Introduction
Osteosarcoma (OS) is a terrible malignancy
characterized by osteoblastic differentiation (1). The tumor affects a wide range of age
groups (2). Recent improvements in
the survival of patients with malignant cancers (including OS) may
contribute to an increase in the incidence of second primary
malignancies (SPMs) (3). SPM is a
serious problem for the survivors of OS during the follow-up period
after treatment (4). The relationship
between subsequent SPM and OS has been well reported (4–7), and both
hematogenic and solid malignancies tend to occur after OS treatment
(7). In OS patients,
2-[18F]-fluoro-2-deoxy-D-glucose positron emission
tomography (FDG-PET) is primarily used for initial cancer staging
(8,9),
to evaluate the response of neoadjuvant chemotherapy (10–12), and
when recurrence or metastasis is clinically suspected (9,13,14).
To date, no accurate protocol of postoperative
FDG-PET for OS patients has been reported, and there is uncertainty
regarding the most appropriate methods for follow-up (15–17). In
addition, no accurate protocol for detecting SPMs after OS
treatment exists. SPM can occur anywhere in the body. Early-stage
malignancy sometimes manifests no clinical symptoms; in contrast,
advanced-stage malignancy generally indicates a poorer prognosis.
Therefore, malignancies, including SPM, should be detected as early
as the clinicians can. We report a case of a patient with triple
primary malignancies (PMs): OS of the jaw, myelodysplastic syndrome
(MDS), and adenocarcinoma of the colorectum. After follow-up
FDG-PET/computed tomography (CT) was performed, rectal cancer was
detected unexpectedly as no clinical symptoms had been
observed.
Case report
A 70-year-old man was referred to the Department of
Oral and Maxillofacial Surgery, University Hospital of the Ryukyus
(Nishihara, Japan) in December 2015, for further evaluation of an
oral mass. The patient had a 10-month history of swelling with
gradual pain in the gums of his lower jaw. He had no previous
malignancies and had never been exposed to ionizing radiation or
been administered chemotherapy. He also had no history of family
cancer syndrome; however, his mother had a history of kidney
cancer. He was a smoker and drinker at the first visit. Written
informed consent was obtained from the patient.
Incisional biopsy was performed in his previous
clinic, and the lesion had previously been pathologically diagnosed
as a spindle cell sarcoma highly suggestive of OS. For details, the
histological findings of the initially diagnosed specimens revealed
an irregular arrangement of spindle- or oval-shaped tumor cells,
accompanying eosinophilic matrix suggesting osteoid formation. Most
of the lesions showed mild atypia with rich osteoid formation
(Fig. 1A). Conversely, tumor cells
showing high-grade atypia with low osteoid formation existed in
part (Fig. 1B). According to
immunohistochemical examination, Ki-67 labeling index was 30–40%
(Fig. 1C). Tumor cells were
positively stained for CD56 and were partially positive for smooth
muscle actin. The cells were negatively stained for CD34, S100, or
bc1-2.
Physical examination showed an elastic-hard,
3.5×2.0-cm mass of the right mandibular premolar gum (Fig. 2A). No lymphadenopathy was found in the
neck region. Panorama X-ray and subsequent contrast-enhanced CT
scans from the head to chest and contrast-enhanced magnetic
resonance imaging (MRI) of the head and neck were performed. A
3.5×3.0×2.5-cm mass without bone resorption or infiltration was
observed. CT scan showed new bone formation on the mandible surface
(Fig. 2B and C). Contrast-enhanced
fat-suppression T1-weighted MRI showed a high-signal mass around
the mandible bone; however, no invasion to the bone was found
(Fig. 2D). The signal of the bone
marrow was considered as a slight bone marrow edema. No other
lesion was detected in the neck, bones, or lungs by the above
tests. Next, FDG-PET/CT and bone scintigraphy were evaluated to
identify the OS staging and any other potential lesions in the
whole body. FDG-PET showed increased FDG uptake in the surface of
right mandible [maximum standardized uptake value (SUVmax)=8.82]
(Fig. 3A). In contrast, no other FDG
uptake was seen in the whole body. Bone scintigraphy showed
abnormal bone intake on the mandibular surface, but no other sign
was found. Based on the above findings, we diagnosed the disease as
jaw surface OS without metastasis at the initial presentation. The
patient was administered a four-drug preoperative regimen; that is,
two cycles of cisplatin and doxorubicin, four cycles of high-dose
methotrexate, and additional oral TS-1 for 7 days preoperatively.
