Introduction
Resveratrol (3,5,4′-trihydroxy-trans-stilbene;
Fig. 1) is a polyphenolic phytoalexin
that is naturally present in food products, including grapes,
mulberries and peanuts (1), with
demonstrated cardioprotective (2),
neuroprotective (3) and
anti-inflammatory effects (4).
Although these effects are partly attributed to its antioxidant
properties, resveratrol has also been demonstrated to produce
anti-carcinogenic effects (5). A
number of previous studies have revealed the anti-proliferative and
apoptotic effects of resveratrol in various cancer cell lines
including breast (6–9), lung and prostate cancer (10–12).
Human cervical carcinoma is the most common type of
cancer in females, accounting for ~8% of all newly diagnosed cancer
cases globally (13). However, the
effects of resveratrol on the regulation of human cervical
carcinoma, and the mechanisms underlying such effects, remain to be
established. In the present study, resveratrol treatment induced
apoptosis in the HeLa human cervical cancer cell line. Furthermore,
resveratrol activated the mitochondrial apoptotic signaling pathway
and upregulated the expression of caspase-3 and −9. In addition,
resveratrol was able to induce cell cycle arrest at the
G2 phase and the expression of p53 was upregulated in
resveratrol-treated HeLa cells. These data indicate that
resveratrol is able to induce cell death in HeLa human cervical
cancer cells through various potential underlying mechanisms.
Materials and methods
Reagents and cell lines
Resveratrol was purchased from The National
Institute for the Control of Pharmaceutical and Biological Products
(Beijing, China), and dissolved in sterile dimethyl sulfoxide
(DMSO) to prepare a stock solution, which was further diluted in
fresh Dulbecco's Modified Eagle's Medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and the final
concentrations of resveratrol used were 0, 5, 10, 20 or 40 µM. The
final concentration of DMSO in all cell cultures was <0.01%. P53
antibody (cat. no. 38007) was purchased from Signalway Antibody
(College Park, MA, USA) and B-cell lymphoma 2 (Bcl-2) (cat. no.
sc-7382), Bcl-2-associated X protein (Bax) (cat. no. sc-7480),
Bcl-extra large (XL; cat. no. sc-8392), caspase-3 (cat. no.
sc-7272), β-actin (cat. no. sc-8432) and were purchased from Santa
Cruz Biotechnology, Inc. (Dallas, TX, USA). Goat anti-mouse
horseradish peroxidase (HRP)-conjugated antibody (cat. no. G-21040;
dilution, 1:1,000) and protein ladder (cat. no. SM0671) were
purchased from Thermo Fisher Scientific, Inc. The P53 antibody was
diluted at a ratio of 1:1,000 when the antibody incubated with the
target protein in the NC membrane, while the primary antibodies
were diluted at a ratio of 1:500. The experimental protocol was
approved by the Research Ethics Committee of Nanjing University of
Chinese Medicine Medical University (Nanjing, China).
Cell culture
The HeLa human cervical cancer line was purchased
from the American Type Culture Collection (Manassas, VA, USA) and
grown in DMEM, supplemented with 10% (v/v) heat-inactivated fetal
bovine serum (FBS), 2 mmol/L-glutamine, 100 U/ml penicillin, and
100 U/ml streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc.). The HeLa cells were incubated at 37°C in a humidified
atmosphere with 5% carbon dioxide. The HeLa cells were subject to
MTT assay upon reaching 70–80% confluency.
Assessment of cell viability using an
MTT assay
Upon reaching ~80% confluency, the HeLa cells were
treated with resveratrol in complete medium for dose-dependent and
time-dependent studies. In the control group, the HeLa cells were
treated with DMSO. The cytotoxicity-based MTT assay was performed
according to previous reference (14). Briefly, 200 µl of complete culture
medium containing 2×104 cells was added to 96-well
microtiter plates and incubated with different concentrations (0,
5, 10, 20 or 40 µM) resveratrol at 37°C in a humidified incubator.
After 12, 24, 36 and 48 h of incubation with resveratrol, MTT was
added and the cell samples were monitored at a wavelength of 595 nm
on a scanning multiwell spectrophotometer. The effect of
resveratrol on growth inhibition was assessed as the percentage of
cell viability, and control cells treated with DMSO were considered
100% viable. Each assay was replicated 4 times and each experiment
was repeated ≥3 times.
Hoechst 33342 staining
The HeLa cells were seeded onto coverslips in 6-well
plates at 5×104 cells per well and incubated at 37°C.
When the cells grown about 70–80% confluency, they were treated
with 20 µM resveratrol. After an incubation at 37°C 48 h, the
medium was aspirated and the coverslips were washed with PBS and
treated with 20 µg/ml Hoechst 33342 at 37°C for 15 min.
