Introduction
Lung cancer was the most common cancer and the
leading cause of cancer-associated mortality in China in 2015
(1), followed by gastric cancer,
esophageal cancer and liver cancer. Non-small cell lung cancer
(NSCLC) accounts for 80–90% of lung cancer cases (2,3). The
primary treatment for stage I–II NSCLC is surgery; however, the
majority of NSCLC diagnoses occur at stage IV (4). In addition to small molecule inhibitors,
tyrosine kinase inhibitors and monoclonal antibodies, immunotherapy
has been used as a primary treatment method (5).
Chimeric antigen receptors (CARs) are fusion
proteins that comprise ≥3 main domains: The antigen-binding domain,
usually a single-chain variable fragment (scFv) of antibody
responsible for recognition and binding; the intracellular domain;
and the transmembrane sequence, which connects the extracellular
region to the intracellular domain (6–8). The CAR
molecule has been developed through three stages according to the
intracellular signaling domain: The first generation of CARs
consisted of only one cluster of differentiation (CD)3ζ chain; the
second generation was comprised of one co-stimulatory molecule and
a CD3ζ chain; and the third generation comprised ≥2 co-stimulatory
molecules with CD3ζ as the last signal transduction region. Once a
CAR molecule is expressed on a CAR-T cell and the tumor antigen is
recognized by the scFv, the CAR-T cell is activated and lyses the
target cells (9). In 2003, the first
preclinical study to demonstrate the effectiveness of anti-CD19 was
published (10), and a series of
studies based on different target molecules have subsequently been
conducted (11–17). Due to antibody specificity, CAR-T
cells may effectively bind to an antigen independently of major
histocompatibility complex restriction (18), and an increasing number of studies
have demonstrated positive outcomes for patients following
treatment with CAR-T cells (19–23).
Programmed death 1 (PD-1) is a receptor that is
involved in apoptosis (24). One
study on PD-1-deficient mice has indicated that PD-1 functions to
negatively regulate immune responses (25). At present, the consensus is that PD-1
acts as an immune-checkpoint receptor and is involved in regulating
T cell activity to inhibit immune responses and prevent
overstimulation in peripheral tissues (26–29).
Programmed death-ligand 1 (PD-L1), one of the two ligands of PD-1,
was identified and subsequently termed PD-L1 (Pdcd1Ig1), the
study of which provided convincing evidence that T cells exhibited
lower proliferative ability when cultured with an anti-CD3 antibody
(30–32).
Recently, several studies have revealed a high
expression level of PD-L1 in patients with NSCLC (33), and have demonstrated its association
with the mechanism underlying tumor immune escape (34,35).
Effective blocking of PD-1 or PD-L1 has a positive effect on the
treatment of cancer together with combination treatment methods
(36–40). CAR-T cells secreting anti-PD-L1
antibodies have also demonstrated promising efficacy (41). Furthermore, the expression of a
dominant negative receptor or switch of co-stimulatory receptor may
achieve the same purpose to prevent inactivation of T cells
(42–44). Based on the results of previous
studies, a CAR was designed comprising an anti-PD-L1 scFv and an
intracellular co-stimulation signal from CD28 and 4-1BB (PD-L1
scFv-CD28-CD137-CD3z), which was inserted into a high-quality
lentivirus for transduction into peripheral blood mononuclear cells
(PBMCs) (45–47). The function of CAR-T cells was
confirmed by a flow cytometric apoptosis assay following co-culture
with NCI-H358 or A549 cells at a ratio of 10:1. The levels of
interferon (IFN)-γ, interleukin (IL)-2 and tumor necrosis factor
(TNF)-α in the supernatant of the co-culture were detected by
ELISA. The results indicated mild antitumor activity and a
protective effect of T cells against PD-L1 by blocking PD-L1.
Materials and methods
Reagents and sequences
RPMI-1640 culture medium and the Dynabeads Human
T-Activator CD3/CD28 kit were purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). The Annexin V Apoptosis kit
with 7-aminoactinomycin (7-AAD) and the carboxyfluorescein
succinimidyl ester (CFSE) Cell Division Tracker kit were obtained
from BioLegend, Inc. (San Diego, CA, USA). Fluorescein
isothiocyanate (FITC)-Protein L was purchased from ACROBiosystems
(Newark, DE, USA). All restriction endonucleases were purchased
from Thermo Fisher Scientific, Inc., and the ligation kit was
obtained from New England BioLabs, Inc. (Ipswich, MA, USA). The
homologous recombination kit and chemically competent cell
Stbl3™ were provided by Beijing TransGen Biotech
Co., Ltd. (Beijing, China). The lentiviral vector pLVX was provided
by Clontech Laboratories, Inc. (Mountainview, CA, USA). DNA
sequences were synthesized by Thermo Fisher Scientific, Inc.
Lentivirus concentrator reagent was obtained from Beijing
Syngentech Co., Ltd. (Beijing, China). All fluorescent antibodies
used for flow cytometric analysis were purchased from BioLegend,
Inc. (PD-L1/PD-1) or BD Biosciences (CD4 and CD8; Franklin Lakes,
NJ, USA).
Cell culture
NSCLC NCI-H358 and A549 cells were provided by ATCC
(Manassas, VA, USA) and cultured in RPMI-1640 medium (cat no.
11875119) with 10% fetal bovine serum (FBS; cat no. 10099141; both
Thermo Fisher Scientific, Inc.) in a 5% CO2 atmosphere
at 37°C. PBMCs (obtained from Mr Jiasen Xie and Miss Zishan Zhou,
Beijing Bio DC Labs, Beijing, China) were activated by Dynabeads
and cultured in X-VIVO™ 15 chemically defined,
serum-free hematopoietic cell medium (cat no. 04-418Q; Lonza Group,
Ltd., Basel, Switzerland) with 5% FBS in a 5% CO2
atmosphere at 37°C.
Construction of vector and production
of lentivirus
The lentiviral vector pLVX (cat no. 631982; Clontech
Laboratories, Inc.) was digested with EcoRI and NotI,
prior to being recovered with a Gel DNA Recovery kit (cat no.
DP209; Tiangen Biotech Co., Ltd., Beijing, China). A scFv fragment,
designated FR1 (Invitrogen; Thermo Fisher Scientific, Inc.), which
comprised scFv and a transmembrane domain, was amplified from a
pUC-vector using the following primers: FR1 forward,
5′-GTGTCGTGAGGATCTATTTCCGGTGAATTCGCCGCCACCATGGCCTTACCAGTGACCGCC-3′
and reverse,
5′-TCTGGACCGCTTAGATCGGTTCCTATGATTTCGGTTCCTATGATTACAATAAAGAGTAAT-3′.
A signal fragment containing a transmembrane domain, CD137
co-stimulatory domain and a CD28 co-stimulatory domain was
amplified from a pUC-vector (Invitrogen; Thermo Fisher Scientific,
Inc.) and termed FR2, using the following primers: FR2 forward,
5′-ATTACTCTTTATTGTAATCATAGGAACCGAAATCATAGGAACCGATCTAAGCGGTCCAGA-3′
and reverse,
5′-GTAATCCAGAGGTTGATTGATCCGCGGCCGCCTACCGTGGGGGGAGGGCCTGCATATGAA-3′.
