Introduction
Multiple myeloma (MM) is a clonal B cell malignancy
which is characterized by the proliferation of plasma cells (PCs)
within the bone marrow (BM). MM is the second most common
hematologic malignancy, but it remains incurable with survival
duration ranging from a few months to over 10 years (1,2). Although
treatment strategies have evolved from traditional chemotherapy and
autologous hematopoietic stem cell trans-plantation to novel
targeted drug therapy, patient outcomes have not improved (3), and the 5-year survival rate remains only
30–40%, mainly due to the development of drug resistance (4). Therefore, it is important to elucidate
the mechanisms regulating the malignant behavior of MM and identify
key genes contributing to cancer progression for improving patient
prognosis. One hot topic of chromosomal abnormalities and other
types of genetic or epigenetic alterations might contribute to
microRNA (miRNA) deregulation in cancer progression (5–7).
miRNAs, a newly discovered class of endogenous,
small, non-coding RNAs, regulate gene expression by base pairing to
partially or fully complementary sites in the 3′-untranslated
regions (3′-UTRs) of target mRNAs (8). miRNAs are involved in various biological
processes, including cellular growth, development, metabolism,
apoptosis and development (9).
Increasing evidence has demonstrated that differential miRNAs
(miR-21, miR-155, miR-17-92, and miR-125a-5p) have key roles in MM,
suggesting the importance of miRNAs in MM progression (8,10,11). Deregulated miRNAs can act as oncogenes
or tumor suppressors upregulated or downregulated in cancer cells
(12–14). miR-20a has an important in various
human cancers, including gastric cancer (15), colorectal cancer (16), non-small cell lung cancer (17) and cervical cancer (18). Importantly, significant expression of
miR-20 has been found in myeloma (19,20).
Several studies have suggested that the dysregulation of miRNAs
initiates the activation of the phosphatase and tensin homolog
(PTEN)/phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)
signaling pathway, which is involved in the progression of breast
cancer, bladder cancer and non-small-cell lung cancer (21–24).
However, the effects of the PTEN/PI3K/Akt signaling pathway and
miRNA dysregulation on MM pathogenesis and progression remain
unknown. Here, we reported for the first time a miRNA that directly
regulated PTEN: miR-20a acts as a negative regulator of PTEN in MM
cell lines. Downregulation of miR-20a expression inhibited MM cell
growth in vivo. Furthermore, PTEN was identified as a
downstream target gene of miR-20a, which bound to the 3′-UTR of
PTEN. Our study provides new insights into the function of miR-20a
during development and suggests that this miRNA has a role in
tumorigenesis. The overall aim of this study was to investigate the
effects of miR-20a on the cell proliferation, migration and
invasion of MM cells.
Materials and methods
Study subjects and sample
collection
The specimens in this study were obtained from 30
patients who were diagnosed according to the NCCN clinical practice
guidelines for MM at the First Affiliated Hospital of Nanchang
University between October 2015 and May 2017 (25). We obtained plasma samples from 30
patients diagnosed with MM. All patients who were receiving
chemotherapy and/or biotherapy were excluded and those with other
types of malignant tumors were also eliminated. To investigate
miR-20a expression in the different genetic subtypes of MM, 30
cases were cytogenetically classified using FISH (Abbott
Diagnostics, Berkshire, UK). Cytogenetic abnormalities involving
13q deletions and immunoglobulin heavy-chain gene rearrangements
were investigated. The patient details were shown in Tables I and II. 8 plasma samples from healthy people were
used as control in the validation set. Additionally, venous blood
was collected in EDTA tubes (BD Biosciences, Franklin Lakes, NJ,
USA). The plasma was transferred to a fresh tube and stored at
−80°C after freezing with liquid nitrogen. The study was approved
by the Local Research Ethics Committee, and written informed
consent was obtained from all study subjects.
 | Table I.Summary of clinical details of
multiple myeloma patients used for quantitative polymerase chain
reaction analysis of plasma samples (n=30). |
Table I.
Summary of clinical details of
multiple myeloma patients used for quantitative polymerase chain
reaction analysis of plasma samples (n=30).
| Clinical
characteristics | n (%) |
|---|
| Sex |
|
| Male | 21 (70) |
|
Female | 9 (30) |
| Age range | 35–75 |
| Mean | 56.5 |
| Durie salmon
stage |
|
| I | 8 (26.67) |
| II | 5 (16.67) |
| III | 17 (56.67) |
| Karyotype |
|
|
t(4:14) | 10 (33.33) |
|
t(11:14) | 5 (3.33) |
|
t(14:16) | 3 (10) |
| del(13q)
as a unique abnormality | 7 (23.33) |
| Normal FISH | 5 (16.67) |
Cell lines and RNA extraction
Human MM cell lines (MM1S, U266, and RPMI-8226) and
normal plasma cells (nPCs) were cultured in RPMI-1640 (Gibco,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum and 1%
penicillin/streptomycin at 5% CO2 atmosphere. When cells
reached 80% confluency, cells at the logarithmic growth phase were
collected.
RNA isolation and quantitative
RT-PCR
Total RNA was extracted from human plasma and human
MM cell lines using the mirVana Paris RNA Isolation kit (Ambion,
Austin, TX, USA) following the manufacture's instructions for
liquid samples. The concentration and purity of extracted RNA were
measured at 260 and 280 nm optical densities. cDNA was synthesized
from RNAs using gene specific primers (Applied Biosystems, Beijing,
China) with the M-MLV Reverse Transcriptase kit (GeneCopoeia,
Rockville, MD, USA) according to the manufacturer's instructions.