No preoperative radiotherapy was performed. Second, FDG-PET/CT was
performed to evaluate the effect of chemotherapy, and the mass of
the lower jaw shrank clinically and radiologically. The FDG uptake
in the right mandible was decreased (SUVmax=5.66) (Fig. 3B). No other indications of lesions
were detected in the whole body. Thrombocytopenia, resulting from
administration of chemotherapy, was controlled by pegfilgrastim.
Subsequently, the patient underwent segmental resection of the
right mandible (that is, wide local resection) with reconstruction
of a vascularized fibular graft and ipsilateral supraomohyoid neck
dissection (April 2016). Histopathological examination revealed
‘OS, post-therapeutic state’, and the surgical margins were
negative. Surgical materials obtained by segmental resection of the
right mandible showed a lot of newly formed woven bone attached to
the existing mandibular bone, and most of the osteocytes and tumor
cells were dead due to chemotherapy. Namely, tumor showed
characteristics of bone formation on the surface of mandibular bone
(Fig. 4). No tumor cell was found in
the bone marrow. According to these findings, the initially
obtained biopsy, clinical and radiological findings, it was
suggested that a part of tumor cells showed high-grade atypia,
although most of the tumor mass showed abundant bone formation,
which arose from the bone surface. Therefore, we considered two
possible diagnoses: high-grade OS of the mandible or parosteal OS
with partial dedifferentiation to high-grade OS (18). However, because of the death of tumor
cells due to chemotherapy, we could not determine which diagnosis
was correct. There was no indication of metastasis to the lymph
nodes. Postoperative chemo/radiotherapy for OS was not performed.
Following the surgery, the patient has not shown recurrence or
metastasis of OS till the time of this writing.
On the other hand, MDS was found because of a
hematoma of the jaw after the surgery, and thrombocytopenia after
the surgery was found during the blood testing performed for
postoperative follow-up. In spite of platelet transfusion, the
thrombocytopenia continued. Therefore, an additional blood test, a
bone marrow examination, and a chromosome analysis were performed
one month after the surgery (May 2016). Wilms' tumor gene was
positive (74 copies/µg RNA) from the blood test. Histopathological
examination of the bone marrow revealed that the marrow was
hypercellular, and micromegakaryocytes were abnormally highly
expressed in cluster of differentiation 41 staining. The
fat-to-cell ratio in the marrow was approximately 2:1, and no OS
cells were found. The chromosome analysis revealed a Y-chromosome
deficiency. On the basis of the examination results, low-risk MDS
(refractory cytopenia of unilineage dysplasia) was diagnosed. The
patient was given only a packed red blood cells and platelets
transfusion, and no other treatment has been required till the time
of this writing.
Eight months after the surgery for OS, the patient
complained that his lower jaw felt different. Local recurrence was
suspected and contrast-enhanced CT was performed; however, no
lesion was found in the head and neck-to-chest region (range of the