Subsequently, the cells were washed with PBS and observed under a
fluorescence microscope (Olympus Corporation, Tokyo, Japan).
Apoptosis detection
Cell apoptosis was evaluated using flow cytometry to
quantify the levels of detectable phosphatidylserine on the outer
membranes of apoptotic cells as previously reported (15). Briefly, the cells were grown to a
density of 2×105 cells in 100 mm culture dishes and
treated with 20 µM resveratrol for 48 h. The HeLa cells were
trypsinized and washed twice with PBS, and then floating and
adherent cells were collected and suspended in binding buffer (cat.
no. 556547; BD Pharmingen; BD Biosciences, Franlink Lakes, NJ, USA)
at 2×105 cells per 100 µl binding buffer. Subsequently,
the HeLa cells were stained using 5 µl Annexin V-fluorescein
isothiocyanate (FITC; cat. no. 556547; BD Pharmingen; BD
Biosciences) in the dark at room temperature for 15 min. Next, the
cells were resuspended in 300 µl binding buffer and stained with 10
µl propidium iodide (PI; 50 µmol/l). Annexin V/PI fluorescence was
analyzed immediately with a flow cytometer. Per sample, ≥10,000
events were counted, and the data presented as the proportion of
early apoptotic cells (FITC+/PI-) and late apoptotic cells
(FITC+/PI+). The percentages of cells in the upper-right phase
(late apoptotic cells), upper-left phase (necrotic cells),
lower-right phase (early-apoptotic cells) and lower-left phase
(viable cells) panels of the resulting histogram were calculated
for comparison among these groups (Fig.
4).
Caspase-3 and −9 activity assay
Caspase-3 or −9 activity was determined using
Caspase Activity Assay kits (Biolife Co., Ltd., Beijing, China).
Briefly, the cells were treated with 0, 5, 10, 20 or 40 µM
resveratrol for 48 h, washed twice with PBS, and digested with
0.25% trypsin-EDTA at 37°C for 1 min. The cells were collected at a
density of 3×106 by centrifugation at 100 × g at 4°C for
3 min and suspended in 100 µl of ice-cold lysis buffer (cat. no.
89900; Thermo Fisher Scientific, Inc.). The cell lysates were
incubated on ice for 10 min and centrifuged at 900 × g for 10 min.
The cell supernatants were collected and the protein concentration
was determined using the Bradford method, based on standard
reference BSA protein concentrations. The supernatant proteins (50
µg) were added to 96-well plates and incubated with caspase-3 and
−9 colorimetric substrate (Biolife Co., Ltd.) for 2 h at 37°C.
Subsequently, the absorbance was measured at a wavelength of 405 nm
using a microplate reader (Titertek Multiskan Plus, Labsystems,
Finland). All experiments were performed in triplicate.
Western blot analysis
Protein samples were dissolved in a sample buffer
containing 0.5 M Tris hydrochloride (pH 6.8), 20% glycerol, 2% SDS,
0.5% bromophenol blue and 10 mM dithiothreitol. The protein
concentrations of cell lysates were evaluated according to the
Bradford method using bovine serum albumin as a standard. The
proteins (40–100 µg) were separated by 12% SDS-polyacrylamide gel
electrophoresis, blotted on nitrocellulose (NC) membranes, and
blocked with a solution of 5% (w/v) skimmed milk powder and 0.1%
(w/v) Tween-20 in PBS (pH 7.5) for 1 h at room temperature. The NC
membranes were incubated with the primary antibodies for 2 h at
room temperature or overnight at 4°C. Subsequently, the membranes
were washed with PBS and incubated with the secondary antibody for
2 h at 4°C (16). Next, HRP substrate
(dilution, 1:1,000) was added and the NC membranes were imaged
(Kodak, Japan). The intensities of the bands were quantitated using
Image Lab™ Image Analysis software on a Gel
Doc™ system (version no. 170-8195; Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Molecular weight-markers were
electroblotted and analyzed simultaneously.