To determine miR-20a expression levels, real-time PCR was performed
with SYBR Green (Takara, Osaka, Japan). β-actin was used as
internal control. PCR conditions were as follows: 95°C for 5 min,
95°C for 45 sec, 55°C for 15 sec, 72°C for 50 sec, for 40 cycles.
The primers used in PCR were as follows: miR-20a forward,
5′-TGCGCTAAAGTGCTTATAGTGC-3′ and reverse,
5′-CCAGTGCAGGGTCCGAGGTATT-3′; PTEN forward,
5′-TTGTGGCAACAGCTGAATCTGCAGTTGGCTAAGAGAGGTT-3′ and reverse,
5′-ATGTAGCAAAACCCTTCGGAAACCTCTCTTAGCCAACTGC-3′; β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGGCACGAAGGCTCATCATT-3′.
Samples were analyzed in triplicate and gene expression was
quantified by normalizing target gene expression to that of the
internal control using the 2−ΔΔCt formula.
Plasmid construction and
transfection
The 5′-flanking regions of the pre-miR-20a-5p and
3′-UTR sequences of PTEN [the mutated (mu) PTEN 3′-UTR was
designated as PTEN] containing the putative miR-20a target sites
were isolated using specific PCR primers. For luciferase assays,
the PTEN 3′-UTR sequence was inserted into the pGL3 luciferase
reporter vector (Promega Corporation, Madison, WI, USA). Target
sites were mutated using the QuickChange Site-Directed Mutagenesis
(Agilent Technologies, Inc., Santa Clara, CA, USA). The miR-20a NC
(5′-CAGUACUUUUGUGUAGUACAA-3′) and the miR-20a inhibitor
(5′-CUACCUGCACUAUAAGCACUUUA-3′) were synthesized by Genepharma
(Shanghai, China). Cell transfection was performed using the
Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions then cells were added
and cultured in complete medium. Cells in the logarithmic growth
phase were seeded in 12-well plates at a density of
1×105 cells, and divided into three groups: miR-20a
inhibitor group (transfected with anti-miR-20a), scramble group
(transfected with small interfering RNA negative control) and blank
group (untransfected cells). Three triplicates were set for each
group. The medium was replaced with normal medium 6 h after
transfection. DMEM containing 10% FBS and G418 was added for
selection 24 h later, and clones were obtained after two weeks. The
selected clones were cultured for the subsequent experiments.
Cell proliferation analysis
For cell proliferation assays, cells were plated
96-well plate (1,500 per well) and examined 48 h after transfection
using the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories,
Shanghai, China). Optical density (OD) values were determined at a
wavelength of 450 nm. The plates were then read on a micro plate
reader using a test wave length of 450 nm. For survival rate assay,
the number of viable cells were counted using Trypan blue
(Solarbio, Beijing, China) on Countstar Cell Counter (Inno-Alliance
Biotech, Inc., Wilmington, NC, USA) according to the manufacturer's
instructions. All assays were performed in triplicate.
Cell migration and invasion
assays
The cells were collected and suspended 72 h after
transfection for the cell migration assays. Then, the cells were
inoculated into the Transwell upper chamber and placed in a 5%
CO2 incubator at 37°C for 48 h. The cells that failed to
penetrate the upper chamber were removed, and the membrane was
fixed in 95% ethanol for 15–20 min and then soaked with water. The
membrane was tinted with crystal violet for 10 min, soaked again in
water, photographed and observed under a high magnification
microscope, followed by cell counting on the back of the membrane.
Five high-power fields were randomly chosen, and the number of
cells penetrating through the polycarbonate membrane was used to
evaluate migration ability. For the invasion assay, Matrigel matrix
was dissolved at 4°C overnight and diluted with 1:3 serum-free DMEM
medium. A total volume of 30 µl of Matrigel was added to the
Transwell upper chamber until it covered the bottom of the upper
chamber. The cell suspension was added to the upper chamber, and
0.5 ml of DMEM containing 10% FBS was added to the lower chamber of
the 24-well plate. The number of cells penetrating through the
Matrigel was determined to assess the cell invasive ability.
Luciferase reporter analysis
For luciferase reporter experiments, the 3′-UTR
segments of PTEN predicted to interact with miR-20a were amplified
by PCR and inserted into pGL3 vector immediately downstream from
the stop codon of luciferase (Promega Corporation). The mutated
(mu) PTEN 3′-UTR without miR-20a binding sites was designated as
PTEN 3′-UTR-mu (forward,
5′-TTGTGGCAACAGCTGAATCTGCAGTTGGCTAAGAGAGGTT-3′ and reverse,
5′-ATGTAGCAAAACCCTTCGGAAACCTCTCTTAGCCAACTGC-3′). To construct the
luciferase reporter vector, 293T cells were cotransfected in
24-well plates with 0.4 mg of the firefly luciferase report vector
and 0.08 mg of the control vector containing Renilla luciferase,
pRL-TK (Promega Corporation), as well as with 100 nM miR-20a
mimics, inhibitor or control miRNA. Luminescence was detected using
the Dual-Luciferase Reporter Assay System (Promega Corporation)
according to the instructions 48 h after transfection. Data were
normalized to the Renilla luminescence and presented relative to
control miRNA transfected group.
Apoptosis analysis
The cells in each group were collected at 24, 48 and
72 h after transfection, and cold PBS was used to wash cells for
three times. The cells were resuspended with 500 µl pre-cooled
binding buffer, and the concentration was adjusted to
5×106 ml. A total of 100 µl of the cell suspension was
added to flow cytometry tubes and 5 µl of Annexin V-fluorescein
isothiocyanate (Beyotime Institute of Biotechnology, Shanghai,
China) was added. After mixture, samples were incubated at room
temperature in the dark for 15 min, and 5 µl of 10 mg/l propidium
iodide (PI) dye (Beyotime Institute of Biotechnology) was added 5
min prior to the measurements. Samples were immediately analyzed
with a FacSort without wash or fixation. Cell Quest FCS 3.0
software (BD Biosciences) was used for data analysis. Each sample
was repeated three times.