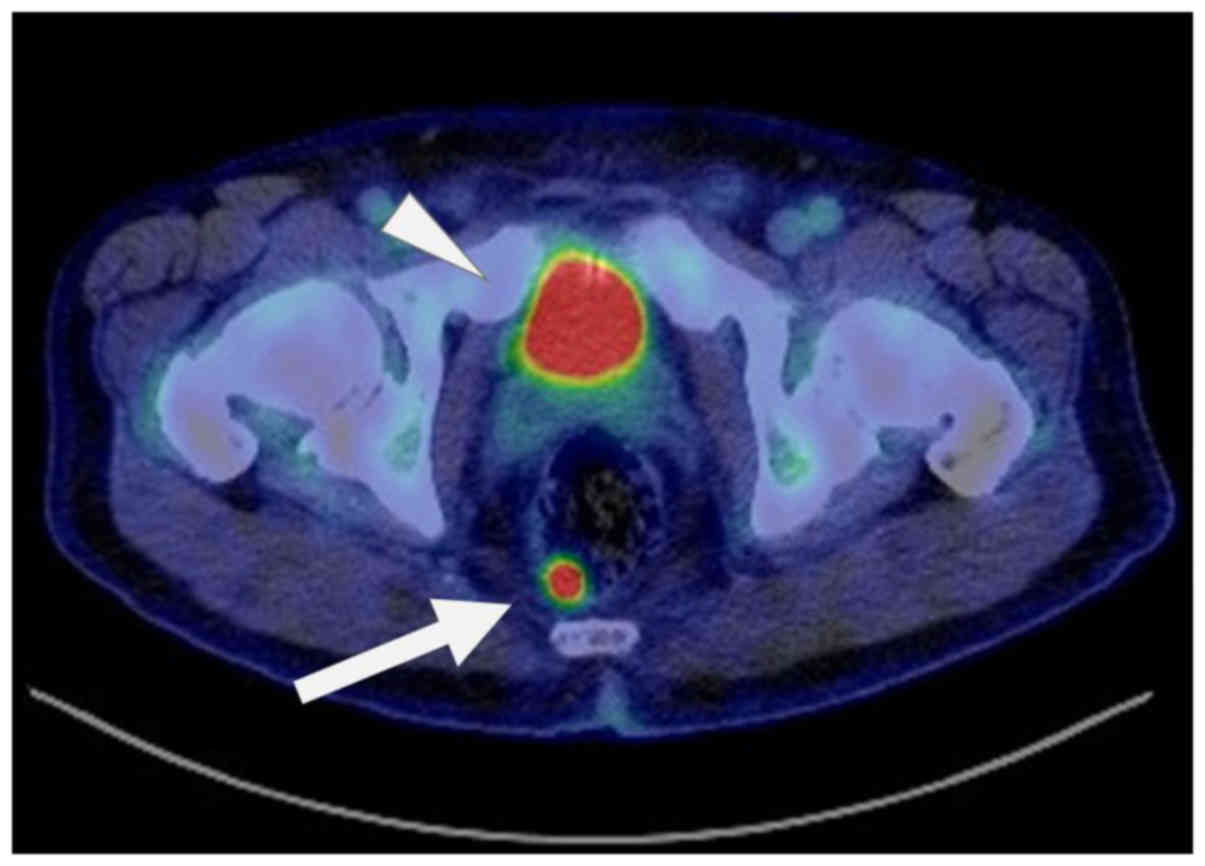
CT scan). Follow-up third FDG-PET/CT was performed 11 months
postoperatively, and abnormal uptake was detected in the rectum
(SUVmax=14.58) (Fig. 5); on the other
hand, no other lesion identified by uptake was found in the whole
body, including the jaw, neck, bone, or lung. A colorectal tumor
was suspected, and subsequent excision by endoscopic mucosal
resection was performed (April 2017). Histopathological examination
showed an adenocarcinoma and showed that the surgical margin was
negative with no vascular invasion. On the other hand, due to the
submucosal tumor invasion depth (3,000 µm), the additional radical
surgery has been considered. Based on the above, MDS and colorectal
cancer were diagnosed after treatment of the jaw cancer. Five
months after the resection of the rectal cancer (one year and five
months after the jaw OS surgery), the patient was free of any
malignant lesion. However, we plan to follow his progress
carefully.
Discussion
This case highlights two important points: i) this
combination of three PMs (jaw OS, MDS, and colorectal
adenocarcinoma) has not been previously reported; and ii) to avoid
overlooking solid SPMs, we suggest that FDG-PET should be performed
in the long-term follow-up of OS patients.
Firstly, to our knowledge, there also have been no
reports of cases involving both hematologic and solid malignancies
after OS. We defined our case as involving three PMs by the Warren
and Gates criteria reported in 1932 (19); that is, each malignancy was distinct
and we excluded disease due to metastasis of one of the other
malignancies. Using the criteria of Lee et al (7), we also discriminated between hematologic
(leukemia, myeloproliferative disease, myelodysplastic disease,
such as MDS, or lymphoma) and solid malignancies (all other
malignancies). Thus, our patient had three PMs, including OS and
subsequent SPMs involving hematologic and solid tumors. When we
then searched PubMed and Google Scholar for English literature
between 1932 and 2017, we found no cases describing the same
combination of three malignancies, indicating the uniqueness of
this case.
Patients with OS tend to develop both hematologic
and solid SPMs after treatment (7).
Compared with cancer-free individuals in the Childhood Cancer
Survivor Study, survivors of OS tended to develop SPMs at a greater
incidence (the standardized incidence ratio was 4.79) (20). Moreover, in a single-institution
study, 26 of 1205 patients with OS of their extremities developed
SPMs after treatment, which was significantly more frequent
compared with the control group (1160 with benign tumors) (21). Cancer survivors are generally at risk
of SPMs (22), with chemotherapy,
radiotherapy, and a family history of cancer recognized to
contribute to SPMs after OS (21,23–25). Our
patient had received preoperative chemotherapy and his mother had a
history of kidney cancer, but he had no history of family cancer
syndrome (25). In addition, smoking
and alcohol consumption are known independent risk factors of two
of the malignancies in our patient (26–29).
Further, old age is a risk factor for cancers such as colorectal
cancer (30).
