regulation of GRP78
expression, presumably via the GSK-3β pathway.
In conclusion, increased GRP78 expression is
associated with the male sex, lymph node metastasis and ER-α36
expression in gastric cancer. GRP78 is regulated by ER-α36-mediated
rapid estrogen signaling via the GSK-3β pathway, which may also be
associated with gastric carcinogenesis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China, (grant nos. 81402315, 81272754
and 81470110), the Natural Science Foundation of Hubei Province
(grant no. 2013CFB215) and the Foundation of Hubei Provincial
Department of Education (grant no. B2014079).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sipponen P and Correa P: Delayed rise in
incidence of gastric cancer in females results in unique sex ratio
(M/F) pattern: Etiologic hypothesis. Gastric Cancer. 5:213–219.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Camargo MC, Goto Y, Zabaleta J, Morgan DR,
Correa P and Rabkin CS: Sex hormones, hormonal interventions and
gastric cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers
Prev. 21:20–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lindblad M, Ye W, Rubio C and Lagergren J:
Estrogen and risk of gastric cancer: A protective effect in a
nationwide cohort study of patients with prostate cancer in Sweden.
Cancer Epidemiol Biomarkers Prev. 13:2203–2207. 2004.PubMed/NCBI
|
|
7
|
Ryu WS, Kim JH, Jang YJ, Park SS, Um JW,
Park SH, Kim SJ, Mok YJ and Kim CS: Expression of estrogen
receptors in gastric cancer and their clinical significance. J Surg
Oncol. 106:456–461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahman Ur MS and Cao J: Estrogen receptors
in gastric cancer: Advances and perspectives. World J
Gastroenterol. 22:2475–2482. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng H, Huang X, Fan J, Wang L, Xia Q,
Yang X, Wang Z and Liu L: A variant of estrogen receptor-alpha,
ER-alpha36 is expressed in human gastric cancer and is highly
correlated with lymph node metastasis. Oncol Rep. 24:171–176.
2010.PubMed/NCBI
|
|
10
|
Wang X, Deng H, Zou F, Fu Z, Chen Y, Wang
Z and Liu L: ER-α36-mediated gastric cancer cell proliferation via
the c-Src pathway. Oncol Lett. 6:329–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: Identification, cloning, and expression of human
estrogen receptor-alpha36, a novel variant of human estrogen
receptor-alpha66. Biochem Biophys Res Commun. 336:1023–1027. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZY and Yin L: Estrogen receptor
alpha-36 (ER-α36): A new player in human breast cancer. Mol Cell
Endocrinol 418 Pt. 3:193–206. 2015. View Article : Google Scholar
|
|
13
|
Wang Z, Zhang X, Shen P, Loggie BW, Chang
Y and Deuel TF: A variant of estrogen receptor-{alpha},
hER-{alpha}36: Transduction of estrogen- and antiestrogen-dependent
membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA.
103:9063–9068. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Huang X, Fu Z, Zou F, Li Y, Wang Z
and Liu L: Biphasic ER-α36-mediated estrogen signaling regulates
growth of gastric cancer cells. Int J Oncol. 45:2325–2330. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi L, Dong B, Li Z, Lu Y, Ouyang T, Li J,
Wang T, Fan Z, Fan T, Lin B, et al: Expression of ER-{alpha}36, a
novel variant of estrogen receptor {alpha}, and resistance to
tamoxifen treatment in breast cancer. J Clin Oncol. 27:3423–3429.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee AS: Glucose-regulated proteins in
cancer: Molecular mechanisms and therapeutic potential. Nat Rev
Cancer. 14:263–276. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Z and Li Z: Glucose regulated protein
78: A critical link between tumor microenvironment and cancer
hallmarks. Biochim Biophys Acta. 1826:13–22. 2012.PubMed/NCBI
|
|
18
|
Lee AS: GRP78 induction in cancer:
Therapeutic and prognostic implications. Cancer Res. 67:3496–3499.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao YR, Eckhardt BL, Cao Y, Pasqualini R,
Argani P, Arap W, Ramsay RG and Anderson RL: Inhibition of
established micrometastases by targeted drug delivery via cell
surface-associated GRP78. Clin Cancer Res. 19:2107–2116. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng HC, Takahashi H, Li XH, Hara T,
Masuda S, Guan YF and Takano Y: Overexpression of GRP78 and GRP94
are markers for aggressive behavior and poor prognosis in gastric
carcinomas. Hum Pathol. 39:1042–1049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J, Jiang Y, Jia Z, Li Q, Gong W,
Wang L, Wei D, Yao J, Fang S and Xie K: Association of elevated
GRP78 expression with increased lymph node metastasis and poor
prognosis in patients with gastric cancer. Clin Exp Metastasis.
