Introduction
Malignant lymphoma arising in the cauda equina is
included in the entity of neurolymphomatosis (NL), which is
characterized by the infiltration of malignant lymphoma cells into
peripheral nerves, nerve roots, plexuses, or cranial nerves
(1,2).
NL is classified as primary and secondary. Primary NL is defined as
NL that is the first manifestation of the hematologic malignancy,
and secondary NL is defined as NL that is the site of relapse or
progression of a previously diagnosed lymphoma or leukemia
(1). NL is a very rare malignant
lymphoma, comprising only 0.2% of non-Hodgkin's lymphoma cases
(3). Furthermore, the frequency of
primary NL is approximately 20% of all NL cases and is less
frequent than secondary NL (4).
Therefore, primary NL is a rare entity. Primary cauda equina
lymphoma (CEL) as primary NL is an extremity rare condition in
which lymphoma cells primarily infiltrate the cauda equina. In
previous reports, only 22 cases of primary CEL have been reported.
Because primary CEL is an uncommon condition, detailed information
about clinical features and image findings has not been reported
yet. Furthermore, the clinical symptoms of CEL vary and may include
mild to severe muscle weakness or numbness of lower limbs, and
bladder and bowel dysfunction. These symptoms resemble lumbar
spinal stenosis, and thus, diagnosis of CEL may take time. As a
result, appropriate treatment may be delayed.
We encountered a case of primary CEL that was
diagnosed following F-18 2-fluoro-2-deoxy-glucose positron emission
tomography (FDG-PET) and cauda equina biopsy. In this report, we
describe an additional case of primary CEL and review the
literature with emphasis on clinical characteristics and imaging
features of primary CEL.
Case report
A 65-year-old man presented with gait disturbance
due to motor palsy in the bilateral lower extremities over the last
5 months. He also had severe numbness in his left sole. The
severity of the symptoms gradually increased, and he was admitted
to our hospital in a wheel chair. He had a history of L3-4
laminectomy with a diagnosis of lumbar spinal stenosis at another
hospital 1.5 years ago. He also had a urinary stent that had been
inserted in another hospital 2 years ago due to a diagnosis of
benign bladder hypertrophy. Neurological examination on admission
showed cauda equina syndrome below the L2 level. Testing of the
motor function of his lower extremities, including the iliopsoas,
quadriceps femoris, tibialis anterior, and gastrocnemius muscles,
showed marked palsy with a score of 3–4 following evaluation of
manual muscle testing. Deep tendon reflexes of the bilateral lower
extremities were diminished. Sensory disturbance was also found in
the bilateral lower extremities. A complete blood count showed
white blood cell (WBC) count; 46.9×102/µl, hemoglobin;
13.1 g/dl, platelets; 21.7×104/µl, and differential WBC
count with 59% neutrophils, 36% lymphocytes, and 3% monocytes. No
lymphoma cells were found in the peripheral blood. Laboratory data
showed that his lactate dehydrogenase was within normal limits (149
U/l), and his C-reactive protein was slightly elevated (0.41
mg/dl). Soluble interleukin 2 receptor (sIL-2R) was slightly
increased (514 U/ml).