Statistical analysis
All data were representative of ≥3 independent
experiments and are presented as the mean ± standard deviation. The
data were analyzed using SPSS version 11.5 for Windows (SPSS, Inc.,
Chicago, IL, USA). One-way analysis of variance or the Student's
t-test was used to compare between the treatment and control
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
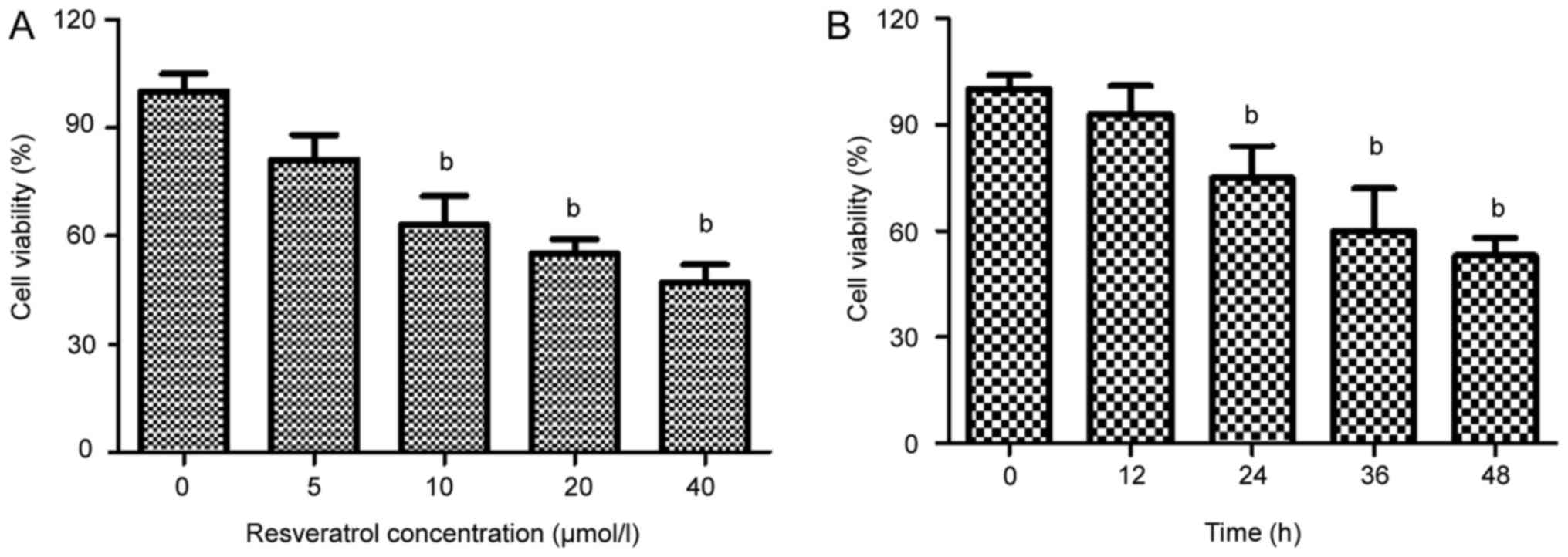
Resveratrol inhibits the proliferation
of HeLa cells
To identify the effects of resveratrol on HeLa cell
survival, the cells were cultured in medium containing various
concentrations of resveratrol (0–40 µmol/l) for 48 h. Resveratrol
treatment induced a concentration-dependent reduction in the
viability of the HeLa cells (Fig.
2A). The survival rate of the HeLa cells treated with 20 µmol/l
resveratrol was reduced after 24 h (Fig.
2B). On the basis of the MTT assay results, resveratrol
treatment at concentrations of 20, 40 and 60 µmol/l, and a 48 h
incubation period, was selected for further mechanistic
studies.
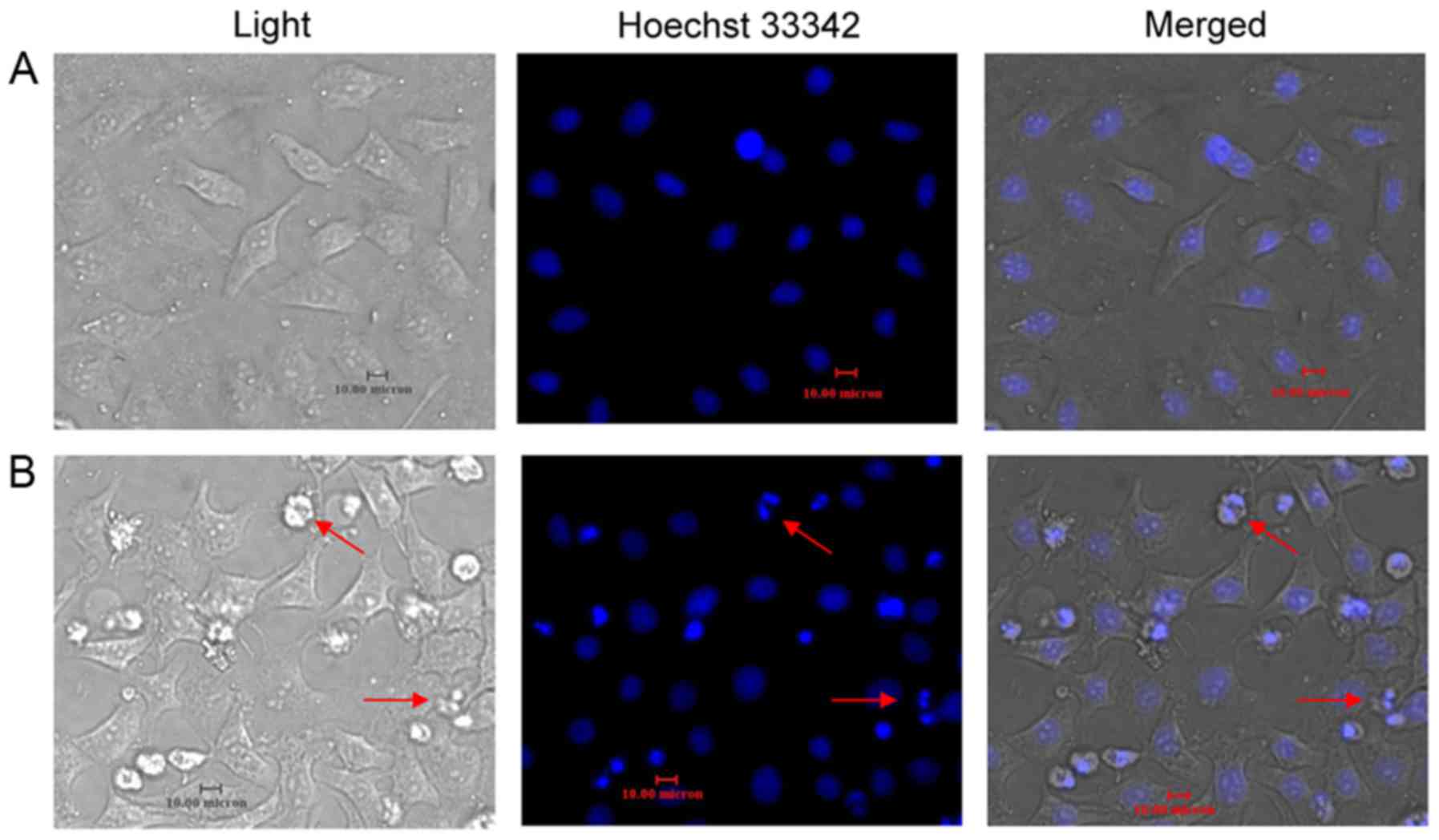
Resveratrol induces HeLa cell