Platinum™ Pfx. The DNA template plasmid pUC-FR1 and
pUC-FR2 were synthesized by Invitrogen; Thermo Fisher Scientific,
Inc. DNA polymerase (cat no. 11708039; Thermo Fisher Scientific,
Inc.) was used according to the manufacturer's protocol, and the
thermocycling conditions were as follows: 94°C for 2 min, 94°C for
15 sec, 58°C for 30 sec, 68°C for 2 min, 30 cycles and 68°C for 5
min. PCR products were subjected to 1% agarose gel electrophoresis
and stained with 0.5 µg/ml ethidium bromide at room temperature for
30 min and observed at 254 nm UV. The specific bands were recovered
with a gel extraction kit. A seamless homologous recombination with
FR1, FR2 and the digested vector pLVX was performed to obtain the
scFv-28Bz expression recombination vector. The recombination
product was transformed into Stbl3™ competent
cells, which were selected on ampicillin plates. The positive
clones were used for sequencing.
The validated vector pLVX-EF1α-scFv-28Bz was used to
produce lentiviral particles with the 2nd generation package system
psPAX2 and pMD2.G. The LipoFiter™ Liposomal Transfection
reagent was purchased from Hanbio Biotechnology Co., Ltd.
(Shanghai, China) and all procedures were performed according to
the manufacturer's protocols. A total of 30 µg plasmid were
transfected (containing 15 µg pLVX-EF1α-scFv-28Bz, 7.5 µg psPAX2
and 7.5 µg pMD2.G). The lentiviruses in the cell culture
supernatant were collected at 48 and 72 h post-transfection and
were concentrated by PEG8000 reagent from the BioGeek™
Lentivirus Concentrate kit (cat no. BG20101L; Beijing Syngentech
Co., Ltd.). In order to ensure the reliability of the system,
parallel experiments were also conducted. The same empty
lentivector of LV-EF1α-scFv-28Bz was constructed to express green
fluorescent protein (GFP) in the same multiple cloning site and
following the same EF1α promoter to produce LV-EF1α-GFP. The
lentivector was packaged in lentiviral particles with the same
packaging system and the particles were transfected into PBMCs as
the LV-EF1α-scFv-28Bz particles would be transfected into PBMCs.
The expression of GFP was confirmed to ensure the reliability of
vector system, packaging system and transfection efficiency.
Cell expansion and transduction
PBMCs were activated by Dynabeads at a bead-to-cell
ratio of 1:1 in X-VIVO™ 15 chemically defined medium
with 30 IU/ml rIL-2 (cat no. Z03074-10; GenScript, Piscataway, NJ,
USA), and incubated in a humidified 5% CO2 incubator at
37°C. A total of 3–5 days later, the activated cells were
resuspended and ~2 million PBMCs were transduced with
pre-conditioned lentiviral particles with or without scFv-28Bz in
serum-free X-VIVO™ 15 chemically defined medium with a final
concentration of 10 µg/ml Polybrene® (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), centrifuged at 270 × g at 32°C for
1 h with a parallel control (virus control - empty lentivirus
particles without scFv-28Bz + PMBC; cell control -
X-VIVO™ 15 medium + PBMC; controls centrifuged at 270 ×
g for 1 h at 32°C) and cultured in a humidified 5% CO2
incubator at 37°C for 24 h. The medium was changed to full-nutrient
medium (X-VIVO™ 15 with 5% FBS) after 24 h and fresh
medium was added according to growth status every 2 days, with a
density of 1–2 million cells/ml.
Detection of CAR molecule in
transduced cells and PD-L1 in tumor cell lines
As reported previously, the protein L has the
ability to bind to the κ light chain of antibodies (48). Flow cytometry was used to confirm the
expression of scFv-28Bz using the FITC-Protein-L reagent (cat no.
RPL-PF141; ACROBiosystems). PBMCs (~2 million) were harvested using
centrifugation at 300 × g for 10 min at 25°C, washed twice with
phosphate-buffered saline (PBS) and incubated with 2 µg
FITC-Protein-L protein at room temperature for 1 h in the absence
of light, with FITC-Protein-L-stained mock cells serving as the
isotype control. Subsequently, the cells were washed three times in
PBS containing 0.5% bovine serum albumin (BSA). NCI-H358 and A549
cells (~2 million) were harvested by digestion with trypsin and
washed twice with PBS. The cell lines were incubated with 2 µg
phycoerythrin (PE)-anti-PD-L1 (PE anti-human CD274 Antibody; 1:10,
cat no. 329706) at room temperature for 30 min, prior to being
washed three times in PBS containing 0.5% BSA. The cells were
analyzed with a BD FACSCalibur flow cytometer and the results were
analyzed with FlowJo software 7.6.1 (FlowJo LLC, Ashland, OR,
USA).
Analysis of subsets of PBMCs and PD-1
expression
CD4+ CAR-T cells provided assistance to
CD8+ CAR-T cells in vitro and in vivo, as
the proportion of CD4+ cells and CD8+ cells in CAR-T are associated
with therapeutic efficacy (49). In
order to compare the cytotoxicity of tumors with different
expression levels of PD-L1, the expression level of PD-1 on T cells
should be similar, to avoid differences in cell viability due to
PD-L1 (30). PBMCs were collected at
14 days post-transduction and were washed using PBS twice. A total
of 2×106 PMBC cells were incubated at room temperature
for 30 min with a mixture of peridinin chlorophyll protein
complex-CD4 (cat no. 347324) and PE-CD8 (cat no. 340046)
antibodies, while the remaining cells were incubated with an
allophycocyanin-conjugated anti-human PD-1 antibody (cat no.
329908) for 30 min at room temperature, with normal immunoglobulin
G (IgG; PerCP-Mouse IgG1 isotype; cat no. 550672; PE-Mouse IgG1
isotype; cat no. 550617; both BD Biosciences; APC-Mouse IgG1
isotype; cat no. 400120; BioLegend, Inc.) used as an isotype
control. All antibodies had a dilution of 1:10. Following washing
three times in PBS containing 0.5% BSA, the cells were analyzed by
a flow cytometer and the results were analyzed with FlowJo software
7.6.1.
Detection of IFN-γ, IL-2 and TNF-α
production by T cells
In order to ensure that the interaction of T cells
with target cells was able to induce the production of cytokines, T
cells were co-cultured with NCI-H358 or A549 cells at a ratio of
1:1 overnight at 37°C in serum-free medium (X-VIVO™ 15 chemically
defined medium), and the supernatants were collected for cytokine
detection by ELISA kits, as follows: Human IFN-γ ELISA kit (cat.
no. DKW12-1000-096), Human IL-2 ELISA kit (cat no. DKW12-1020-096)
and Human TNF-α ELISA kit (cat no. DKW12-1720-096; all Dakewe
Biotech Co., Ltd., Shenzhen, China).
Flow cytometric analysis of cell
apoptosis
As a reliable way to detect lytic activities of
effector cells (50,51), flow cytometry has the advantages of
good repeatability and sensitivity. CFSE-labeled NCI-H358 or A549
cells (1×106 cells/well) were seeded onto 12-well
culture plates on day 0. PBMCs (1×107 cells/well) were
plated into the 12-well culture plates for co-culturing with
NCI-H358 or A549 cells for 3.5 h at 37°C and 5% CO2 in a
cell incubator. Subsequently, all the cells from each well were
collected, and were centrifuged for 10 min at 200 × g at 25°C and
washed three times in PBS. The cells were resuspended in 100 µl
binding buffer with Annexin V/7-AAD (cat no. 640930; Biolegend,
Inc.) and incubated for 30 min at room temperature in the dark.
Following being washed with PBS containing 0.5% BSA, the cell
mixtures were collected and resuspended in 100 µl PBS.