Magnetic resonance (MR) images of the lumbar spine
demonstrated swollen cauda equina occupying the dural sac from the
L1-S1 level that was isointense on T1-weighted MR images and
hypointense on T2-weighted MR images compared to the spinal cord
(Fig. 1A). Gadolinium-enhanced
T1-weighted MR images revealed swelling of the cauda equina nerve
roots with diffuse enhancement (Fig. 1B
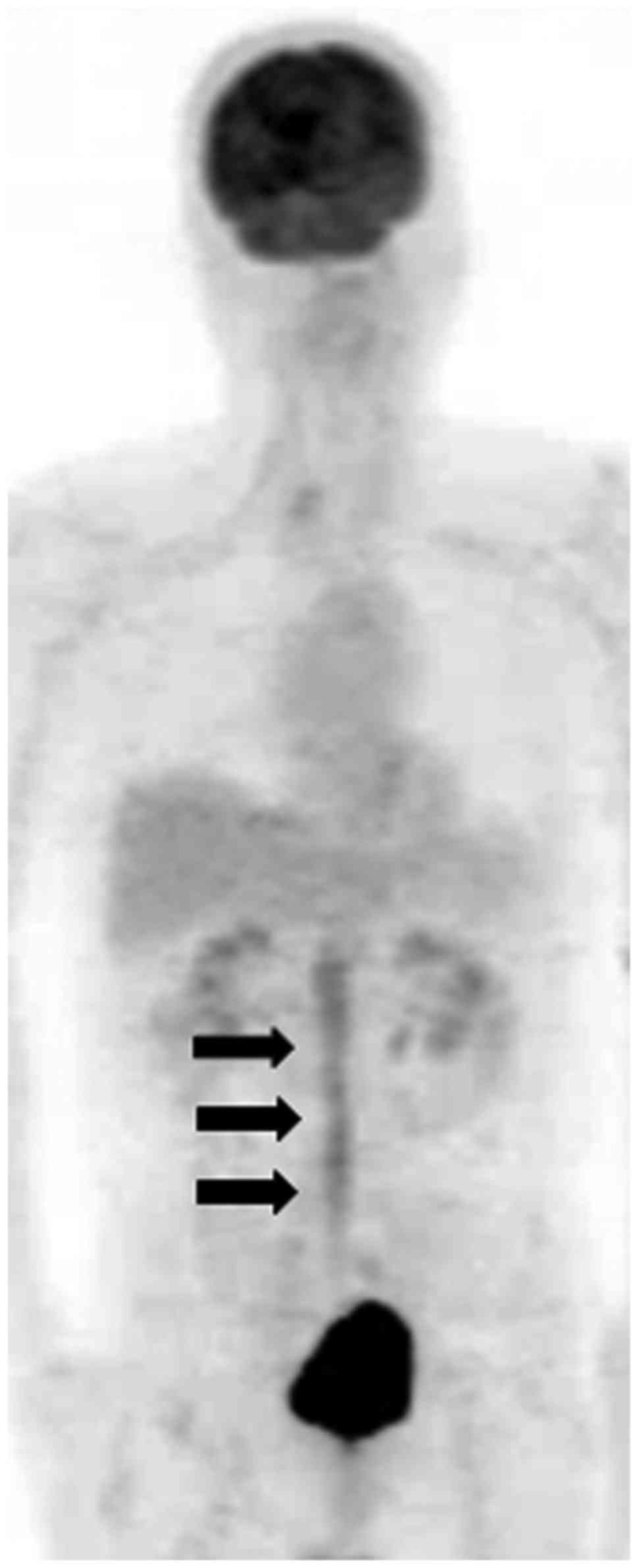
and C). FDG-PET showed diffuse accumulation of FDG in the cauda
equina with a maximum standardized uptake value (SUV) of 4.9
(Fig. 2). Examination of
cerebrospinal fluid (CSF) following a lumbar spinal tap showed that
the cell count was 443/µl, protein was 2470 mg/dl, and glucose was
less than 2 mg/dl. sIL-2R in CSF was remarkably increased to 2,033
U/ml, and cytology of the CSF was defined as stage IV. To obtain
more detailed pathological information, we performed a cauda equina
biopsy during motor evoked potential monitoring. After L4-5 partial
laminectomy and incision of the dural sac, the cauda equina was
observed as extremely swollen with grayish tumors present. The
tumors infiltrated extensively into the cauda equina and adhered
strongly to the nerve root. We performed the biopsy by cutting one
of the cauda equina. The patient did not have any further motor
deficits, severe leg pain, or numbness after surgery. Pathological
examination was performed (Fig. 3).
The pathological examination revealed that atypical cells with
irregular large nuclei and little cytoplasm had infiltrated into
the nerve (Fig. 3A and B).
Immunohistochemistry revealed that the atypical large cells were
positive for cluster of differentiation (CD)20, B-cell lymphoma 2
(BCL2), BCL6, multiple myeloma oncogene 1 (MUM-1), and negative for
CD3, CD5 and CD10 (Fig. 3C-E, G, H).
The nerve region which was showed by ‘single asterisk’ in the
Fig. 3A was positive by S-100
staining (Fig. 3F). Thus, we
diagnosed the pathology as diffuse large B-cell lymphoma (DLBCL),
non-germinal center type that originated in the cauda equina.
 | Figure 3.Histopathological examination and
immunohistochemistry of the cauda equina following a biopsy. (A)
Hematoxylin and eosin staining revealed lymphoma cells (*)
infiltrated into the nerve (**) (magnification, ×100). (B) Lymphoma
cells presented a large nuclei and little cytoplasm with a high
magnification view (magnification, ×200). Immunohistochemistry
showed that the atypical large lymphoma cells were negative for
(C), CD3, (D) CD5, and (E) CD10 (magnification, ×200). (F) The
nerve region infiltrated by lymphoma cells in figure A was positive
by S-100 staining (magnification, ×100). Immunostaining for (G)
CD20, (H) BCL2, and (I) MUM-1 were diffusely strong positive for
the atypical large lymphoma cells (magnification, ×200).