apoptosis
After 48 h of incubation with resveratrol (20
µmol/l), Hoechst 33342 staining revealed increased apoptotic bodies
and nuclear condensations in the HeLa cells (Fig. 3). Apoptosis was quantified by
flow-cytometric analysis using Annexin V FITC/PI double staining in
the HeLa cells treated with 20 µmol/l resveratrol at various time
points. Resveratrol (0–40 µmol/l) produced a
concentration-dependent increase in the apoptotic cell population
after 48 h of incubation. After 24 h of treatment with resveratrol,
the counts of early (lower-right quadrant) and late (upper-right
quadrant) apoptotic cells were increased in a
concentration-dependent manner (Fig.
4). Almost 35% of the HeLa cells had advanced to late apoptotic
stages upon treatment with 60 µmol/l resveratrol, compared with the
vehicle-treated controls (Fig.
4).
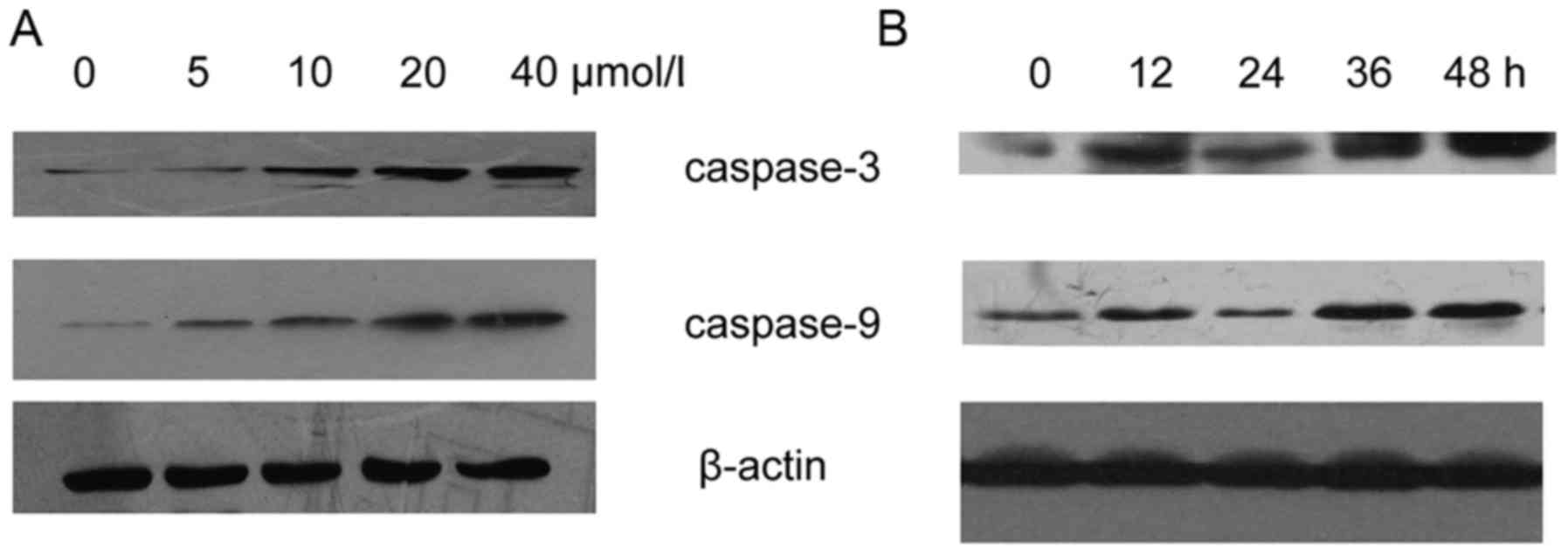
Resveratrol upregulates the expression
and activity of caspase-3 and −9 in HeLa cells
Following treatment with varying concentrations of
resveratrol for 48 h, the expression levels of caspase-3 and −9
were increased in the HeLa cells (Fig.