Non-co-cultured target cells served as a parallel control to detect
spontaneous cell death. The labeled cells were analyzed using a
flow cytometer. Data were collected and analyzed with FlowJo
software 7.6.1 to determine the percentage of apoptotic cells. The
specific cytotoxicity of PBMCs was equal to the total death of
co-cultured target cells following subtraction of the total
spontaneous death of non-co-cultured target cells [specific
cytotoxicity=target cellco-cultured (Q1+Q2+Q3) - target
cellnon-co-cultured (Q1+Q2+Q3)].
Statistical analysis
Experiments were repeated ≥3 times and all the
results are presented as the mean ± standard deviation. The
GraphPad Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA,
USA) was used for statistical analyses. The pairwise mean
comparisons were performed using an unpaired Student's t-test in
the cell apoptosis assay and one-way analysis of variance, followed
by Tukey's multiple comparisons test, was applied to analyze the
data following ELISA detection. P<0.05 was considered to
indicate a statistically significant difference.
Results
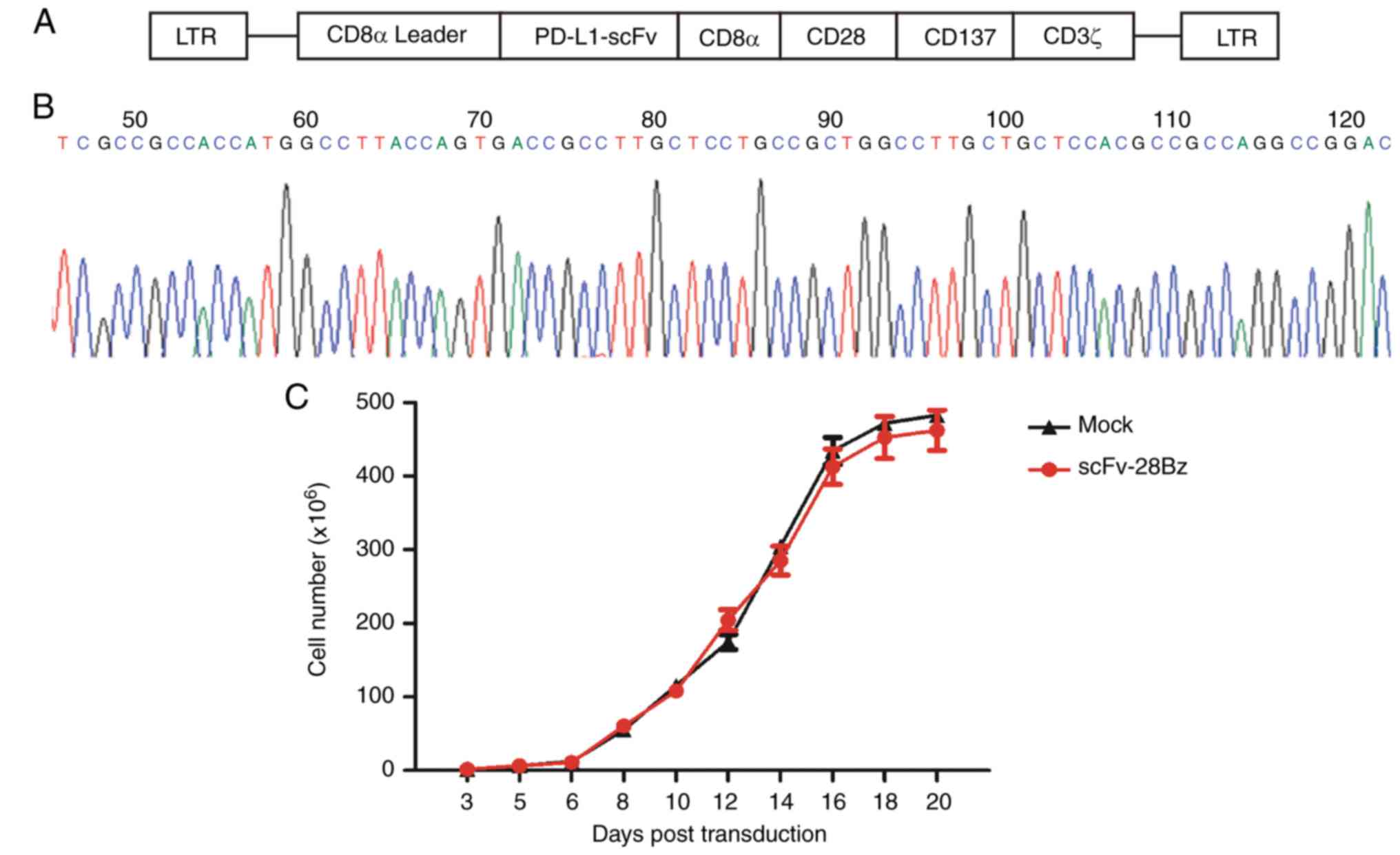
Successful construction of
PD-L1-specific scFv-28Bz and efficient expansion of cells
One type of lentiviral vector (LV-EF1α-scFv-28Bz)
was constructed successfully. As demonstrated in Fig. 1A, the vector contained PD-L1 scFv, a
scFv from MPDL3280A (52), and
transmembrane domains from CD8α, a CD28 co-stimulatory domain, a
CD137 co-stimulatory domain and a CD3ζ intracellular domain
(Fig. 1A). The 5′ terminal sequencing
results are depicted in Fig. 1B. The
PBMCs were cultured in X-VIVO™ 15 chemically defined
medium with 5% FBS post-transduction, and the cell numbers were
counted; according to cell growth status, fresh medium was
supplemented to maintain a density of 1–2 million cells/ml. As
depicted in Fig. 1C, the total cell
number reached 200–300 million at day 14–21 post-transduction.