Immunostaining for (J) BCL6 was weakly positive for the atypical
large tumor cells (magnification, ×200). CD, cluster of
differentiation; BCL2, B-cell lymphoma 2; MUM-1, multiple myeloma
oncogene 1. |
He was treated with intravenous chemotherapy using
high-dose cytarabine plus high-dose methotrexate for four cycles in
4 months. The severe adverse events of this regimen were Grade 3–4
anemia and neutropenia, which required blood transfusion and
administration of granulocyte-colony stimulating factor. The
chemotherapy was effective. After chemotherapy was over, we
recommended radiotherapy to the patient, but because the patient
strongly refused, we followed up with him without radiotherapy. His
motor function was restored following rehabilitation to correct the
muscle weakness in the lower limbs, and he was able to walk without
any assistance 6 months after induction of chemotherapy. MR images
of the lumbar spine did not show any enhancement in the cauda
equina at 3 months after completion of chemotherapy (Fig. 4). He has maintained complete remission
for more than 6 years after the initial diagnosis of CEL.
This study was approved by the Ethics Committee of
the Toyama University Hospital (Toyama, Japan), Database of
Musculoskeletal Disease (no. 21-22) and Database of Musculoskeletal
Tumor (no. 24-40). The patient gave his written consent for this
report.
Discussion
Lymphoma cells rarely infiltrate nervous system
tissues. When malignant lymphoma occurs in the central nervous
system, it is called primary central nervous system lymphoma
(PCNSL), and PCNSL accounts for 4–6% of all malignant lymphomas
(5). When malignant lymphoma
infiltrates the peripheral nervous system, it is called NL
(1). Among primary NLs, invasion of
the cauda equina by lymphoma cells is extremely rare. Twenty-three
cases of primary CEL, including our case, have been reported so far
(2,6–25). We
reviewed these 23 cases regarding the clinical characteristics and
imaging of primary CEL and summarized these data in Table I. Among the reported cases of primary
CEL, the youngest patient was 11 years old (18), but the median age of CEL patients is
55 years. The male-to-female ratio is 1.3:1, which is similar to
that of PCNSL (5). PCNSL has an
increased incidence in immunocompromised hosts, such as patients
with acquired immunodeficiency syndrome, post-organ
transplantation, and congenital immunodeficiencies (26). In this series, immunocompromised hosts
included only two cases (8.7%), one with acquired immunodeficiency
syndrome and one with Epstein-Barr virus infection (8,11). The
duration from onset of symptoms to diagnosis was 6 months on
average. The shortest duration was 3 days (18), but in some cases, 3 years elapsed
before diagnosis of primary CEL (13,14).
Symptom onset varies from acute to subacute progression. Major
symptoms include lower back pain, muscle weakness, and numbness of
lower limbs, with or without mild to severe urinary dysfunction. In
some cases, the symptoms progress rapidly (18–20).
Neurological examination showed cauda equina syndrome in 17 cases
including our case (74%) (2,6–10,12,13,15,17–21,23,24),
radiculopathy in three cases (13%) (16,22), and
other symptoms (polyradiculoneuropathy, irritation, and facial
numbness) in three cases (13%) (11,14,25).
Because CEL involves some cauda equina roots, many patients with
CEL present with cauda equina syndrome.
 | Table I.The clinical characteristics of
primary cauda equina lymphoma. |
Table I.