5A). The expression levels of caspase-3 and −9 were upregulated
in the resveratrol-treated (20 µmol/l) HeLa cells (Fig. 5B). Activation of caspase cascades was
observed in the process of apoptosis. Subsequently, the HeLa cells
were incubated with various concentrations of resveratrol for 48 h
to examine caspase-3 and −9 activity. The results revealed that
caspase-3-like and caspase-9-like cysteine protease activity
increased during the process of resveratrol-induced apoptosis
(Fig. 6).
Resveratrol alters the expression
levels of Bcl-2 family proteins in the HeLa cells
The effect of resveratrol on the expression levels
of the pro-apoptotic protein Bax and the anti-apoptotic proteins
Bcl-2 and Bcl-XL was examined. Following treatment with
resveratrol, the expression levels of Bax were increased, whereas
the expression levels of Bcl-2 and Bcl-XL were decreased in a
dose-dependent and time-dependent manner in the HeLa cells
(Fig. 7).
 | Figure 7.Western blot analysis of Bax, Bcl-2
and Bcl-XL protein expression levels in resveratrol-treated HeLa
cells. (A) Cells were exposed to 0, 5, 10, 20 and 40 µmol/l of
resveratrol for 48 h. (B) Cells were treated with 20 µmol/l
resveratrol for 0, 12, 24, 36 and 48 h. All cells were lysed, equal
quantities of protein were separated using 12% SDS-PAGE and protein
expression was detected by western blotting. Bcl-2, B-cell lymphoma
2; Bax, B-cell lymphoma 2-associated X protein; Bcl-2 XL, B-cell
lymphoma 2-extra large. |
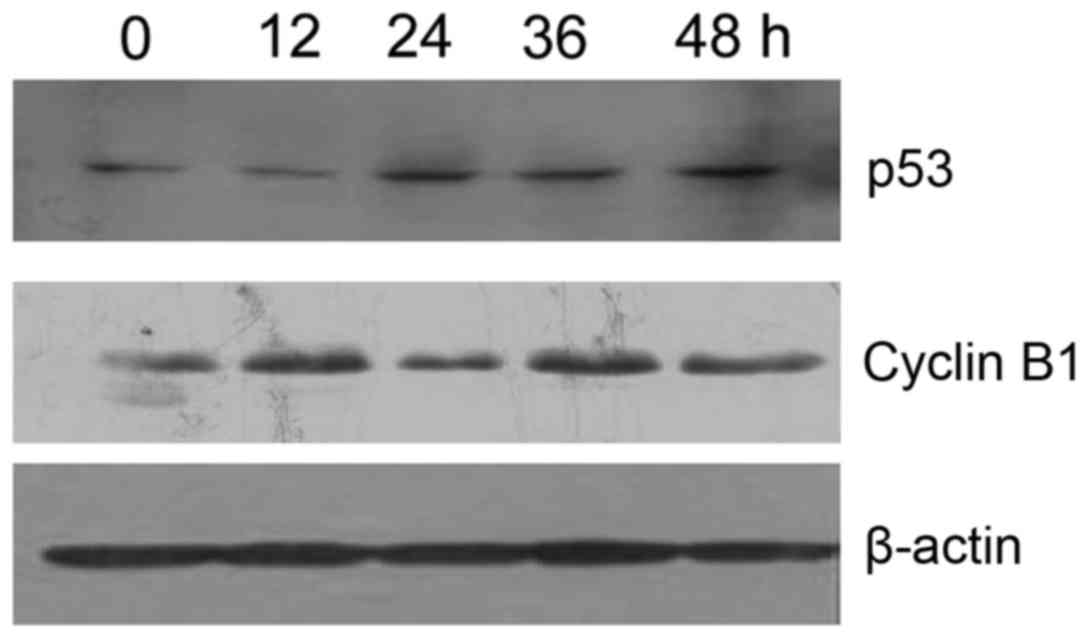
Resveratrol upregulates p53 expression
and downregulates cyclin B1 expression in HeLa cells
Western blot analysis revealed that p53 expression
levels were gradually increased and Cyclin B1 expression levels
were decreased (P<0.05) in a time-dependent manner in the
resveratrol-treated (20 µmol/l) HeLa cells, as compared with in the
vehicle control cells (Fig. 8).