Other reports of triple to quintuple PMs involving
OS as index malignancy (21,24,31–33)
(Table I) have been associated with
intervals of 1–26 years between the diagnoses of OS and the SPMs
(31–33), indicating that the occurrence of SPM,
particularly solid malignancies, does not decrease over time, after
OS treatment. Studies have generally reported that the average
interval between OS and subsequent SPMs was 6.0–7.6 years (5,7,21,24).
Further, in large series, it has been shown that most SPMs occur
>10 years after OS diagnosis, whereas most local or distant
recurrences occur ≤5 years, with as few as 5% of patients with OS
developing their first local recurrence or distant metastasis
greater than or equal to 5 years after initial treatment (20,34). In a
smaller study of OS survivors, the cumulative incidences of SPMs at
10, 20, and 30 years were 2.1, 4.0, and 7.4%, respectively, with
solid malignancy developing at all times (7). Therefore, a long follow-up period is
needed after primary treatment for OS to detect both SPMs as well
as recurrence, metastasis, or multiple OS (9,24,35,36). SPMs
after OS can be fatal (6,7), and together with metastasis,
chemotherapy response, tumor characteristics, patient
characteristics, surgical margins, and toxicity, SPMs are an
important prognostic factor (37).
Indeed, the prognosis is poor once SPMs occur (20). In these patients, given that advanced
malignancy generally indicates a poorer prognosis, we recommend
monitoring to detect SPMs early.
 | Table I.Triple to quintuple primary
malignancies involving OS as index malignancy. |
Table I.
Triple to quintuple primary
malignancies involving OS as index malignancy.
|
|
| Type of cancer (age
at onset) |
|---|
|
|
|
|
|---|
| Author, year | Sex | First | Second | Third | Fourth | Fifth | (Refs.) |
|---|
| Kubota et
al, 1997 | Male | Non-Hodgkin's
lymphoma (4Y and 9M) | OS of right femur
(7Y and 2M) | MDS (16Y and
4M) | – | – | (31) |
| Bacci et al,
2006 | NA | OS | NA | NA | – | – | (21) |
| Kimura et
al, 2001 | Female | OS of left femur
(9Y) | Paget Ca. of left
breast (19Y) | Paget Ca. of right
breast (24Y) | OS of right femur
(25Y) | Lung adeno Ca.
(26Y) | (32) |
| Yonemoto et
al, 2004 | NA | OS (29Y) | NA | Uterine
leiomyosarcoma (35Y) | – | – | (24) |
| Kousaka et
al, 2014 | Female | OS of left lower
leg (15Y) | Tongue squamous
cell Ca. (23Y) | Papillary thyroid
Ca. (40Y) | Duct Ca. of right
breast (41Y) | – | (33) |
| Present study | Male | OS of jaw
(70Y) | MDS | Rectal adeno
Ca. | – | – | – |
The surface OS of jaw is rare (38). Pathologically, we considered two
possible histological diagnoses: high-grade surface OS and
parosteal OS with partial dedifferentiation to high grade OS.
Although the current case could not be clearly diagnosed due to
tumor mass degeneration of surgical materials post chemotherapy.
According to the findings of osteoid formation with tumor cell
atypia showed in Fig. 1A-C, it was
possible that the tumor was high-grade surface OS. However, the
incidence of the high-grade surface OS at age 70 years was very
unusual. Therefore, we should consider the possibility of parosteal
OS with partial dedifferentiation to high-grade OS. Namely, all of
the surface OSs of jaw tend to develop approximately 20–30 years
old (39). Among those, parosteal OS
occurs at a relatively higher rate in elderly patients, similar to
our patient (38). In contrast,
high-grade surface OS of jaw is very rare (38). Therefore, the current case may be
parosteal OS with dedifferentiation of high-grade surface OS
(40). In OS of the jaw (particularly
the surface-type), metastasis is rare but can occur (41,42). The
most common site is the lung (41,42).
FDG-PET is a useful tool for detecting metastasis of OS (43). Conversely, SPM was detected by testing
our patient.
Another issue is that FDG-PET should be performed
during the long-term follow-up of OS to avoid overlooking solid
SPMs. To date, the importance of detecting SPMs during follow-up
after OS treatment has not been emphasized. In our case, MDS and
colorectal cancer were metachronous according to Moertel's
definition (i.e., recognized ≥6 months after diagnosis) (44). MDS was easily diagnosed because of the
hematoma observed after surgery and during the postoperative
routine blood test (1 month after resection), which prompted early
diagnostic bone marrow aspiration. By contrast, no clinical
symptoms of colorectal cancer were present, and the tumor was found
incidentally by FDG-PET/CT. Recent improvements in patient survival
(including from OS) may lead to increased rates of SPMs (3). Further, old age is a risk factor of SPMs
such as colorectal cancer (30).