The clinical characteristics of
primary cauda equina lymphoma.
|
|
|
|
|
|
| sIL-2R (U/ml) |
|
|
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Author, year | Age sex | Past history | Initial
symptom | Duration
(months) | Diagnosis
method | Serum | CSF | CSF | Other site | Subtype | Treatment | Out-come | F.U time
(months) | (Refs.) |
|---|
| Mauney and Sciotto,
1983 | 68 F | None | CES | 6 | Tumor excision | NA | NA | Leukocytes↑,
protein↑ | B-cell |
| RT | Alive | 3 | (6) |
| Toner et al,
1987 | 59 M | None | CES | 3.5 | Biopsy | NA | NA | Leukocytes↑,
protein↑ glucose↓ |
| B-cell | RT, CT | Alive | 22 | (7) |
| Klein et al,
1990 | 29 F | AIDS | CES | 0.5 | Tumor
resection | NA | NA | Leukocytes↑,
protein↑ glucose↓ |
| B-cell | NA | DOD | 1.25 | (8) |
| Knopp et al,
1994 | 69 F | None | CES | 0.75 | Biopsy | NA | NA | Leukocytes↑,
protein↑ |
| NA | NA | NA | NA | (9) |
| Ooi et al,
1996 | 16 M | None | CES | 0.75 | Biopsy | NA | NA | NA |
| T-cell | CT (i.t.) RT | DOD | 8 | (10) |
| Giobbia et
al, 1999 | 30 F | EBV | cauda equina
irritation | 2 | Cytology of
CSF | NA | NA | Leukocytes↑,
protein↑ glucose↓ |
| DLBCL | RT | Alive | 12 | (11) |
| Zagami and Granot,
2003 | 71 F | None | CES | 1 | 8th thoracic
laminectomy | NA | NA | Leukocytes↑ | 3rd cranial
nerve | DLBCL | CT (i.t.) | DOD | 15 | (12) |
| Kumar and Dyck,
2005 | 60 M | None | CES | 36 | Biopsy | NA | NA | Protein↑ | Bone marrow | Lympho-blastic | CT (i.v.) | Alive | 6 | (13) |
| Tajima et
al, 2007 | 67 F | None |
polyradiculo-neuropathy | 36 | Biopsy | 735 | 12333 | Pleocytosis,
protein↑ sIL-2R↑ |
| DLBCL | CT (i.t.) RT | Alive | 36 | (14) |
| Khong et al,
2008 | 16 M | None | CES | 1.5 | Biopsy | NA | NA | NA |
| DLBCL | CT (i.v.) RT | Alive | 12 | (2) |
| Morita et
al, 2009 | 67 M | None | CES | 2 | Tumor
resection | NA | NA | NA |
| NK/T-cell | RT | DOD | 14 | (15) |
| Beitzke et
al, 2010 | 69 M | None | lt. L5-S1
radiculopathy | 21 | Biopsy | NA | NA | Leukocytes↑,
protein↑ |
| DLBCL | CT | DOD | Soon | (16) |
| Teo et al,
2012 | 58 M | None | CES | 2 | Biopsy | NA | NA | Negative |
| DLBCL | CT (i.v.) RT | Alive | 24 | (17) |
| Cugati et
al, 2012 | 11 M | None | CES | 0.1 | Tumor excision | NA | NA | NA |
| B-cell | RT, CT | Alive | 12 | (18) |
| Iwasaki et
al, 2012 | 69 M | None | CES | 0.25 | Biopsy | NA | NA | NA |
| DLBCL | CT (i.v.) RT | Died (NED) | 18 | (19) |
| Nishida et
al, 2012 | 47 M | None | CES | 0.5 | Cytology of
CSF | 233 | NA | Leukocytes↑,
protein↑ glucose→ |
| DLBCL | CT (i.v., i.t.)