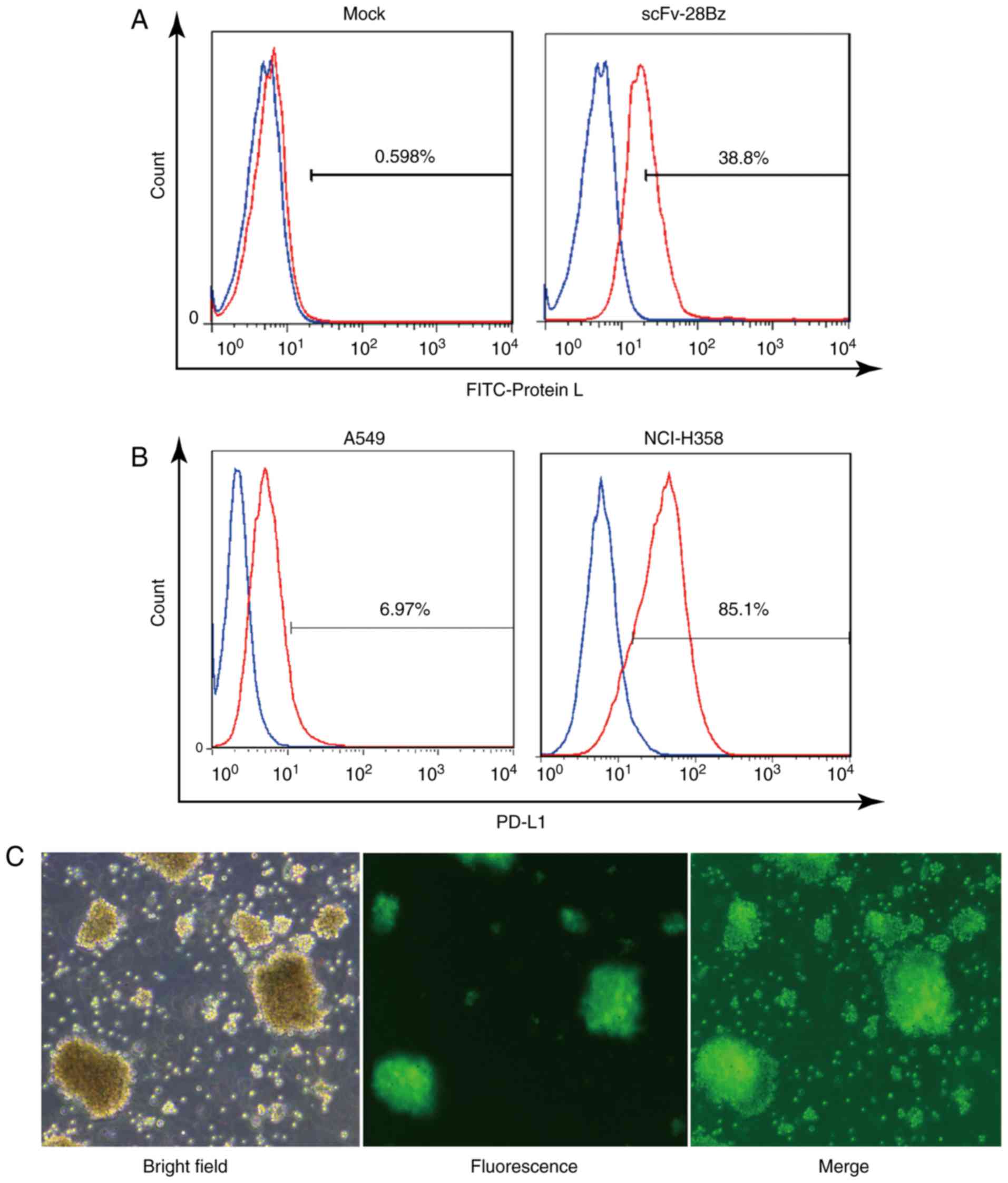
Expression of CAR on transduced cells
and differential PD-L1 expression in tumor cell lines
The FITC-Protein L may effectively bind to the
k-light chain of scFv, as reported by Zheng et al (48). As depicted in Fig. 2A, according to FITC-Protein L
staining, the scFv-28Bz-positive cells accounted for ~39% of the
total cells, compared with <1% in the non-transgenic control,
which indicated that scFv-28Bz was efficiently expressed on T
cells. PD-L1 was expressed on 6.97% of A549 cells and 85.1% of
NCI-H358 cells, as depicted in Fig.
2B. Therefore, A549 was selected to represent negative PD-L1
expression, while NCI-H358 was used as the PD-L1-positive cell
line. As a systematic parallel experimental control, the
LV-EF1α-GFP virus had a high infection efficiency in PBMCs, as
depicted in Fig. 2C. The transfection
efficiency of the viral system ensured the reliability of the
expression of the CAR on the PBMCs.
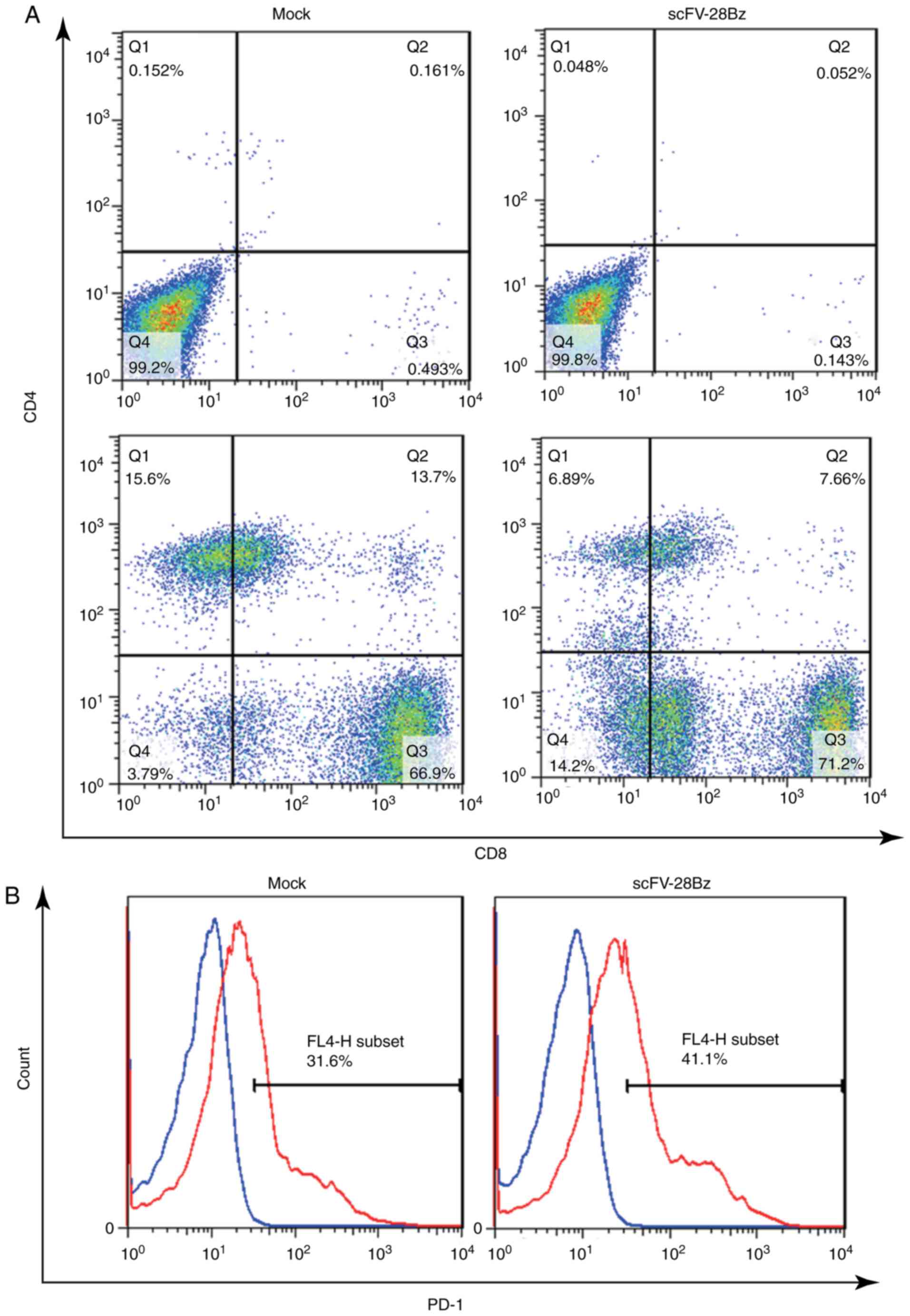
CD4+ and CD8+
cells account for the majority of PBMCs, and PD1 is highly
expressed in these cells
On day 14 post-transduction, the cells were
collected to analyze the subsets of CD4+ and
CD8+ cells and the expression of PD-1. As depicted in
Fig. 3A, the CD4+ subset
accounted for 10–30% of the total number of cells, and the
CD8+ subset accounted for 70–90% of the total number of
cells. The expression of PD-1 was 30–50%, as depicted in Fig. 3B.