Therefore, PET/CT studies are recommended to screen for SPMs
(22).
PET/CT is useful for detecting SPMs in survivors of
OS. However, they are not routinely included in OS follow-up
protocols because few studies have used them for that purpose
(14,45). Indeed, FDG-PET tends to have been
reserved for initial cancer staging and for evaluating the response
of neoadjuvant chemotherapy (8–12), even
though PET is especially useful after treatment for OS because it
is not adversely affected by metal plates or implants (14). These scans can also detect three
important disease patterns for which whole body examination is
needed (5,7,21,24,35,46): i)
recurrence or metastasis (9,13,14); ii)
multiple synchronous or metachronous OS (35,46); and
iii) synchronous or metachronous SPMs (5,7,21,24). CT
and MRI are inadequate because their coverage range is incomplete.
Although OS is associated with a high incidence of lung metastases,
indicating that chest CT is probably the best screening tool in
most cases (14,47), this can fail to identify SPMs at other
sites. In the present case, we could not detect the rectal lesion
by contrast-enhanced CT that was performed because local recurrence
was suspected 8 months after OS treatment.
In daily practice, clinicians check local, regional,
and distant sites where OS recurrences typically occur. Further,
regarding SPMs for patients with head and neck cancers, they tend
to recur locally in the head and neck, esophagus, or lung (48). SPMs at other sites (e.g., the
colorectum) are relatively rare (49). In the current case, the early-stage
colorectal SPM was found incidentally by FDG-PET/CT 11 months after
treatment. OS is commonly associated with pain or swelling as an
early symptom (1,50), and recurrence may be suspected based
on clinical symptoms (14). However,
some SPMs are not associated with clinical symptoms, and it is
known that early-stage colorectal cancer can be clinically silent
(51). In practice, clinicians should
consider the benefits and risks of performing FDG-PET/CT for
patients after OS treatment. Although we have emphasized the
benefits, the following are equally important considerations when
choosing FDG-PET/CT: i) the test is generally expensive for
patients (47); ii) physiological
accumulation makes the detection of malignant lesions difficult,
especially in the kidney or bladder (the major excretion route of
FDG; Fig. 3); and iii) background
activity may obscure the presence of lesions (52).
To date, no protocol exists which guides the use of
FDG-PET after treatment for OS. Some studies have reported that
follow-up PET/CT was useful even when there was no clinical
evidence of recurrence or metastasis (14,53–56).
However, no literature has emphasized the importance of detecting
SPMs during follow-up for OS. We recommend that PET/CT should be
performed during the follow-up of OS, specifically to detect
SPMs.
In conclusion, the specific combination of triple
PMs in this case (i.e., jaw OS, MDS, and colorectal adenocarcinoma)
has not been reported previously. Based on our research, we
recommend that FDG-PET be performed during the long-term follow-up
of OS to avoid overlooking solid SPMs. However, our conclusions are
based on a single case report, which limits their generalizability.
Further cases are needed to help develop a protocol that describes
the optimal role of FDG-PET or FDG-PET/CT scans in the
identification of hidden synchronous or metachronous SPMs during
the follow-up of patients with OS.
Acknowledgements
The authors would like to thank Dr. Hirofumi
Matsumoto (Department of Pathology, University Hospital of the
Ryukyus, Nishihara, Japan), for the pathological advice. The
authors would also like to thank Ms. Chinatsu Toguchi and Ms. Ai
Tokeshi (Department of Pathology, University Hospital of the
Ryukyus) for their technical support.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NM and TM acquired the data, performed the
literature review and edited the manuscript. AA substantially
contributed to the concept and design of the study. KN, TN, IT, TO,
FN and AM acquired the data and contributed clinical advice. MS,
IT, TO and AA revised the manuscript. MS, KK and NY evaluated the
specimens. MS gave histopathological advice. TM had a major role in
writing the manuscript.
Ethics approval and consent to
participate
The present case report was submitted for ethical
review to the Ethics Committee of The University of the Ryukyus
(Nishihara, Japan), which waived the requirement for review per
institutional protocol as the case report does not contain content
that requires ethical approval. The Ethics Committee approved the
submission and publication of the manuscript. Written informed
consent was obtained from the patient.
Consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SPM
|
second primary malignancy
|
|
OS
|
osteosarcoma
|
|
FDG-PET
|
2-[18F]-fluoro-2-deoxy-D-glucose positron emission
tomography
|
|
PM
|
primary malignancy
|
|
MDS
|
myelodysplastic syndrome
|
|
CT
|
computed tomography
|
|
MRI
|
magnetic resonance imaging
|
|
SUVmax
|
maximum standardized uptake value
|
References
|
1
|
Zhao L, Zhang J, Tan H, Wang W, Liu Y,
Song R and Wang L: Gene function analysis in osteosarcoma based on
microarray gene expression profiling. Int J Clin Exp Med.
8:10401–10410. 2015.PubMed/NCBI
|
|
2
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick RL: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim JS, Chung CY, Park HC, Myung DS, Cho
SB, Lee WS, Min JJ and Joo YE: Synchronous quadruple primary tumors
of thyroid, breast, pancreas and stomach: A case report. Anticancer
Res. 33:2135–2138. 2013.PubMed/NCBI
|
|
4
|
Aung L, Gorlick RG, Shi W, Thaler H,
Shorter NA, Healey JH, Huvos AG and Meyers PA: Second malignant
neoplasms in long-term survivors of osteosarcoma: Memorial
sloan-kettering cancer center experience. Cancer. 95:1728–1734.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pratt CB, Meyer WH, Luo X, Cain AM, Kaste
SC, Pappo AS, Rao BN, Fleming ID and Jenkins JJ III: Second
malignant neoplasms occuring in survivors of osteosarcoma. Cancer.
80:960–965. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SH, Shin KH, Seok SO, Cho YJ, Noh JK,
Suh JS and Yang WI: Secondary malignant neoplasms after
osteosarcoma: Early onset and cumulative alkylating agent dose
dependency. Ann Surg Oncol. 22:859–865. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JS, DuBois SG, Boscardin WJ, Wustrack
RL and Goldsby RE: Secondary malignant neoplasms among children,
adolescents and young adults with osteosarcoma. Cancer.
120:3987–3993. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quartuccio N, Treglia G, Salsano M,
Mattoli MV, Muoio B, Piccardo A, Lopci E and Cistaro A: The role of
Fluorine-18-Fluorodeoxyglucose positron emission tomography in
staging and restaging of patients with osteosarcoma. Radiol Oncol.
47:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costelloe CM, Chuang HH and Daw NC: PET/CT
of Osteosarcoma and ewing sarcoma. Semin Roentgenol. 52:255–268.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harrison DJ, Parisi MT and Shulkin BL: The
Role of 18F-FDG-PET/CT in pediatric sarcoma. Semin Nucl Med.
47:229–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Byun BH, Kong CB, Lim I, Choi CW, Song WS,
Cho WH, Jeon DG, Koh JS, Lee SY and Lim SM: Combination of 18F-FDG
PET/CT and diffusion-weighted MR imaging as a predictor of
histologic response to neoadjuvant chemotherapy: Preliminary
results in osteosarcoma. J Nucl Med. 54:1053–1059. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Byun BH, Kong CB, Lim I, Kim BI, Choi CW,
Song WS, Cho WH, Jeon DG, Koh JS and Lim SM: Early response
monitoring to neoadjuvant chemotherapy in osteosarcoma using
sequential 18F-FDG PET/CT and MRI. Eur J Nucl Med Mol Imaging.
41:1553–1562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hurley C, McCarville MB, Shulkin BL, Mao
S, Wu J, Navid F, Daw NC, Pappo AS and Bishop MW: Comparison of
(18) F-FDG-PET-CT and bone scintigraphy for evaluation of osseous
metastases in newly diagnosed and recurrent osteosarcoma. Pediatr
Blood Cancer. 63:1381–1386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Angelini A, Ceci F, Castellucci P,
Graziani T, Polverari G, Trovarelli G, Palmerini E, Ferrari S,
Fanti S and Ruggieri P: The role of 18F-FDG PET/CT in the detection
of osteosarcoma recurrence. Eur J Nucl Med Mol Imaging.
44:1712–1720. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rothermundt C, Seddon BM, Dileo P, Strauss
SJ, Coleman J, Briggs TW, Haile SR and Whelan JS: Follow-up
practices for high-grade extremity Osteosarcoma. BMC Cancer.
16:3012016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Paioli A, Rocca M, Cevolani L, Rimondi E,
Vanel D, Palmerini E, Cesari M, Longhi A, Eraldo AM, Marchesi E, et
al: Osteosarcoma follow-up: chest X-ray or computed tomography?
Clin Sarcoma Res. 7:32017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Quartuccio N, Fox J, Kuk D, Wexler LH,
Baldari S, Cistaro A and Schöder H: Pediatric bone sarcoma:
Diagnostic performance of 18F-FDG PET/CT versus conventional
imaging for initial staging and follow-up. AJR Am J Roentgenol.