Western blotting
Whole cell protein extracts were obtained from MM
cell lines. Cell lysates were loaded and polyacrylamide gel
electrophoresis separated. Proteins were transferred by Trans-Blot
Turbo Transfer Starter System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) for 7 min. After protein transfer, the membranes
were blotted with the primary antibodies. The cells in the
logarithmic growth phase with 80% confluency were collected, and
radio immunoprecipitation assay lysis buffer containing protease
inhibitors (Beyotime Institute of Biotechnology, Haimen, China) was
used for conventional extraction of total cellular protein. A
bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology) was used for protein quantification. An equal amount
of protein was separated by running on 10% SDS gel electrophoresis
under denaturing and nonreducing conditions and then transferred to
a nitrocellulose membrane. 5% skim milk was added to block the
membrane in a sealed container for 1 h. The membranes were
incubated overnight at 4°C using the following antibodies:
anti-PTEN-7 (1:300), anti-p13k (1:1,000), anti-p-p13k (1:500),
anti-AKT (1:500) and anti-p-AKT (1:500) (all from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Anti-β-actin (1:200; Abcam,
Cambridge, MA, USA) was used as a loading control.
Statistical analysis
Each experiment was performed at least three times
and values are reported as mean ± SD. Differences between two
groups were evaluated by a Student's t-test, and differences among
multiple groups were evaluated by one-way analysis of variance and
a Newman-Keuls post-hoc test using SPSS 19.0 software (SPSS, Inc.,
Chicago, IL, USA). P-values <0.05 were considered to indicate a
statistically significant difference.
Results
miR-20a was overexpressed in plasma
from MM patients and MM cell lines
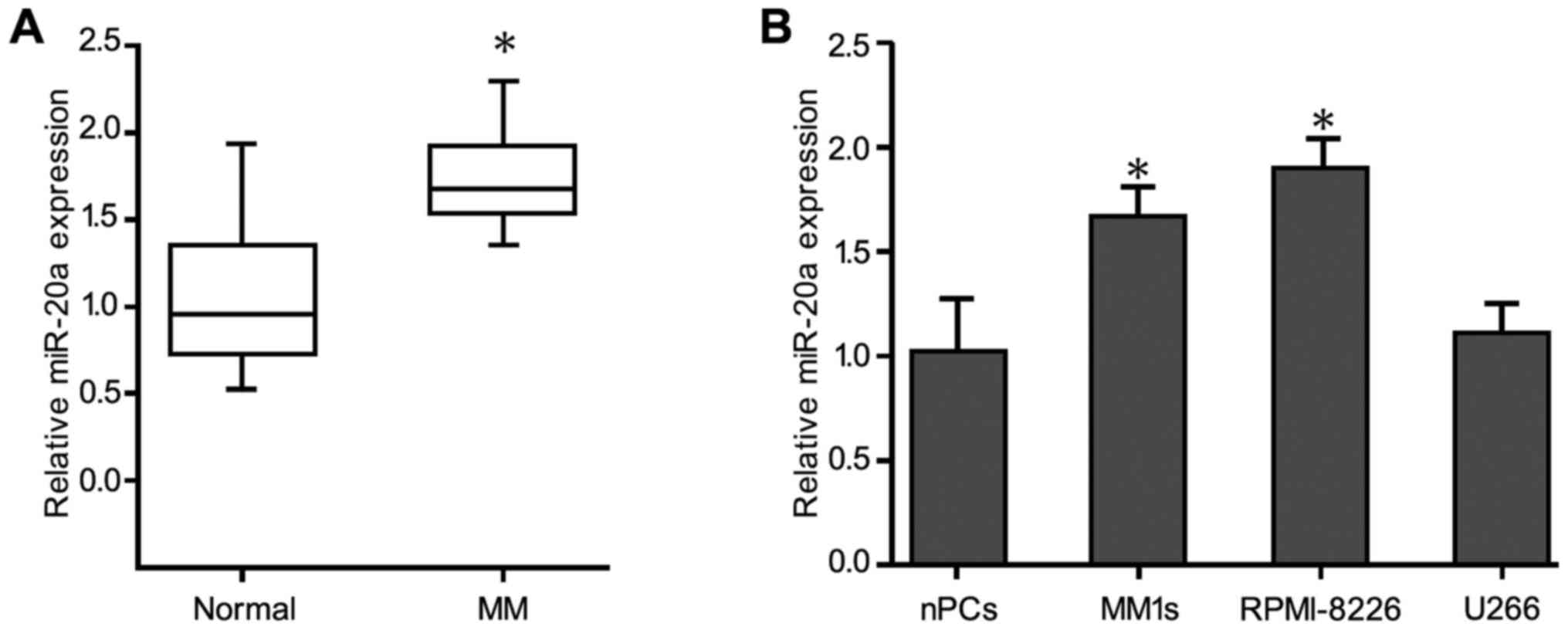
Real-time quantitative PCR revealed that miR-20a was
significantly upregulated in 30 plasma samples from MM patients
compared with healthy control subjects (Fig. 1A). The levels of miR-20a expression in
the MM patients and the control subjects were 1.70±0.02 and
0.94±0.03, respectively, and miR-20a expression in the MM patients
was significantly higher than that in the normal individuals
(P<0.05). Consistent with these data, miR-20a expression in the
U266, MM1 sec, and RPMI-8226 MM cell lines, was significantly
increased compared with the normal healthy bone marrow-derived
plasma cells (Fig. 1B). In addition,
miR-20a expression in stage III patients (1.80±0.31) was
significantly higher than that in stage I/II patients (1.63±0.03)
(P<0.05) (Table I). However,
miR-20a expression was not associated with the patient age, sex or
karyotype (P>0.05) (Table
II).
 | Table II.Relationship between the miR-20a
expression and the clinical pathological characteristics of
patients with multiple myeloma. |
Table II.