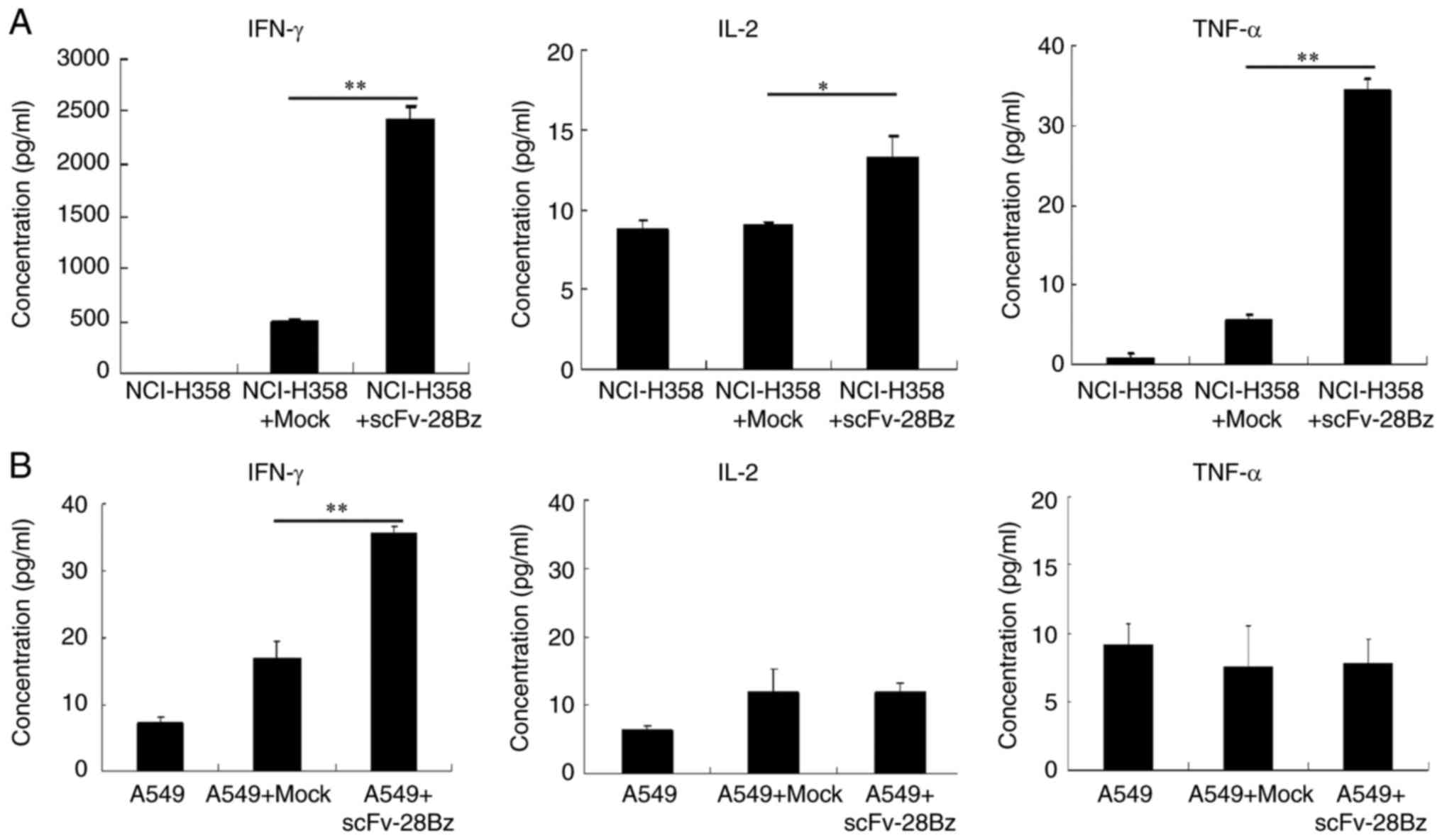
IFN-γ, IL-2 and TNF-α production in T
cells
The results revealed that the co-culture of
transduced T cells with NCI-H358 cells induced significantly
increased production of IFN-γ, compared with mock T cells with
NCI-H358 (P<0.01; Fig. 4A), but
the levels of IL-2 and TNF-α were low. The levels of cytokines in
the supernatants of co-cultured cells with A549 cells were <40
pg/ml (Fig. 4B).
Transduced T cells exhibit a mild
ability to kill NCI-H358 cells, but not A549 cells
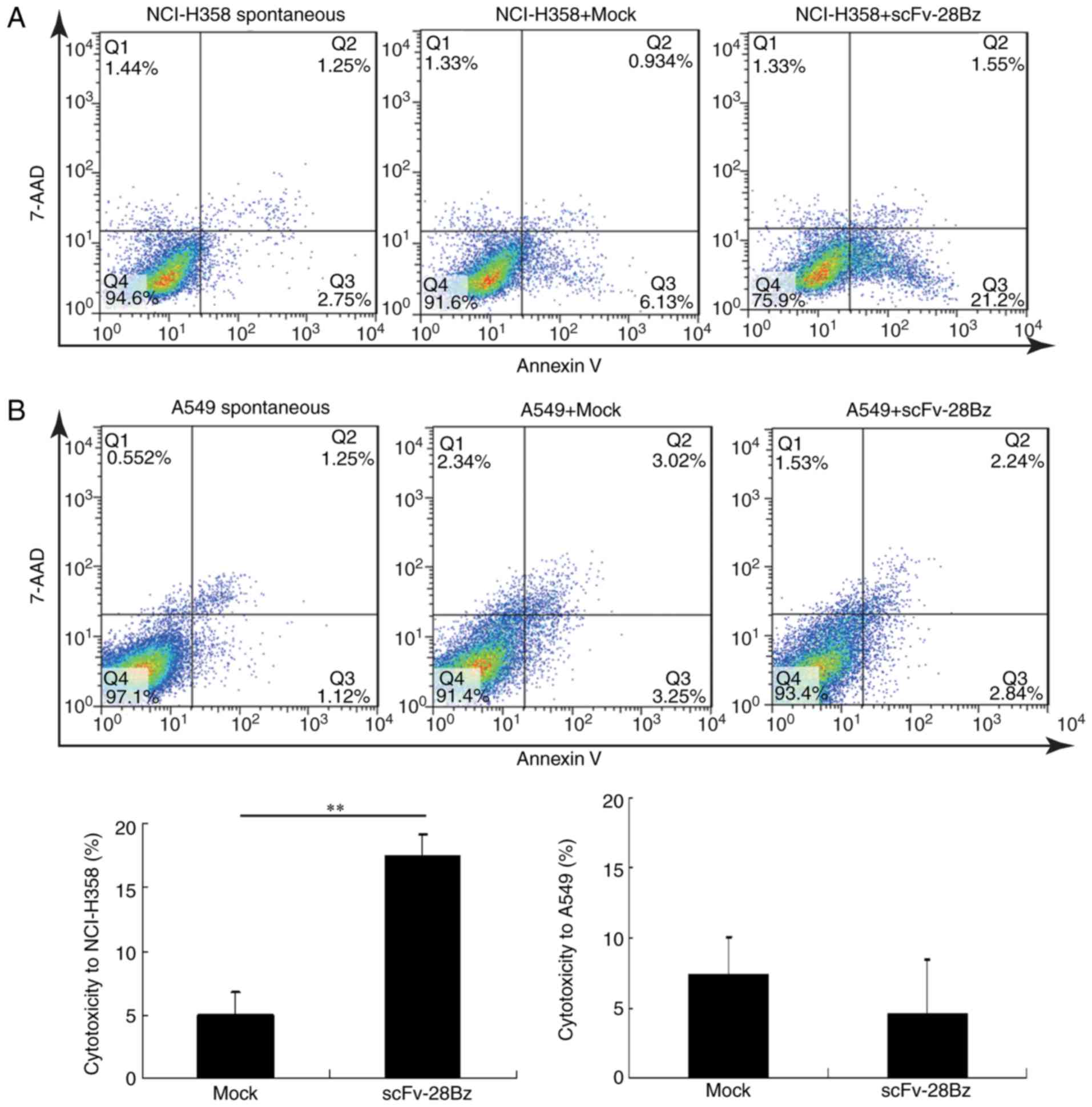
The cytotoxicity percentages of mock and scFv-28Bz T
cells against NCI-H358 cells were 5±1.7 and 17.3±1.8%, respectively
(Fig. 5A), and scFv-28Bz was
significantly higher, compared with the mock control group
(P<0.01). The cytotoxicity percentages of mock and scFv-28Bz T
cells against A549 cells were 7.3±2.77 and 4.5±3.96%, respectively,
and there was no significant difference between these groups
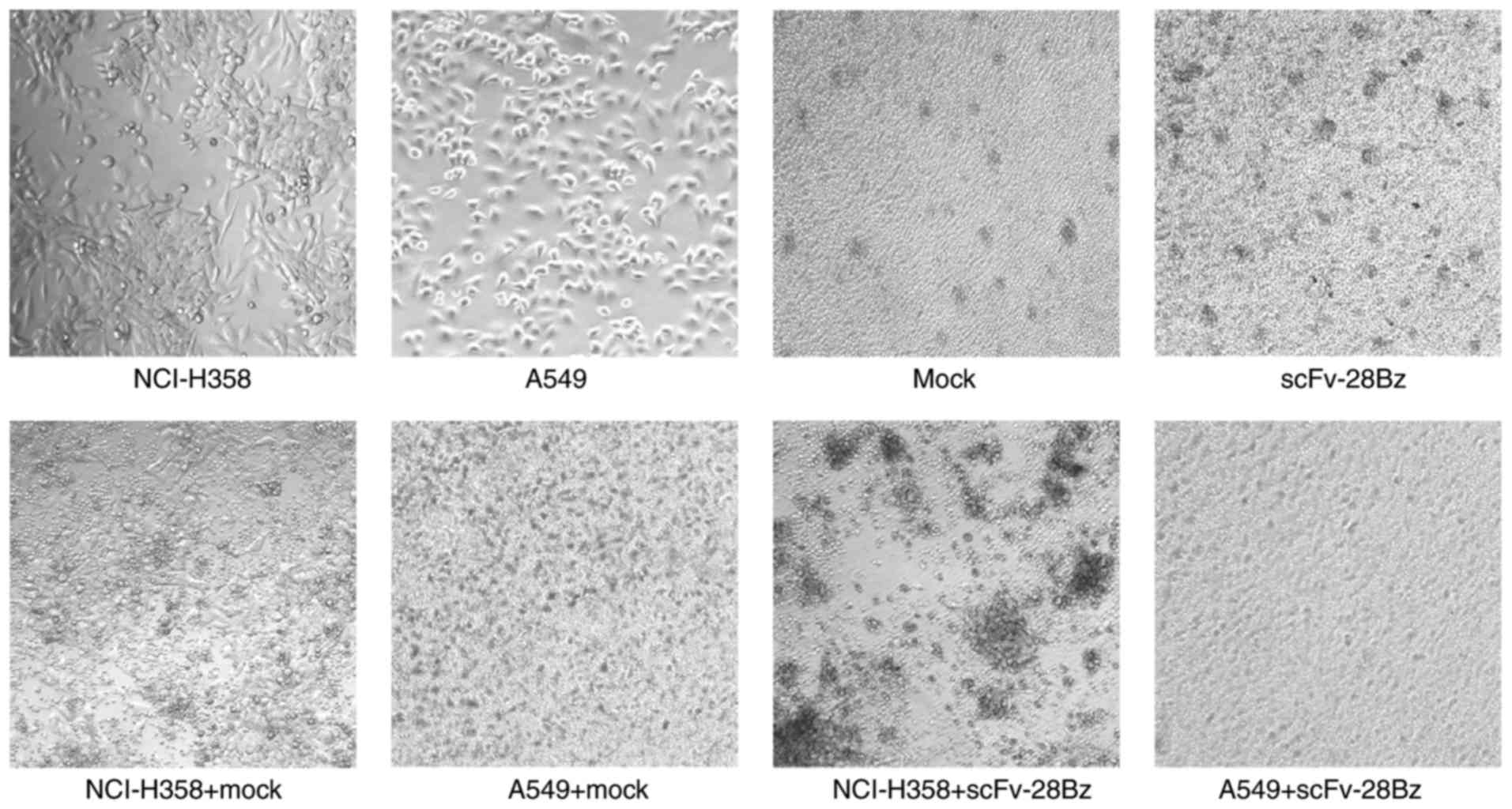
(Fig. 5B). The morphology of
co-cultured cells also demonstrated specific cytotoxicity of CAR-T
cells against PD-L1+ NCI-H358 cells, but no significant
effect on A549 cells expressing low PD-L1 levels (Fig. 6).
Discussion
Lung cancer is a lethal disease, the
etiopathogenesis of which is varied and complex (53–55). There
are several types of lung cancer, and different treatment methods
are adopted depending on the type and level of development;
however, more effective treatment programs are required (3,56). In
recent years, immunotherapy has provided a good option for the
treatment of lung cancer, and is expected to relieve the pain
induced by cancer (3). Previous CAR-T
cell studies based on CD19 as a target have demonstrated positive
effects (19,20,45,57).
Furthermore, increasing evidence on immune checkpoints has provided
a potential novel approach to tumor therapy (36,37,58–61).