RT | Alive | 18 | (20) |
| Nakashima et
al, 2014 | 59 M | None | CES | 7 | Biopsy | 458 | NA | Atypical lymphoid
cell protein↑ |
| DLBCL | RT, CT | Alive | 12 | (21) |
| Broen et al,
2014 | 71 F | None | rt. S1
radiculopathy | 15 | Biopsy | NA | NA | Leukocytes↑,
protein↑ glucose↓ |
| DLBCL | CT (i.v.,
i.t.) | Alive | NA | (22) |
| Broen et al,
2014 | 75 F | None | lt. L5
radiculopathy | 5 | Biopsy | NA | NA | Leukocytes↑,
protein↑ glucose→ |
| DLBCL | Steroid only | DOD | 11 | (22) |
| Shin et al,
2016 | 79 F | DM | CES | 0.5 | Biopsy | NA | NA | NA |
| DLBCL | CT, RT | Alive | NA | (23) |
| Belcastro et
al, 2016 | 47 M | None | CES | 4 | Biopsy | NA | NA | Protein↑,
glucose↓ |
| DLBCL | CT (i.t.) | DOD | 2 | (24) |
| Wang et al,
2016 | 65 M | None | Headache, facial
numbness, lower limb numbness | NA | Cytology of
CSF | NA | NA | Leukocytes↑,
protein↑ glucose↓ | Cranial | B-cell nerve | NA | NA | NA | (25) |
| Our case | 65 M | None | CES | 6 | Biopsy | 514 | 2033 | Leukocytes↑,
protein↑ glucose↓, sIL-2R↑ |
| DLBCL | CT (i.v.) | Alive | 81 |
|
sIL-2R is expressed after B cell activation and is a
strong independent prognostic factor even for long-term observation
during treatment for DLBCL (27). The
serum level of sIL-2R is not a specific and highly sensitive marker
of PCNSL, but serial evaluation of sIL-2R may be useful for
monitoring therapeutic effectiveness (28). Among the previous reports of primary
CEL, only a case presented by Tajima et al showed elevation
of sIL-2R in serum and CSF (14). Our
present case also had elevated levels of sIL-2R slightly in serum
and markedly in CSF. Because sIL-2R in CSF was markedly elevated
similar to the report by Tajima et al, the sIL-2R level,
especially in CSF, could be quite useful for diagnosis of CEL
(14). However, only a few reports of
the level of sIL-2R in CSF are available, and the utility of
measuring sIL-2R in CSF and its cut-off value are not known in CEL
patients. Examination of CSF is helpful for the diagnosis of
meningitis, malignant tumors of the spinal cord including CEL, and
meningeal dissemination. In these diseases, CSF findings generally
show increased leukocytes, elevated protein levels, and decreased
glucose. In previously reported cases, an increase in leukocytes
was observed in 82.4% of patients, elevation of protein level in
88.2%, and a decline in glucose in 41.2% (Table I). However, distinguishing spinal cord
tumors from infections with these CSF findings is difficult.
Nishida et al reported that cytology and immunophenotyping
of CSF are effective diagnostic tools for CEL (20). In addition to general CSF examinations
such as the cell count and protein and glucose levels, if atypical
cells are observed with CSF cytology, immunophenotyping analysis
could improve the accuracy of diagnosis of CEL. In this review of
reported articles, only three cases were diagnosed with cytology
and CD20 immunotyping of CSF (11,20,25).
Furthermore, because a lumbar puncture for obtaining CSF is quickly
performed, Nishida et al reported that cytology of CSF is
useful for monitoring molecular disease recurrence (20). However, the majority of reported cases
were diagnosed with histopathological examination of tumors
obtained during surgery or biopsy of the tumor that affected the
cauda equina nerve. Broen et al recommended an early biopsy
of the nerve root for a definitive diagnosis (22). The possibility of irreversible nerve
damage due to nerve root biopsy is a concern, but the effectiveness
of obtaining a definitive diagnosis is thought to exceed that risk.
In particularly, biopsy of the infiltrated nerve is also
recommended for diagnosis of chronic inflammatory demyelinating
polyradiculopathy (29). In our
present case, we were able to obtain a definitive diagnosis
following a biopsy of the cauda equina. The major histopathological
type of CEL was the diffuse large B-cell type, which makes up
approximately 82.6% of reported cases of CEL. The T-cell type and
natural killer/T cell type are rare subtypes (10,15).
The imaging findings of these 23 cases of primary
CEL are summarized in Table II. MR
images are useful for assessing the location of lesions and
morphology of the cauda equina. Multiple levels of the spine are
involved, from T11 to S1. In more than 60% of the 23 reported
cases, CEL extended to the L1 to L4 level (Fig. 5). The most common MR finding of CEL is
swelling or enlargement of the cauda equina root (2,8–25). Enlarging nerve roots in CEL exhibit
mostly isointensity or low intensity relative to the signal of the
spinal cord on T1-weighted images (2,10,15,20,21,25),
and high signal intensity may be observed in rare cases (8,9). On
T2-weighted images, enlarging nerve roots of CEL show low or
isointensity relative to the spinal cord in 78% of cases (2,13–15,18,21–23,25),
and approximately 20% of cases show slight hyperintensity (17,19,20). In
all cases in which contrast MR imaging was performed, the enlarged
cauda equina exhibited a contrast effect (2,9–16,19–25). The
features of MR images of CEL are enlargement of the cauda equina
with iso- or low intensity relative to the spinal cord signal on
both T1- and T2-weighted images and the presence of enhancement of
the cauda equina on contrast MR images. The findings of MR images
in our case also showed enlargement of the cauda equina with
isointensity on T1-weighted images, low intensity on T2-weighted
images, and enhancement in contrast images, which were consistent
with the representative findings of CEL. Enlargement of the cauda
equina on MR imaging is observed not only in CEL, but also in
chronic inflammatory demyelinating polyradiculopathy,
neurofibromatosis, malignant peripheral nerve sheath tumors, and
metastatic cauda equina carcinoma tumors (25). Distinguishing between the
above-mentioned diseases and CEL with only MR image findings is
difficult. Therefore, elevation of protein and sIL-2R levels in the
CSF, CSF cytology, and CD20 immunophenotyping of CSF are considered
useful for adjunctive diagnosis of CEL. Ultimately, a nerve root
biopsy of the cauda equina can lead to a definitive diagnosis of
lymphoma (22).