Discussion
Chemotherapy using non-toxic natural components is a
promising therapeutic strategy for the treatment of carcinoma.
Recently, numerous studies have attempted to identify novel
anticancer treatments obtained from natural products (17). Resveratrol, a natural polyphenolic
phytoalexin, has been demonstrated to possess antioxidant,
anti-atherosclerotic, anti-inflammatory (18) and anticancer properties (19–21). The
chemopreventive efficacy of resveratrol has been identified in
hepatocellular, lung, skin and prostate cancer, functioning through
a number of underlying regulatory mechanisms (22–24).
However, the precise mechanism of action of resveratrol in human
cervical carcinoma remains to be investigated, limiting its
therapeutic applications. In the present study, it was demonstrated
that resveratrol significantly inhibited HeLa cell growth and
induced apoptosis in a dose- and time-dependent manner, and the
cells exhibited hallmarks of apoptosis, including cell shrinkage,
DNA fragmentation and formation of apoptotic bodies.
Numerous external and internal stimuli may trigger
cell apoptosis via the activation of caspases. In the process of
caspase-9 activation, cytochrome c binds to apoptosis
activating factor 1 and procaspase-9 to form an apoptosome complex,
which further activates the downstream effector caspase-3 (25). Caspase-8 and −9 are regarded as
initiator caspases, and activate additional effector caspases,
including caspase-6 and −7 (26). The
activation of caspases leads to the cleavage of a set of proteins,
including poly (ADP-ribose) polymerase, and the disassembly of cell
components, including the fragmentation of DNA (27). The overexpression of Bcl-2 or Bcl-XL
results in the inhibition of cytochrome c release and
termination of the apoptotic response, whereas the overexpression
of Bax or its Bcl-2 homologous domain 3 promotes cytochrome
c release (28,29). The present study revealed that
resveratrol-treatment was able to significantly increase the
activation of caspase-3 and −9, decrease Bcl-2 and Bcl-XL protein
levels and increase Bax protein levels (P<0.05). These findings
suggest that Bcl-2 family proteins, as well as caspase-3 and −9,
are involved in the process of resveratrol-induced apoptosis.
The cell cycle includes four phases progressing from
quiescence (G0 phase) to proliferation (G1,
S, G2 and M phases), which are driven by the sequential
activation of cyclin-dependent kinase (CDK) and its cofactor
cyclins. CDK-cyclin B1 complexes are essential for the
phosphorylation of a variety of proteins involved in mitotic
events, including nuclear envelope breakdown, chromosomal
condensation, spindle formation and the attachment of chromosomes
to spindle fibers (30). Therefore,
cell cycle proteins, including cyclin B1 and CDK1, are associated
with the G2/M phase of the cell cycle (31). p53, a tumor suppressor gene, is
activated during cellular stresses, including hypoxia,
carcinogenesis and oxidative stress, functioning by inhibiting cell
cycle progression and activating the DNA repair machinery to
promote cell survival and maintain genome integrity. A
p53-dependent arrest occurring at the G2 phase of the
cell cycle is associated with a proteasome-dependent decrease in
cyclin B1 protein levels (32,33). In
addition, a p53-dependent increase in p21 protein levels is
associated with a decrease in cyclin B1 protein levels (34,35). The
results of the present study revealed that resveratrol was able to
induce G2/M phase arrest in HeLa cells. To investigate
the association between G2/M phase arrest and cyclin B1
expression levels, the effect of resveratrol on cyclin B1 proteins
was examined. The results revealed that resveratrol treatment
significantly decreased (P<0.05) the expression levels of cyclin
B1 protein in HeLa cells, leading to a significant reduction
(P<0.05) in the formation of CDK1-cyclin B complexes and
G2/M phase cell cycle arrest. In summary, the present
study demonstrated that resveratrol is able to increase the
expression levels of p53 in HeLa cells in order to inhibit cell
cycle progression and activate DNA repair machinery to promote cell
survival and maintain genome integrity.