Relationship between the miR-20a
expression and the clinical pathological characteristics of
patients with multiple myeloma.
| Characteristics | Cases, % | miR-20a
expression | P-value |
|---|
| Age, years |
|
| 0.053 |
|
≤50 | 14 | 1.67±0.03 |
|
|
>50 | 16 | 1.73±0.04 |
|
| Sex |
|
| 0.112 |
|
Male | 21 | 1.68±0.05 |
|
|
Female | 9 | 1.77±0.05 |
|
| Durie-salmon
stage |
|
| <0.05
(P=0.004) |
| I/II
phase | 13 | 1.63±0.03 |
|
| III
phase | 17 | 1.80±0.31 |
|
| Extramedullary
infiltration |
|
| 0.413 |
|
Yes | 13 | 1.68±0.03 |
|
| No | 17 | 1.72±0.04 |
|
| Karyotype |
|
| 0.282 |
| Normal
FISH | 12 | 1.74±0.03 |
|
|
Abnormal FISH | 18 | 1.68±0.03 |
|
Effect of miR-20a expression on
cellular growth, migration, invasion and apoptosis
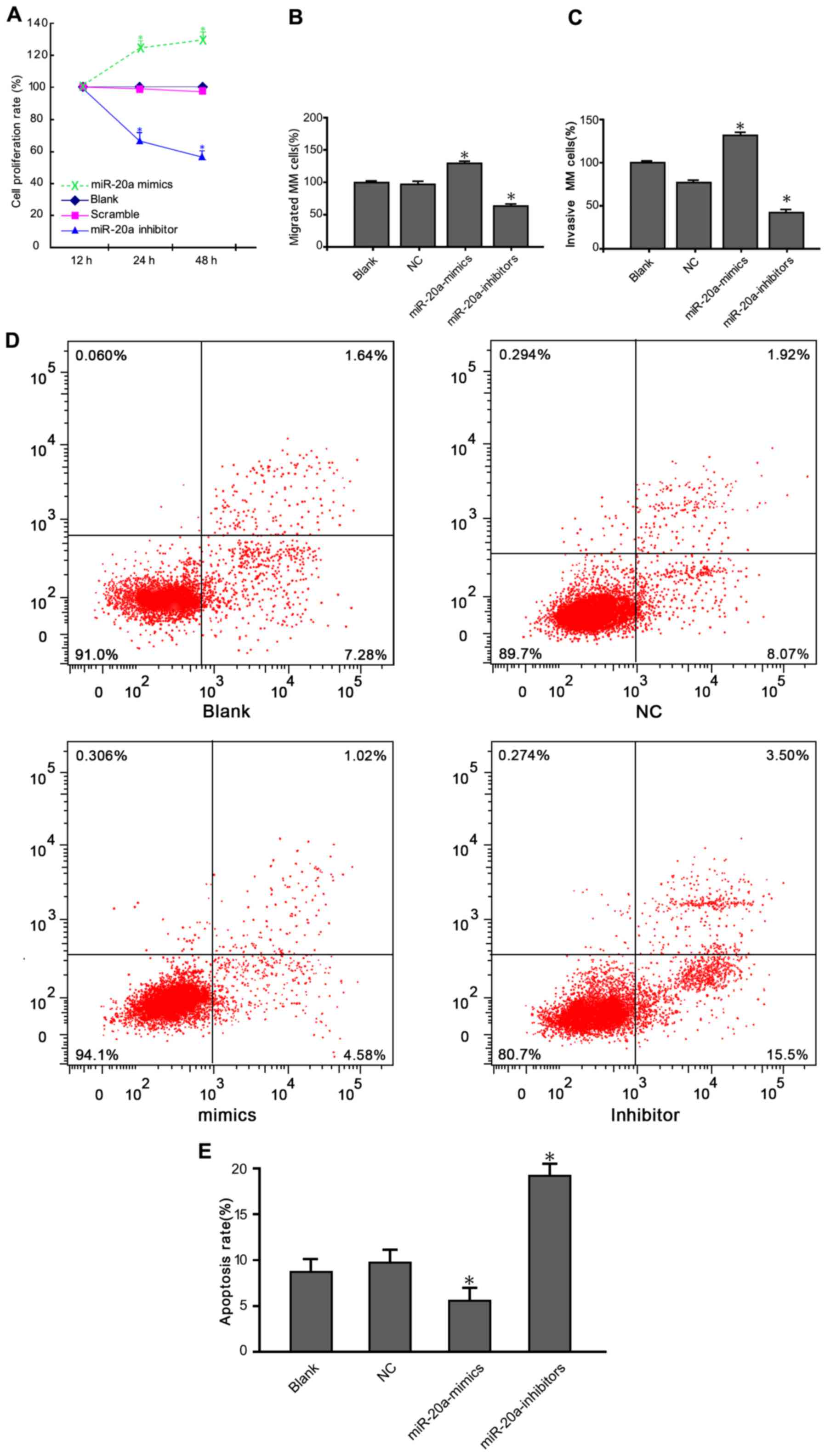
CCK-8 assay results showed that the viability of
U266 and RPMI-8226 cells was reduced following transfection with a
miR-20a inhibitor (Fig. 2A). Cell
growth in the miR-20a inhibitor group was significantly decreased
compared with blank and negative control (NC) groups (P<0.05).