23:401–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng CC, Lu N, Peng CL, Chang CC, Mai FD,
Chen LY, Liao MH, Wang WM and Chang J: Targeting to overexpressed
glucose-regulated protein 78 in gastric cancer discovered by 2D
DIGE improves the diagnostic and therapeutic efficacy of
micelles-mediated system. Proteomics. 12:2584–2597. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luvsandagva B, Nakamura K, Kitahara Y,
Aoki H, Murata T, Ikeda S and Minegishi T: GRP78 induced by
estrogen plays a role in the chemosensitivity of endometrial
cancer. Gynecol Oncol. 126:132–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fernandez PM, Tabbara SO, Jacobs LK,
Manning FC, Tsangaris TN, Schwartz AM, Kennedy KA and Patierno SR:
Overexpression of the glucose-regulated stress gene GRP78 in
malignant but not benign human breast lesions. Breast Cancer Res
Treat. 59:15–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Z, Deng H, Wang X, Yang X, Wang Z and
Liu L: Involvement of ER-α36 in the malignant growth of gastric
carcinoma cells is associated with GRP94 overexpression.
Histopathology. 63:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou J, Deng M, Li X, Liu W, Chu X, Wang J,
Chen F and Meng S: Chaperone gp96 mediates ER-α36 cell membrane
expression. Oncotarget. 6:31857–31867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang L, Guo Y, Zhang X, Meng J and Wang
ZY: A positive cross-regulation of HER2 and ER-α36 controls ALDH1
positive breast cancer cells. J Steroid Biochem Mol Biol.
127:262–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avila MF, Cabezas R, Torrente D, Gonzalez
J, Morales L, Alvarez L, Capani F and Barreto GE: Novel
interactions of GRP78: UPR and estrogen responses in the brain.
Cell Biol Int. 37:521–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang XM, Huang X, Fu ZQ, Zou F, Zhang SK
and Wang ZY: Construction of lentiviral vector-mediated siRNA
knockdown of ER-α36 and its action on gastric cancer cell growth.
Chin J Pathophysiol. 12:2113–2119. 2014.(In Chinese).
|
|
30
|
Wang M, Pan JY, Song GR, Chen HB, An LJ
and Qu SX: Altered expression of estrogen receptor alpha and beta
in advanced gastric adenocarcinoma: Correlation with prothymosin
alpha and clinicopathological parameters. Eur J Surg Oncol.
33:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takano N, Iizuka N, Hazama S, Yoshino S,
Tangoku A and Oka M: Expression of estrogen receptor-alpha and
-beta mRNAs in human gastric cancer. Cancer Lett. 176:129–135.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gan L, He J, Zhang X, Zhang YJ, Yu GZ,
Chen Y, Pan J, Wang JJ and Wang X: Expression profile and
prognostic role of sex hormone receptors in gastric cancer. BMC
Cancer. 12:5662012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang JM, Park S and Kim SJ, Kim H, Lee B,
Kim J, Park J, Kim ST, Yang HK, Kim WH and Kim SJ: KIAA1324
suppresses gastric cancer progression by inhibiting the oncoprotein
GRP78. Cancer Res. 75:3087–3097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Beurel E and Jope RS: The paradoxical pro-
and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic
apoptosis signaling pathways. Prog Neurobiol. 79:173–189. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McCubrey JA, Steelman LS, Bertrand FE,
Davis NM, Sokolosky M, Abrams SL, Montalto G, D'Assoro AB, Libra M,
Nicoletti F, et al: GSK-3 as potential target for therapeutic
intervention in cancer. Oncotarget. 5:2881–2911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dembowy J, Adissu HA, Liu JC, Zacksenhaus
E and Woodgett JR: Effect of glycogen synthase kinase-3
inactivation on mouse mammary gland development and oncogenesis.
Oncogene. 34:3514–3526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grisouard J, Medunjanin S, Hermani A,
Shukla A and Mayer D: Glycogen synthase kinase-3 protects estrogen
receptor alpha from proteasomal degradation and is required for
full transcriptional activity of the receptor. Mol Endocrinol.
21:2427–2439. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Song L, De Sarno P and Jope RS: Central
role of glycogen synthase kinase-3beta in endoplasmic reticulum
stress-induced caspase-3 activation. J Biol Chem. 277:44701–44708.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu ZC, Fu ZQ, Song J, Zhang JY, Wei YP,
Chu J, Han L, Qu N, Wang JZ and Tian Q: Bip enhanced the
association of GSK-3β with tau during ER stress both in vivo and in
vitro. J Alzheimers Dis. 29:727–740. 2012.PubMed/NCBI
|
|
40
|
Lau MY, Han H, Hu J and Ji C: Association
of cyclin D and estrogen receptor α36 with hepatocellular adenomas
of female mice under chronic endoplasmic reticulum stress. J
Gastroenterol Hepatol. 28:576–583. 2013. View Article : Google Scholar : PubMed/NCBI
|