In conclusion, the results of the present study
support the hypothesis that resveratrol downregulates the
expression levels of the essential signaling proteins Bcl-2 and
Bcl-XL, which are involved in the proliferation and survival of
HeLa cells. Furthermore, resveratrol treatment promotes apoptosis
by increasing the levels of caspase-3 and −9 and p53 protein
expression in HeLa cells. In summary, resveratrol may induce cell
cycle arrest and apoptosis in HeLa cells through activation of the
mitochondrial apoptosis signaling pathway, accompanied by the
upregulation of p53 expression and downregulation of cyclin B1
expression. Therefore, resveratrol may be a promising novel
inhibitor of human cervical cancer.
Acknowledgements
The authors would like to thank Professor Li Zhang
for her guidance, and Professor Li-Yun Shi and laboratory members
for discussion and insightful comments.
Funding
This study was supported by the National Natural
Science Foundation of China (grant nos. 30371727, 30973940,
30772766 and 81001599).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL contributed to study design, performed
experiments, data analysis and writing of the manuscript. RLQ made
substantial contributions to the conception and design of the
study, performed experiments and writing of the manuscript. YL
contributed to study design, performed experiments and critically
revised the manuscript. YC performed experiments, and data analysis
and interpretation. YB analyzed data and contributed to writing of
the manuscript. YF and XJG performed experiments and data analysis.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Brisdelli F, D'Andrea G and Bozzi A:
Resveratrol: A natural polyphenol with multiple chemopreventive
properties. Curr Drug Metab. 10:530–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pineda-Sanabria SE, Robertson IM and Sykes
BD: Structure of trans-resveratrol in complex with the cardiac
regulatory protein troponin C. Biochemistry. 50:1309–1320. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khurana S, Venkataraman K, Hollingsworth
A, Piche M and Tai TC: Polyphenols: Benefits to the cardiovascular
system in health and in aging. Nutrients. 5:3779–3827. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bollmann F, Art J, Henke J, Schrick K,
Besche V, Bros M, Li H, Siuda D, Handler N, Bauer F, et al:
Resveratrol post-transcriptionally regulates pro-inflammatory gene
expression via regulation of KSRP RNA binding activity. Nucleic
Acids Res. 42:12555–12569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fresco P, Borges F, Diniz C and Marques
MP: New insights on the anticancer properties of dietary
polyphenols. Med Res Rev. 26:747–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh CK, George SJ and Ahmad N:
Resveratrol-based combinatorial strategies for cancer management.
Ann N Y Acad Sci. 1290:113–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Amicis F, Giordano F, Vivacqua A,
Pellegrino M, Panno ML, Tramontano D, Fuqua SA and Andò S:
Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation,
inhibits estrogen receptor alpha gene expression via p38MAPK/CK2
signaling in human breast cancer cells. FASEB J. 25:3695–3707.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Damianaki A, Bakogeorgou E, Kampa M, Notas
G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Martin PM
and Castanas E: Potent inhibitory action of red wine polyphenols on
human breast cancer cells. J Cell Biochem. 78:429–441. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pandey PR, Okuda H, Watabe M, Pai SK, Liu
W, Kobayashi A, Xing F, Fukuda K, Hirota S, Sugai T, et al:
Resveratrol suppresses growth of cancer stem-like cells by
inhibiting fatty acid synthase. Breast Cancer Res Treat.
130:387–398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bae S, Lee EM, Cha HJ, Kim K, Yoon Y, Lee
H, Kim J, Kim YJ, Lee HG, Jeung HK, et al: Resveratrol alters
microRNA expression profiles in A549 human non-small cell lung
cancer cells. Mol Cells. 32:243–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hsieh TC and Wu JM: Differential effects
on growth, cell cycle arrest, and induction of apoptosis by
resveratrol in human prostate cancer cell lines. Exp Cell Res.
249:109–115. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li G, Rivas P, Bedolla R, Thapa D, Reddick
RL, Ghosh R and Kumar AP: Dietary resveratrol prevents development
of high-grade prostatic intraepithelial neoplastic lesions:
Involvement of SIRT1/S6K axis. Cancer Prev Res (Phila). 6:27–39.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Torre LA, Bray F, Siege RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Berridge MV, Herst PM and Tan AS:
Tetrazolium dyes as tools in cell biology: New insights into their
cellular reduction. Biotechnol Annu Rev. 11:127–152. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple mothed for measuring
thymocyte apoptosis by propidum iodide staining and flow cytometry.
J Immonuol Methods. 139:271–279. 1991. View Article : Google Scholar
|
|
16
|
Khan A, Khan AA, Dwivedi V, Ahmad MG,
Hakeem S and Owais M: Tuftsin augments antitumor efficacy of
liposomized etoposide against fibrosarcoma in Swiss albino mice.