We next investigated whether treatment with the miR-20a inhibitor
inhibited MM cell migration using a Transwell assay. Consistent
with the CCK-8 assays results, cells migration rate transfected
with the miR-20a inhibitor was reduced (Fig. 2B). The number of migrated cells in the
miR-20a inhibitor group was significantly lower than that in the
blank and NC groups (P<0.05). Cell invasion assay showed that
the number of cells penetrating through the Matrigel into the back
of the Transwell membrane was significantly lower in the treatment
group compared with the blank and NC groups (Fig. 2C) (P<0.05). Moreover, apoptosis
rates for the cells in the blank group, NC group, miR-20a mimics
group and miR-20a-inhibitor group was 8.92, 9.99, 5.62 and 19.9% 48
h after transfection, respectively (Fig.
2D). Compared with the two control groups, the apoptotic rate
in the miR-20a-inhibitor group was significantly increased
(P<0.05) (Fig. 2E).
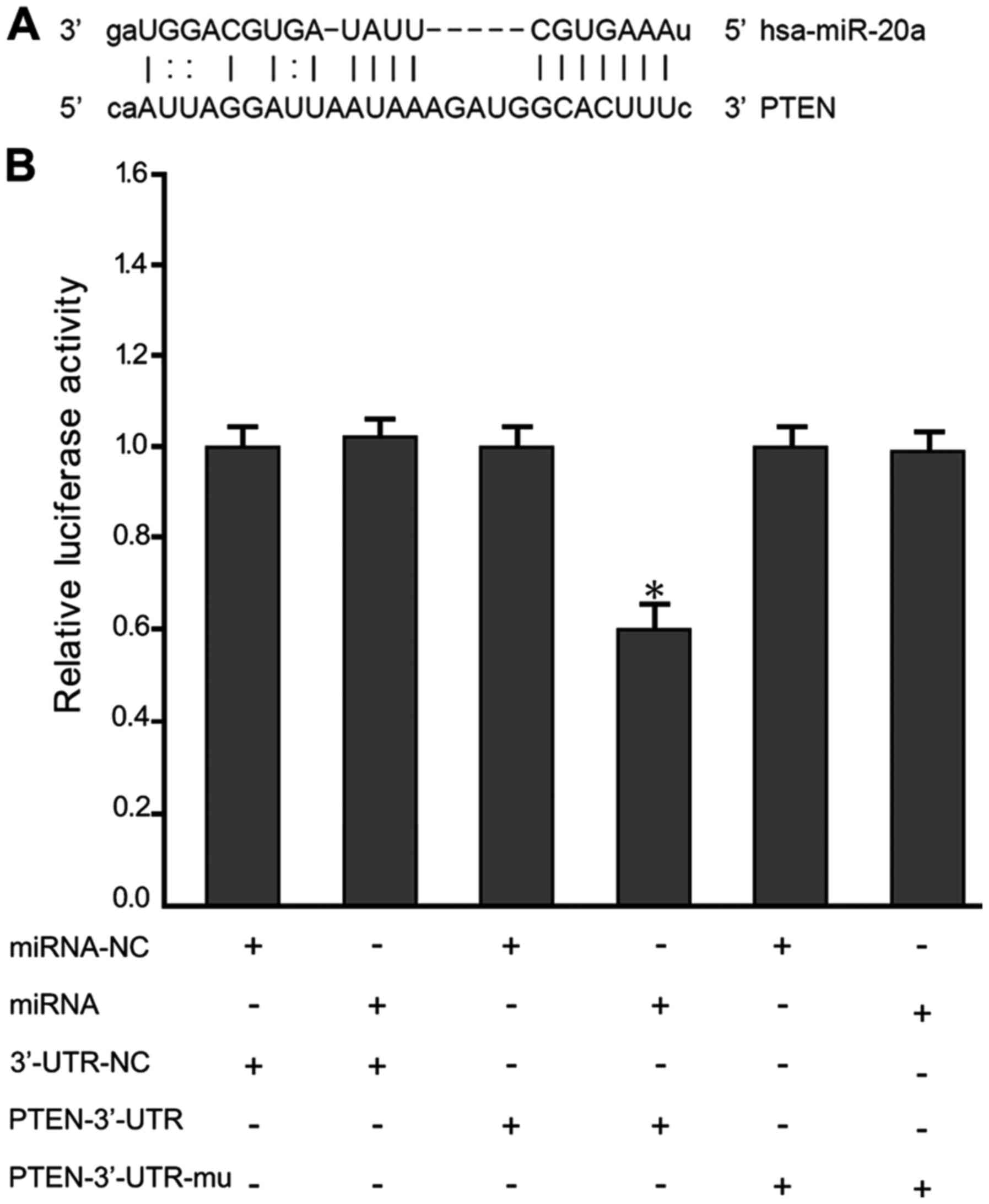
PTEN as a target gene of miR-20a
To further explore the mechanisms that miR-20a
regulated MM cell growth and metastasis, we identified candidate
targets of miR-20a using the TargetScan program. Among the
identified genes, we chose to further investigate PTEN (Fig. 3A). To determine whether miR-20a binds
to the 3′-UTR of PTEN, we utilized a luciferase reporter vector
containing 3′-UTR of PTEN. As expected, miR-20a directly bound to
the 3′-UTR and remarkably reduced the vector's luciferase activity.