In 2014, PD-L1 immunotherapy utilizing a monoclonal antibody,
MPDL3280A, was demonstrated to be effective in the treatment of
metastatic urothelial bladder cancer, and this therapy received
breakthrough designation status by the US Food and Drug
Administration in June 2014 (62).
Using the monoclonal antibody MPDL3280A, another study also
demonstrated the inhibition of a variety of cancer types with high
PD-L1 expression, which suppressed the pre-existing immunity
towards the tumor antigen (52).
Therefore, the light chain and heavy chain of MPDL3280A was
selected to produce a CAR together with an intracellular domain. To
use PD-L1 as a novel target for the treatment of solid tumors with
CAR-T cells, it was determined that T cells expressing scFv-28z did
not attack nearby T cells, despite the fact that T cells expressed
low PD-L1 (63), and the number of T
cells was effectively amplified. This indicated that it may be safe
to select PD-L1 as a target. The data indicated that CAR-T cells
were able to release IFN-γ at a high level when co-cultured with
NCI-H358 cells, but not when co-cultured with <100 pg/ml A549
cells, which demonstrated a PD-L1-specific interaction with scFv.
Furthermore, low concentrations of IL-2 and TNF-α were observed,
reflecting that exposure to an antigen for a long time may lead to
low cytokine production by T cells, which requires reversal by
multiple simultaneous treatments (64). Similarly, the apoptosis assay revealed
mild specific cytotoxicity against PD-L1-positive cells at an
effector-to-target T ratio of 10:1. The results demonstrated that
effectively blocking the PD-1 pathway may reactivate T cell
activity that has been decreased due to PD-L1 expression in the
solid tumor microenvironment. From another perspective, the
moderate killing effect may be an ideal result of target blocking.