Mol Med. 13:266–276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang J, Cai C, Wang Q, Lin P, Zhao Z and
Cheng F: Systems pharmacology-based discovery of natural products
for precision oncology through targeting cancer mutated genes. CPT
Pharmacometrics Syst Pharmacol. 6:177–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Soleas GL, Grass L, Josephy PD, Goldberg
DM and Diamandis EP: A comparison of the anticarcinogentic
properties of four red wine polyphenols. Clin Biochem. 35:119–124.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
She QB, Bode AM, Ma WY, Chen NY and Dong
Z: Resveratrol-induced activation of P53 and apoptosis is mediated
by extracellular-single-regulated protein kinase and P38 kinase.
Cancer Res. 61:1604–1610. 2001.PubMed/NCBI
|
|
20
|
Surh YJ, Hurh YJ, Kang JK, Lee E, Kong G
and Lee SJ: Resveratrol, an antioxidant present in red wine,
induces apoptosis in human promyelocytic leukemia (HL-60) cells.
Cancer Lett. 140:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clément MV, Hirpara JL, Chawdhury SH and
Pervaiz S: Chemopreventive agent resveratrol, a natural product
derived from grapes, triggers CD95 signaling-dependent apoptosis in
human tumor cells. Blood. 92:996–1002. 1998.PubMed/NCBI
|
|
22
|
Seeni A, Takahashi S, Takeshita K, Tang M,
Sugiura S, Sato SY and Shirai T: Suppression of prostate cancer
growth by resveratol in the transgenic rat for adenocarcinoma of
prostate (TRAP) model. Asisan Pac J cancer Prev. 9:7–14. 2008.
|
|
23
|
Liu H, Zang C, Fenner MH, Liu D, Possinger
K, Koeffler HP and Elstner E: Growth inhibition and apoptosis in
human Philadelphia chromosome-positive lymphoblastic leukemia cell
lines by treatment with the dual PPARalpha/gamma ligand TZD18.
Blood. 107:3683–3692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roy P, Kalra N, Prasad S, George J and
Shukla Y: Chemopreventive potential of Resveratrol in mouse skin
tumors through regulation of mitochondrial and PI3K/AKT signaling
pathways. Pharm Res. 26:211–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kidd VJ: Proteolytic activities that
mediate apoptosis. Annu Rev Physiol. 60:533–573. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kurokawa M and Kornbluth S: Caspases and
kinases in a death grip. Cell. 138:838–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kook S, Zhan X, Cleghorn WM, Benovic JL,
Gurevich VV and Gurevich EV: Caspase-cleaved arrestin-2 and BID
cooperatively facilitate cytochrome C release and cell death. Cell
Death Differ. 21:172–184. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiong S, Mu T, Wang G and Jiang X:
Mitochondria-mediated apoptosis in mammals. Protein Cell.
5:737–749. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu J, Li Y, Ren W and Hu WX: Apoptosis of
HL-60 cells induced by extracts from Narcissus tazetta var.
chinensis. Cancer Lett. 242:133–140. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hiraoka D, Aono R, Hanada S, Okumura E and
Kishimoto T: Two new competing pathways establish the threshold for
cyclin-B-Cdk1 activation at the meiotic G2/M transition. J Cell
Sci. 129:3153–3166. 2016.PubMed/NCBI
|
|
31
|
Pal HC, Sharma S, Elmets CA, Athar M and
Afaq F: Fisetin inhibits growth, induces G2/M arrest and
apoptosis of human epidermoid carcinoma A431 cells: Role of
mitochondrial membrane potential disruption and consequent caspases
activation. Exp Dermatol. 22:470–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhattacharya S, Ray RM and Johnson LR:
Cyclin-dependent kinases regulate apoptosis of intestinal
epithelial cells. Apoptosis. 19:451–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Z, Wang CZ, Du GJ, Qi LW, Calway T,
He TC, Du W and Yuan CS: Genistein induces G2/M cell cycle arrest
and apoptosis via ATM/p53-dependent pathway in human colon cancer
cells. Int J Oncol. 43:289–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Charrier-Savournin FB, Château MT, Gire V,
Sedivy J, Piette J and Dulic V: p21-mediated nuclear retention of
cyclin B1-Cdk1 in response to genotoxic stress. Mol Biol Cell.
15:3965–3976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chae SW, Sohn JH, Kim DH, Choi YJ, Park
YL, Kim K, Cho YH, Pyo JS and Kim JH: Overexpressions of cyclin B1,
cdc2, p16 and p53 in human breast cancer: The clinicopathologic
correlations and prognostic implications. Yonsei Med J. 52:445–453.
2011. View Article : Google Scholar : PubMed/NCBI
|