204:153–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lazar A and Mertens F: Parosteal
osteosarcomaWHO classification of tumors of soft tissue and bone.
Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: IARC Press;
Lyon: pp. 292–293. 2013
|
|
19
|
Warren S and Gates O: Multiple primary
malignant tumors: a survey of the literature and a statistical
study. Am J Cancer. 16:1358–1414. 1932.
|
|
20
|
Nagarajan R, Kamruzzaman A, Ness KK,
Marchese VG, Sklar C, Mertens A, Yasui Y, Robison LL and Marina N:
Twenty years of follow-up of survivors of childhood osteosarcoma: A
report from the Childhood Cancer Survivor Study. Cancer.
117:625–634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bacci G, Ferrari C, Longhi A, Ferrari S,
Forni C, Bacchini P, Palmerini E, Briccoli A, Pignotti E,
Balladelli A, et al: Second malignant neoplasm in patients with
osteosarcoma of the extremities treated with adjuvant and
neoadjuvant chemotherapy. J Pediatr Hematol Oncol. 28:774–780.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Almangush A, Coletta RD, Bello IO, Bitu C,
Keski-Säntti H, Mäkinen LK, Kauppila JH, Pukkila M, Hagström J,
Laranne J, et al: A simple novel prognostic model for early stage
oral tongue cancer. Int J Oral Maxillofac Surg. 44:143–150. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bacci G, Ferrari S, Bertoni F, Ruggieri P,
Picci P, Longhi A, Casadei R, Fabbri N, Forni C, Versari M and
Campanacci M: Long-term outcome for patients with nonmetastatic
osteosarcoma of the extremity treated at the istituto ortopedico
rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2
protocol: An updated report. J Clin Oncol. 18:4016–4027. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yonemoto T, Tatezaki S, Ishii T, Hagiwara
Y and Inoue M: Multiple primary cancers in patients with
osteosarcoma: Influence of anticancer drugs and genetic factors. Am
J Clin Oncol. 27:220–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hauben EI, Arends J, Vandenbroucke JP, van
Asperen CJ, Van Marck E and Hogendoorn PC: Multiple primary
malignancies in osteosarcoma patients. Incidence and predictive
value of osteosarcoma subtype for cancer syndromes related with
osteosarcoma. Eur J Hum Genet. 11:611–618. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu P, Holman CD, Jin J and Zhang M:
Alcohol consumption and risk of myelodysplastic syndromes: A
case-control study. Cancer Causes Control. 27:209–216. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tong H, Hu C, Yin X, Yu M, Yang J and Jin
J: A meta-analysis of the relationship between cigarette smoking
and incidence of myelodysplastic syndromes. PLoS One. 8:e675372013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson CM, Wei C, Ensor JE, Smolenski DJ,
Amos CI, Levin B and Berry DA: Meta-analyses of colorectal cancer
risk factors. Cancer Causes Control. 24:1207–1222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Parajuli R, Bjerkaas E, Tverdal A, Le
Marchand L, Weiderpass E and Gram IT: Cigarette smoking and
colorectal cancer mortality among 602,242 Norwegian males and
females. Clin Epidemiol. 7:137–145. 2014.
|
|
30
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kubota M, Sawada M, Watanabe K, Koishi S,
Kataoka A, Usami I, Lin YW, Okuda A, Akiyama Y and Furusho K:
Myelodysplastic syndrome presenting as third malignancy after
non-Hodgkin's lymphoma and osteosarcoma. Ann Hematol. 74:95–97.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimura K, Shinmura K, Hasegawa T, Beppu Y,
Yokoyama R and Yokota J: Germline p53 mutation in a patient with
multiple primary cancers. Jpn J Clin Oncol. 31:349–351. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kousaka J, Fujii K, Yorozuya K, Mouri Y,
Yoshida M, Nakano S, Fukutomi T, Takahashi E and Yokoi T: A case of
quadruple primary malignancies including breast, tongue and thyroid
cancers and osteosarcoma in a young female without karyotype
abnormality. Breast Cancer. 21:500–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hauben EI, Bielack S, Grimer R, Jundt G,
Reichardt P, Sydes M, Taminiau AH and Hogendoorn PC:
Clinico-histologic parameters of osteosarcoma patients with late
relapse. Eur J Cancer. 42:460–466. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jaffe N, Pearson P, Yasko AW, Lin P,
Herzog C and Raymond K: Single and multiple metachronous
osteosarcoma tumors after therapy. Cancer. 98:2457–2466. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Currall VA and Dixon JH: Synchronous
multifocal osteosarcoma: Case report and Literature review.