In contrast, cells with mutant PTEN 3′-UTRs displayed much higher
luciferase activity (Fig. 3B).
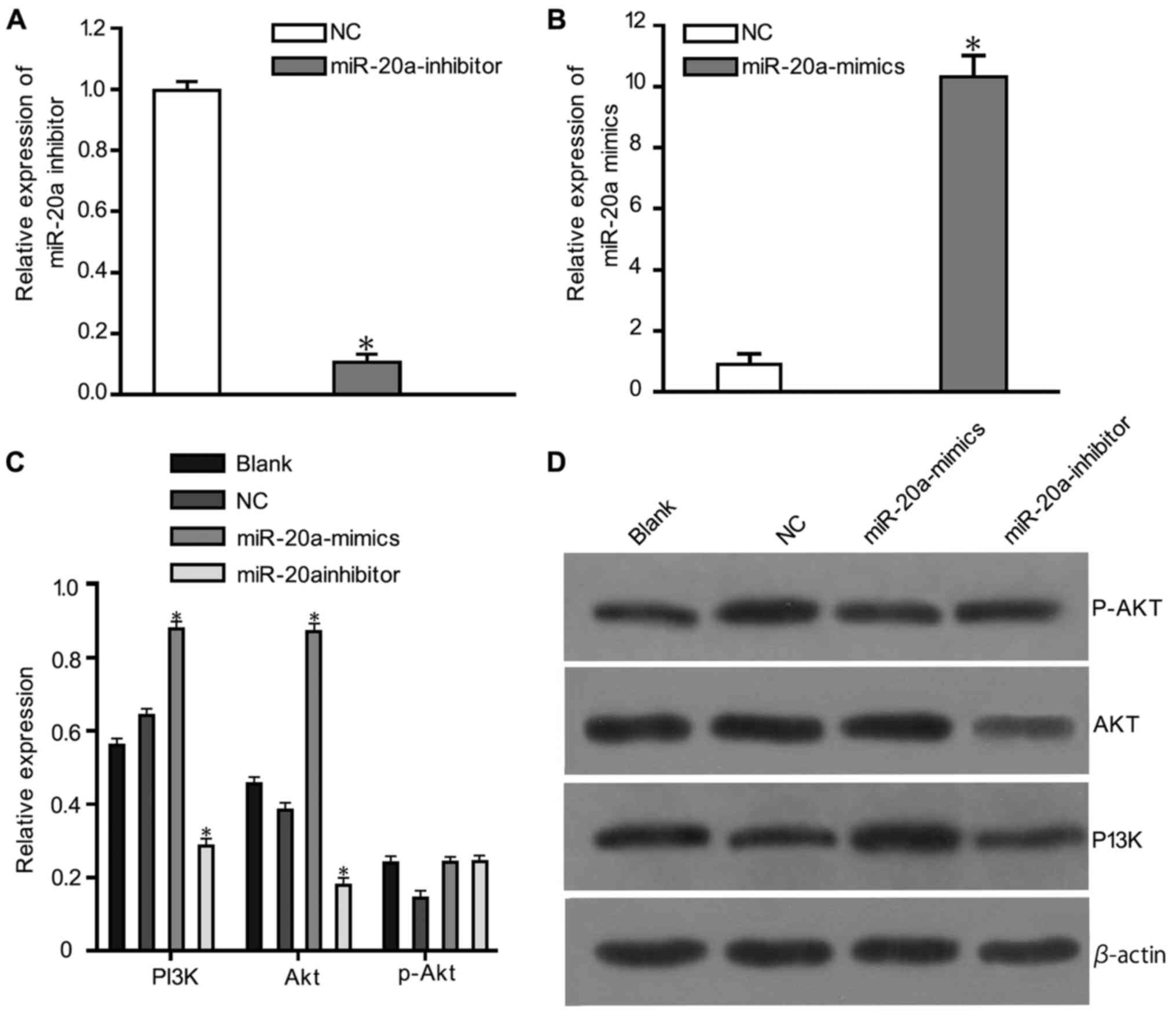
PTEN, PI3K, AKT and p-AKT protein
expression
The relative expression of miR-20a and inhibitor was
shown in Fig. 4A and B. Western
blotting results (Fig. 4) showed that
PTEN expression in the miR-20a-mimic group was downregulated
compared with the blank and NC groups (P<0.05). p-PI3K and p-Akt
expression levels were also markedly downregulated in the
miR-20a-mimics group compared with the blank and NC groups
(P<0.05). There were no statistically significant differences in
the protein expression between the blank and NC groups.
Discussion
Increasing studies focusing the molecular biology of
cancer have revealed that the PTEN/PI3K/AKT pathway is an important
oncogenic pathway that is frequently activated during
tumorigenesis, and plays a crucial role in the growth,
proliferation, migration, and invasion of malignant cells (26,27). MM is
a plasma cell disorder with a relatively high incidence rate among
hematological malignancies. Despite significant progress in
elucidating the biology of MM and identifying treatment options,
the disease remains incurable, highlighting the urgent need for
digging novel and effective therapies (28). As miRNAs have important functions in
many cancers, therapeutic modulation of miRNAs might be a valuable
strategy for cancer study. The overall aim of current study was to
explore the roles of miR-20a and the PTEN/PI3K/AKT signaling
pathway in the proliferation, migration and invasion of MM cells.
To the best of our knowledge, this was the first examination has
been reported in the present day.