Furthermore, the high level of PD-1 expression in T cells, as
depicted in Fig. 3, demonstrated that
complete blocking of the signaling pathway by only one treatment
may not be easy to implement, and that a variety of blocking
methods, including combination with PD-1 blocking, may lead to
improved results.
Studies on tumor therapy have indicated that the
presence or absence of invasive T cells is critical in determining
the response of patients to treatment. The inhibitory effect of the
tumor microenvironment on immunity may manifest as the absence of
infiltrating cells (65,66). If tumor-specific T cells are unable to
effectively reach the tumor cells and to accumulate in their
vicinity, it is not possible to remove the tumor cells (18). By contrast, PD-L1-targeted T cells may
avoid being weakened, and accumulate in the vicinity of tumor cells
and infiltrate into the tumor to exert cytotoxic effect. As a
therapeutic method, PD-L1 CAR-T presented its specificity against
tumor cells with a high expression of PD-L1. PD-L1 CAR may be
considered to be a shield and a probe that is directed toward tumor
cells with high expression of PD-L1. This is of clinical
significance for adoptive cell immunotherapy. During the T-cell
immunotherapy, effective defense against PD-L1 in the tumor
microenvironment is a prerequisite for maintaining a continuous
antitumor effect (28,62,67–69).
In summary, PD-L1-targeted CAR lentiviral vectors
and efficiently transduced T cells were constructed. The CAR-T
cells exerted a mild cytotoxic effect against a PD-L1-positive lung
cancer cell line in vitro; however, this approach requires
further optimization in order to obtain improved results.
The results indicated the possibility of treating
tumors by targeting immune checkpoints in the tumor
microenvironment, however it remains unknown whether CAR-T cells
attack normal tissues expressing PD-L1. As the results of the
present study demonstrate, it is confirmed that CAR-T does not
attack nearby T-cells with low PD-L1 expression. In addition, PD-L1
CAR may be further developed to a dual target CAR to achieve
improved safety and specificity.
Acknowledgements
The authors would like to thank Mr Hongbao Yao
(Beijing Bio DC Labs, Beijing, China) for his technical assistance
with the flow cytometry operation and Dr Xiaobin Chen (Beijing Bio
DC Labs) for reviewing the manuscript.
Funding
The present study was supported by the National High
Technology Research and Development Program of China (grant no.
2014AA022206).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and ZZ designed the experiments, analyzed the
data and wrote the manuscript. SJ and XL contributed to the initial
idea and approved the final version to be published.
Ethics statement and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018.PubMed/NCBI
|
|
4
|
Tanoue LT and Detterbeck FC: New TNM
classification for non-small-cell lung cancer. Expert Rev
Anticancer Ther. 9:413–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lemjabbar-Alaoui H, Hassan OU, Yang YW and
Buchanan P: Lung cancer: Biology and treatment options. Biochim
Biophys Acta. 1856:189–210. 2015.PubMed/NCBI
|
|
6
|
Eshhar Z, Waks T, Gross G and Schindler
DG: Specific activation and targeting of cytotoxic lymphocytes
through chimeric single chains consisting of antibody-binding
domains and the gamma or zeta subunits of the immunoglobulin and
T-cell receptors. Proc Natl Acad Sci USA. 90:720–724. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kochenderfer JN and Rosenberg SA: Treating
B-cell cancer with T cells expressing anti-CD19 chimeric antigen
receptors. Nat Rev Clin Oncol. 10:267–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson LA and June CH: Driving
gene-engineered T cell immunotherapy of cancer. Cell Res. 27:38–58.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Wu Z, Liu Y and Han W: New
development in CAR-T cell therapy. J Hematol Oncol. 10:532017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper LJ, Topp MS, Serrano LM, Gonzalez
S, Chang WC, Naranjo A, Wright C, Popplewell L, Raubitschek A,
Forman SJ and Jensen MC: T-cell clones can be rendered specific for
CD19: Toward the selective augmentation of the
graft-versus-B-lineage leukemia effect. Blood. 101:1637–1644. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stirrups R: CAR T-cells for relapsed
B-cell ALL in children and young adults. Lancet Oncol. 19:e1442018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilbert JA: CAR T-cells for relapsed
B-cell ALL in adults. Lancet Oncol. 19:e1432018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zuo BL, Yan B, Zheng GX, Xi WJ, Zhang X,
Yang AG and Jia LT: Targeting and suppression of HER3-positive
breast cancer by T lymphocytes expressing a heregulin chimeric
antigen receptor. Cancer Immunol Immunother. 67:393–401. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Byrd TT, Fousek K, Pignata A, Szot C,
Samaha H, Seaman S, Dobrolecki L, Salsman VS, Oo HZ, Bielamowicz K,
et al: TEM8/ANTXR1-Specific CAR T cells as a targeted therapy for
triple-negative breast cancer. Cancer Res. 78:489–500. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banerjee K, Kumar S, Ross KA, Gautam S,
Poelaert B, Nasser MW, Aithal A, Bhatia R, Wannemuehler MJ,
Narasimhan B, et al: Emerging trends in the immunotherapy of
pancreatic cancer. Cancer Lett. 417:35–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park YP, Jin L, Bennett KB, Wang D,
Fredenburg KM, Tseng JE, Chang LJ, Huang J and Chan EKL: CD70 as a
target for chimeric antigen receptor T cells in head and neck
squamous cell carcinoma. Oral Oncol. 78:145–150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Till BG, Jensen MC, Wang J, Qian X, Gopal
AK, Maloney DG, Lindgren CG, Lin Y, Pagel JM, Budde LE, et al:
CD20-specific adoptive immunotherapy for lymphoma using a chimeric
antigen receptor with both CD28 and 4-1BB domains: Pilot clinical
trial results. Blood. 119:3940–3950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fesnak AD, June CH and Levine BL:
Engineered T cells: The promise and challenges of cancer
immunotherapy. Nat Rev Cancer. 16:566–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalos M, Levine BL, Porter DL, Katz S,
Grupp SA, Bagg A and June CH: T cells with chimeric antigen
receptors have potent antitumor effects and can establish memory in
patients with advanced leukemia. Sci Transl Med. 3:95ra732011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DW, Kochenderfer JN, Stetler-Stevenson
M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M,
Shah NN, et al: T cells expressing CD19 chimeric antigen receptors
for acute lymphoblastic leukaemia in children and young adults: A
phase 1 dose-escalation trial. Lancet. 385:517–528. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brentjens RJ, Davila ML, Riviere I, Park
J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska
M, et al: CD19-targeted T cells rapidly induce molecular remissions
in adults with chemotherapy-refractory acute lymphoblastic
leukemia. Sci Transl Med. 5:177ra382013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Correction: Mesothelin-specific chimeric
antigen receptor mRNA-engineered T cells induce antitumor activity
in solid malignancies. Cancer Immunol Res. 3:2172015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brentjens RJ, Santos E, Nikhamin Y, Yeh R,
Matsushita M, La Perle K, Quintás-Cardama A, Larson SM and Sadelain
M: Genetically targeted T cells eradicate systemic acute
lymphoblastic leukemia xenografts. Clin Cancer Res. 13:5426–5435.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishida Y, Agata Y, Shibahara K and Honjo
T: Induced expression of PD-1, a novel member of the immunoglobulin
gene superfamily, upon programmed cell death. EMBO J. 11:3887–3895.