Sarcoma. 2006:539012006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van den Berg H, Schreuder WH and de Lange
J: Osteosarcoma: A comparison of jaw versus nonjaw localizations
and review of the literature. Sarcoma. 2013:3161232013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kumar VS, Barwar N and Khan SA: Surface
osteosarcomas: Diagnosis, treatment and outcome. Indian J Orthop.
48:255–261. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bertoni F, Bacchini P, Staals EL and
Davidovitz P: Dedifferentiated parosteal osteosarcoma: The
experience of the Rizzoli Institute. Cancer. 103:2373–2382. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bertoni F, Dallera P, Bacchini P,
Marchetti C and Campobassi A: The Istituto Rizzoli-Beretta
experience with osteosarcoma of the jaw. Cancer. 68:1555–1563.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sawair FA, Cheng J, Hao N, Maruyama S,
Hoshina H, Takagi R, Koyama J, Hayashi T and Saku T: Periosteal
osteosarcoma of the jaw bones: a clinicopathologic review. Oral Med
Pathol. 12:3–10. 2007. View Article : Google Scholar
|
|
43
|
Brenner W, Bohuslavizki KH and Eary JF:
PET imaging of osteosarcoma. J Nucl Med. 44:930–942.
2003.PubMed/NCBI
|
|
44
|
Moertel CG, Dockerty MB and Baggenstoss
AH: Multiple primary malignant neoplasms. I. Introduction and
presentation of data. Cancer. 14:221–230. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim MS, Sim YS, Lee SY and Jeon DG: Occult
thyroid carcinoma detected by FDG-PET scan in elderly osteosarcoma
patients: Report of two cases. Ann Nucl Med. 21:529–532. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gavane S, Price AP, Magnan H, Mahajan S
and Pandit-Taskar N: Multifocal osteosarcoma: Unusual presentation
and imaging findings. Clin Nucl Med. 42:e202–e206. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kneisl JS, Patt JC, Johnson JC and Zuger
JH: Is PET useful in detecting occult nonpulmonary metastases in
pediatric bone sarcomas? Clin Orthop Relat Res. 450:101–104. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jain KS, Sikora AG, Baxi SS and Morris LG:
Synchronous cancers in patients with head and neck cancer: Risks in
the era of human papillomavirus-associated oropharyngeal cancer.
Cancer. 119:1832–1837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Coyte A, Morrison DS and McLoone P: Second
primary cancer risk-the impact of applying different definitions of
multiple primaries: Results from a retrospective population-based
cancer registry study. BMC Cancer. 14:2722014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yildiz FR, Avci A, Dereci O, Erol B,
Celasun B and Gunhan O: Gnathic osteosarcomas, experience of four
institutions from Turkey. Int J Clin Exp Pathol. 7:2800–2808.
2014.PubMed/NCBI
|
|
51
|
Kahi CJ, Imperiale TF, Juliar BE and Rex
DK: Effect of screening colonoscopy on colorectal cancer incidence
and mortality. Clin Gastroenterol Hepatol. 7:770–775. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang HY, Ding HJ, Chen JH, Chao CH, Lu YY,
Lin WY and Kao CH: Meta-analysis of the diagnostic performance of
[18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging.
12:464–474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chang KJ, Kong CB, Cho WH, Jeon DG, Lee
SY, Lim I and Lim SM: Usefulness of increased 18F-FDG uptake for
detecting local recurrence in patients with extremity osteosarcoma
treated with surgical resection and endoprosthetic replacement.
Skeletal Radiol. 44:529–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Franzius C, Daldrup-Link HE, Wagner-Bohn
A, Sciuk J, Heindel WL, Jürgens H and Schober O: FDG-PET for
detection of recurrences from malignant primary bone tumors:
Comparison with conventional imaging. Ann Oncol. 13:157–160. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Piperkova E, Mikhaeil M, Mousavi A, Libes
R, Viejo-Rullan F, Lin H, Rosen G and Abdel-Dayem H: Impact of PET
and CT in PET/CT studies for staging and evaluating treatment
response in bone and soft tissue sarcomas. Clin Nucl Med.
34:146–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fuglø HM, Jørgensen SM, Loft A, Hovgaard D
and Petersen MM: The diagnostic and prognostic value of 18F-FDG
PET/CT in the initial assessment of high-grade bone and soft tissue
sarcoma. A retrospective study of 89 patients. Eur J Nucl Med Mol
Imaging. 39:1416–1424. 2012. View Article : Google Scholar : PubMed/NCBI
|