Initially, we demonstrated that miR-20a was
upregulated in plasma from MM patients and MM cells. Notably,
miR-20a, a member of the miR-17-92 cluster, has been shown to
function as an oncomir in many human cancers. A study performed by
Wang et al demonstrated that miR-20a is highly expressed in
brainstem gliomas and potentially associated with gliomagenesis
(29). Li et al have reported
that miR-20a expression was remarkably increased in the breast
cancer of patients (30). Moreover,
previous research has also shown that high levels of miR-20a are
associated with shortened progression-free survival (PFS) (31). In the current study, miR-20a
expression in plasma was significantly higher in stage III MM
patients than stage I/II patients, suggesting a key role for this
miRNA in survival prediction. Considering endogenously expression
of miR-20a in stage III MM patients, we chose to utilize RPMI-8226
cells for subsequent loss-of-function studies. Our results showed a
marked reduction in RPMI-8226 cell viability after transfection of
with a miR-20a inhibitor. The transfected cells also showed
inhibited migration, however, apoptosis was promoted. These data
suggests that the upregulation of miR-20a in MM is related to the
development and progression of the disease.
Recent research has shown that activation of
PI3K/AKT reduces the p21Cip1 levels and increases CCND1 expression
(32,33), which regulates cell cycle progression
through the G1 phase. In addition, activated PI3K can catalyze
3,4-phosphatidylinositol trisphosphate phosphorylation and
subsequently activate the protein kinase AKT to promote cell growth
and proliferation (26). PTEN, a
tumor suppressor gene, was confirmed as a target of miR-20a by a
luciferase assay. Notably, one prior study speculated that miR-20a
plays an oncogenic role in hepatocellular carcinoma, at least
partially by negatively regulating PTEN (27). In the present study, we found that
PTEN expression decreased after overexpression of miR-20a,
suggesting that PTEN is regulated by miR-20a. These data were
further confirmed by a luciferase activity assay. Our results
suggest that PTEN is a direct target of miR-20a. The PTEN/PI3K/AKT
signaling pathway is known to have important roles in regulating
biological processes including cell growth and proliferation,
metabolism, and apoptosis, which makes the pathway an attractive
candidate for drug discovery (34).
Chen et al reported that PTEN and activated AKT are
associated with intrahepatic metastasis, tumor grade, and a high
proliferation index. Furthermore the PI3K/PTEN/AKT pathway is a
diagnostic and prognostic indicator of invasion and metastasis
hepatocellular carcinoma as well as a therapeutic target for this
cancer (35). Our current correlation
analyses of miR-20a, PTEN, PI3K and Akt proteins expression also
confirmed the relationships existing among these proteins.
In conclusion, we report that miR-20a regulates the
proliferation and migration of MM cells by modulating the
PTEN/PI3K/AKT signaling pathway. Meanwhile, this pathway may serve
as a novel therapeutic target for MM. Unraveling the relationship
between miR-20a and the PTEN/PI3K/Akt signaling pathway may help
identify new molecular markers and potential therapeutic targets
that will provide a theoretical basis for future studies. The
effects of miR-20a on MM cell proliferation, migration and invasion
should be confirmed using animal models in future study. Performing
such experiment will bring us closer to the goal of developing new
genetic and therapeutic strategies for treating MM.
Acknowledgements
International Collaboration Fund from National
Science and Technology Committee of China (no. 2011DFA32820).
Innovation fund project in Jiangxi Province (no. YC2016-B018). The
National Natural Science Fund Project (no. 81460037).
References
|
1
|
Sonneveld P, Avet-Loiseau H, Lonial S,
Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle
RA, et al: Treatment of multiple myeloma with high-risk
cytogenetics: A consensus of the international myeloma working
group. Blood. 127:2955–2962. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abdi J, Chen G and Chang H: Erratum: Drug
resistance in multiple myeloma: Latest findings and new concepts on
molecular mechanisms. Oncotarget. 6:73642015.PubMed/NCBI
|
|
3
|
Mimura N, Hideshima T and Anderson KC:
Novel therapeutic strategies for multiple myeloma. Exp Hematol.
43:732–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kumar SK, Lee JH, Lahuerta JJ, Morgan G,
Richardson PG, Crowley J, Haessler J, Feather J, Hoering A, Moreau
P, et al: Risk of progression and survival in multiple myeloma
relapsing after therapy with IMiDs and bortezomib: A multicenter
international myeloma working group study. Leukemia. 26:149–157.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lionetti M, Biasiolo M, Agnelli L,
Todoerti K, Mosca L, Fabris S, Sales G, Deliliers GL, Bicciato S,
Lombardi L, et al: Identification of microRNA expression patterns
and definition of a microRNA/mRNA regulatory network in distinct
molecular groups of multiple myeloma. Blood. 114:e20–e26. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNAs and
chromosomal abnormalities in cancer cells. Oncogene. 25:6202–6210.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo X, Gu J, Zhu R, Feng M, Zhu X, Li Y
and Fei J: Integrative analysis of differential miRNA and
functional study of miR-21 by seed-targeting inhibition in multiple
myeloma cells in response to berberine. BMC Syst Biol. 8:822014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seckinger A, Meißner T, Moreaux J, Benes
V, Hillengass J, Castoldi M, Zimmermann J, Ho AD, Jauch A,
Goldschmidt H, et al: miRNAs in multiple myeloma-a survival
relevant complex regulator of gene expression. Oncotarget.