1992.PubMed/NCBI
|
|
25
|
Nishimura H, Nose M, Hiai H, Minato N and
Honjo T: Development of lupus-like autoimmune diseases by
disruption of the PD-1 gene encoding an ITIM motif-carrying
immunoreceptor. Immunity. 11:141–151. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keir ME, Butte MJ, Freeman GJ and Sharpe
AH: PD-1 and its ligands in tolerance and immunity. Annu Rev
Immunol. 26:677–704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwai Y, Ishida M, Tanaka Y, Okazaki T,
Honjo T and Minato N: Involvement of PD-L1 on tumor cells in the
escape from host immune system and tumor immunotherapy by PD-L1
blockade. Proc Natl Acad Sci USA. 99:12293–12297. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taube JM, Klein A, Brahmer JR, Xu H, Pan
X, Kim JH, Chen L, Pardoll DM, Topalian SL and Anders RA:
Association of PD-1, PD-1 ligands, and other features of the tumor
immune microenvironment with response to anti-PD-1 therapy. Clin
Cancer Res. 20:5064–5074. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharpe AH, Wherry EJ, Ahmed R and Freeman
GJ: The function of programmed cell death 1 and its ligands in
regulating autoimmunity and infection. Nat Immunol. 8:239–245.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dong H, Zhu G, Tamada K and Chen L: B7-H1,
a third member of the B7 family, co-stimulates T-cell proliferation
and interleukin-10 secretion. Nat Med. 5:1365–1369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Inoue Y, Yoshimura K, Mori K, Kurabe N,
Kahyo T, Mori H, Kawase A, Tanahashi M, Ogawa H, Inui N, et al:
Clinical significance of PD-L1 and PD-L2 copy number gains in
non-small-cell lung cancer. Oncotarget. 7:32113–32128. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Konishi J, Yamazaki K, Azuma M, Kinoshita
I, Dosaka-Akita H and Nishimura M: B7-H1 expression on non-small
cell lung cancer cells and its relationship with tumor-infiltrating
lymphocytes and their PD-1 expression. Clin Cancer Res.
10:5094–5100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DS, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt We, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fehrenbacher L, Spira A, Ballinger M,
Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D,
Artal-Cortes A, Lewanski C, et al: Atezolizumab versus docetaxel
for patients with previously treated non-small-cell lung cancer
(POPLAR): A multicentre, open-label, phase 2 randomised controlled
trial. Lancet. 387:1837–1846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suarez ER, de Chang K, Sun J, Sui J,
Freeman GJ, Signoretti S, Zhu Q and Marasco WA: Chimeric antigen
receptor T cells secreting anti-PD-L1 antibodies more effectively
regress renal cell carcinoma in a humanized mouse model.
Oncotarget. 7:34341–34355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cherkassky L, Morello A, Villena-Vargas J,
Feng Y, Dimitrov DS, Jones DR, Sadelain M and Adusumilli PS: Human
CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist
tumor-mediated inhibition. J Clin Invest. 126:3130–3144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kobold S, Grassmann S, Chaloupka M,
Lampert C, Wenk S, Kraus F, Rapp M, Düwell P, Zeng Y, Schmollinger
JC, et al: Impact of a new fusion receptor on PD-1-mediated
immunosuppression in adoptive T cell therapy. J Natl Cancer Inst.
107:2015. View Article : Google Scholar
|
|
44
|
Liu X, Ranganathan R, Jiang S, Fang C, Sun
J, Kim S, Newick K, Lo A, June CH, Zhao Y and Moon EK: A chimeric
switch-receptor targeting PD1 augments the efficacy of
second-generation CAR T cells in advanced solid tumors. Cancer Res.
76:1578–1590. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19–28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6:224ra252014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Savoldo B, Ramos CA, Liu E, Mims MP,
Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al:
CD28 costimulation improves expansion and persistence of chimeric
antigen receptor-modified T cells in lymphoma patients. J Clin
Invest. 121:1822–1826. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Long AH, Haso WM, Shern JF, Wanhainen KM,
Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara
VR, et al: 4-1BB costimulation ameliorates T cell exhaustion
induced by tonic signaling of chimeric antigen receptors. Nat Med.
21:581–590. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng Z, Chinnasamy N and Morgan RA:
Protein L: A novel reagent for the detection of chimeric antigen
receptor (CAR) expression by flow cytometry. J Transl Med.
10:292012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sommermeyer D, Hudecek M, Kosasih PL,
Gogishvili T, Maloney DG, Turtle CJ and Riddell SR: Chimeric
antigen receptor-modified T cells derived from defined CD8+ and
CD4+ subsets confer superior antitumor reactivity in vivo.
Leukemia. 30:492–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu X, Zhang Y, Cheng C, Cheng AW, Zhang
X, Li N, Xia C, Wei X, Liu X and Wang H: CRISPR-Cas9-mediated
multiplex gene editing in CAR-T cells. Cell Res. 27:154–157. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fischer K, Andreesen R and Mackensen A: An
improved flow cytometric assay for the determination of cytotoxic T
lymphocyte activity. J Immunol Methods. 259:159–169. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Travis WD, Brambilla E and Riely GJ: New
pathologic classification of lung cancer: Relevance for clinical
practice and clinical trials. J Clin Oncol. 31:992–1001. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Porter DL, Hwang WT, Frey NV, Lacey SF,
Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, et al:
Chimeric antigen receptor T cells persist and induce sustained
remissions in relapsed refractory chronic lymphocytic leukemia. Sci
Transl Med. 7:303ra1392015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Patel SP and Kurzrock R: PD-L1 expression
as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther.
14:847–856. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
McDermott DF and Atkins MB: PD-1 as a
potential target in cancer therapy. Cancer Med. 2:662–673.
2013.PubMed/NCBI
|
|
60
|
Baruch K, Deczkowska A, Rosenzweig N,
Tsitsou-Kampeli A, Sharif AM, Matcovitch-Natan O, Kertser A, David
E, Amit I and Schwartz M: PD-1 immune checkpoint blockade reduces
pathology and improves memory in mouse models of Alzheimer's
disease. Nat Med. 22:135–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
John LB, Devaud C, Duong CP, Yong CS,
Beavis PA, Haynes NM, Chow MT, Smyth MJ, Kershaw MH and Darcy PK:
Anti-PD-1 antibody therapy potently enhances the eradication of
established tumors by gene-modified T cells. Clin Cancer Res.
19:5636–5646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Powles T, Eder JP, Fine GD, Braiteh FS,
Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et
al: MPDL3280A (anti-PD-L1) treatment leads to clinical activity in
metastatic bladder cancer. Nature. 515:558–562. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Keir ME, Francisco LM and Sharpe AH: PD-1
and its ligands in T-cell immunity. Curr Opin Immunol. 19:309–314.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gargett T, Yu W, Dotti G, Yvon ES, Christo
SN, Hayball JD, Lewis ID, Brenner MK and Brown MP: GD2-specific CAR
T cells undergo potent activation and deletion following antigen
encounter but can be protected from activation-induced cell death
by PD-1 blockade. Mol Ther. 24:1135–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Joyce JA and Fearon DT: T cell exclusion,
immune privilege, and the tumor microenvironment. Science.
348:74–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Feig C, Jones JO, Kraman M, Wells RJ,
Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL,
et al: Targeting CXCL12 from FAP-expressing carcinoma-associated
fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic
cancer. Proc Natl Acad Sci USA. 110:20212–20217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sharma P and Allison JP: The future of
immune checkpoint therapy. Science. 348:56–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zou W and Chen L: Inhibitory B7-family
molecules in the tumour microenvironment. Nat Rev Immunol.
8:467–477. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|