6:39165–39183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leotta M, Biamonte L, Raimondi L,
Ronchetti D, Di Martino MT, Botta C, Leone E, Pitari MR, Neri A,
Giordano A, et al: A p53-dependent tumor suppressor network is
induced by selective miR-125a-5p inhibition in multiple myeloma
cells. J Cell Physiol. 229:2106–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chi J, Ballabio E, Chen XH, Kušec R,
Taylor S, Hay D, Tramonti D, Saunders NJ, Littlewood T, Pezzella F,
et al: MicroRNA expression in multiple myeloma is associated with
genetic subtype, isotype and survival. Biol Direct. 6:232011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esquela-Kerscher A: The lin-4 microRNA:
The ultimate micromanager. Cell Cycle. 13:1060–1061. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garzon R and Marcucci G: Potential of
microRNAs for cancer diagnostics, prognostication and therapy. Curr
Opin Oncol. 24:655–659. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang R, Fu Y, Zeng Y, Xiang M, Yin Y, Li
L, Xu H, Zhong J and Zeng X: Serum miR-20a is a promising biomarker
for gastric cancer. Biomed Rep. 6:429–434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang G, Chen X, Cai Y, Wang X and Xing C:
miR-20a-directed regulation of BID is associated with the TRAIL
sensitivity in colorectal cancer. Oncol Rep. 37:571–578. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Mao F, Shen T, Luo Q, Ding Z,
Qian L and Huang J: Plasma miR-145, miR-20a, miR-21 and miR-223 as
novel biomarkers for screening early-stage non-small cell lung
cancer. Oncol Lett. 13:669–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong Y, Sun F, Dong P, Watari H, Yue J,
Yu MF, Lan CY, Wang Y and Ma ZB: iASPP induces EMT and cisplatin
resistance in human cervical cancer through miR-20a-FBXL5/BTG3
signaling. J Exp Clin Cancer Res. 36:482017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peng J, Thakur A, Zhang S, Dong Y, Wang X,
Yuan R, Zhang K and Guo X: Expressions of miR-181a and miR-20a in
RPMI8226 cell line and their potential as biomarkers for multiple
myeloma. Tumour Biol. 36:8545–8552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Corrigan-Cummins M, Barber EA,
Saleh LM, Zingone A, Ghafoor A, Costello R, Zhang Y, Kurlander RJ,
Korde N, et al: Aberrant levels of miRNAs in bone marrow
microenvironment and peripheral blood of myeloma patients and
disease progression. J Mol Diagn. 17:669–678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo M, Tan X, Mu L, Luo Y, Li R, Deng X,
Chen N, Ren M, Li Y, Wang L, et al: MiRNA-21 mediates the
antiangiogenic activity of metformin through targeting PTEN and
SMAD7 expression and PI3K/AKT pathway. Sci Rep. 7:434272017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu MH, Yang L, Liu XJ, Nie ZY and Luo JM:
Targeted suppression of miRNA-21 inhibit K562 cells growth through
PTEN-PI3K/AKT signaling pathway. Zhonghua Xue Ye Xue Za Zhi.
37:982–986. 2016.(In Chinese). PubMed/NCBI
|
|
23
|
Yang X, Cheng Y, Li P, Tao J, Deng X,
Zhang X, Gu M, Lu Q and Yin C: A lentiviral sponge for miRNA-21
diminishes aerobic glycolysis in bladder cancer T24 cells via the
PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 36:383–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang F, Li L, Chen Z, Zhu M and Gu Y:
MicroRNA-214 acts as a potential oncogene in breast cancer by
targeting the PTEN-PI3K/Akt signaling pathway. Int J Mol Med.
37:1421–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anderson KC: Progress and paradigms in
multiple myeloma. Clin Cancer Res. 22:5419–5427. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Z, Fang S, Di Y, Ying W, Tan Y and Gu
W: Modulation of NF-κB/miR-21/PTEN pathway sensitizes non-small
cell lung cancer to cisplatin. PLoS One. 10:e01215472015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Zheng L, Ding Y, Li Q, Wang R,
Liu T, Sun Q, Yang H, Peng S, Wang W and Chen L: MiR-20a induces
cell radioresistance by activating the PTEN/PI3K/Akt signaling
pathway in hepatocellular carcinoma. Int J Radiat Oncol Biol Phys.
92:1132–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bates SE: Multiple myeloma: Multiplying
therapies. Clin Cancer Res. 22:54182016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Zhang H, Zhang A, Han L, Wang K,
Liu R, Yang S, Pu P, Shen C, Kang O and Yu C: Upregulation of
miR-20a and miR-106b is involved in the acquisition of malignancy
of pediatric brainstem gliomas. Oncol Rep. 28:1293–1300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li S, Qiang Q, Shan H, Shi M, Gan G, Ma F
and Chen B: miR-20a and miR-20b negatively regulate autophagy by
targeting RB1CC1/FIP200 in breast cancer cells. Life Sci.
147:143–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao X, Zhang R, Qu X, Zhao M, Zhang S, Wu
H, Jianyong L and Chen L: MiR-15a, miR-16-1 and miR-17-92 cluster
expression are linked to poor prognosis in multiple myeloma. Leuk
Res. 36:1505–1509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Medema RH, Kops GJ, Bos JL and Burgering
BM: AFX-like Forkhead transcription factors mediate cell-cycle
regulation by Ras and PKB through p27kip1. Nature. 404:782–787.
2000. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Roy SK, Srivastava RK and Shankar S:
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of
FOXO transcription factor, leading to cell cycle arrest and
apoptosis in pancreatic cancer. J Mol Signal. 5:102010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Carnero A, Blanco-Aparicio C, Renner O,
Link W and Leal JF: The PTEN/PI3K/AKT signalling pathway in cancer,
therapeutic implications. Curr Cancer Drug Targets. 8:187–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen JS, Wang Q, Fu XH, Huang XH, Chen XL,
Cao LQ, Chen LZ, Tan HX, Li W, Bi J and Zhang LJ: Involvement of
PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: Association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|