 | Table II.Imaging findings of primary cauda
equina lymphoma. |
Table II.
Imaging findings of primary cauda
equina lymphoma.
|
| MR imaging | PET/CT |
|
|---|
|
|
|
|
|
|---|
| Author, year | Level | T1WI | T2WI | Enhancement | Swelling of cauda
equina | FDG
accumulation | SUV max | (Refs.) |
|---|
| Mauney and Sciotto,
1983 | NA | NA | NA | NA | NA | None |
| (6) |
| Toner et al,
1987 | NA | NA | NA | NA | NA | None |
| (7) |
| Klein et al,
1990 | L1-L2 | Focally high | NA | NA | + | None |
| (8) |
| Knopp et al,
1994 | L1-L3 | Heterogeneously
high | NA | + | + | None |
| (9) |
| Ooi et al,
1996 | L2-L4 | Isointense | NA | + | + | None |
| (10) |
| Giobbia et
al, 1999 | L5-S1 | NA | NA | + | + | None |
| (11) |
| Zagami and Granot,
2003 | Below cornus | NA | NA | + | + | None |
| (12) |
| Kumar and Dyck,
2005 | L2-S1 | NA | Low | + | + | None |
| (13) |
| Tajima et
al, 2007 | T12-L3 | NA | Low | + | + | None |
| (14) |
| Khong et al,
2008 | T12-L3 | Low | Low | + | + | No findings | NA | (2) |
| Morita et
al, 2009 | L3-L5 | Isointense | Low | + | + | None |
| (15) |
| Beitzke et
al, 2010 | T12-S1 | NA | NA | + | + | Increased | NA | (16) |
| Teo et al,
2012 | T11-L4 | NA | Slightly high | NA | + | None |
| (17) |
| Cugati et
al, 2012 | L2-L3 | Isointense | Isointense | NA | + | None |
| (18) |
| Iwasaki et
al, 2012 | T12-L1 | NA | Slightly high | + | + | None |
| (19) |
| Nishida et
al, 2012 | T12-L2 | Low | Slightly high | + | + | Increased | NA | (20) |
| Nakashima et
al, 2014 | T12-S1 | Low | Low | + | + | Increased | NA | (21) |
| Broen et al,
2014 | L2-L5 | NA | Low | + | + | Increased | NA | (22) |
| Broen et al,
2014 | T12-L5 | NA | Low | + | + | None |
| (22) |
| Shin et al,
2016 | L3-L5 | NA | Low | + | + | None |
| (23) |
| Belcastro et
al, 2016 | L2-L4 | NA | NA | + | + | Increased | NA | (24) |
| Wang et al,
2016 | T11-L5 | Low | Isointense | + | + | Increased | 9.6 | (25) |
| Our case | L1-S1 | Low | Low | + | + | Increased | 4.9 |
|
FDG-PET/CT examination of CEL was performed in eight
cases including our case among those reported after 2008 (2,16,20–22,24,25).
The findings of FDG-PET/CT show increasing FDG accumulation in
tumor lesions in seven cases (87.5%). However, the maximum SUV was
reported only for the two cases described by Wang et al
(25) and our case; the maximum SUV
were 9.6 and 4.9, respectively. These values are very low compared
with the median SUV max of 21 (range 8.2–47.1) of general DLBCL
(30). Because the maximum SUV for
CEL was reported in only two cases including our case, the average
or cut-off of the maximum SUV for CEL is unclear. On the other
hand, the maximum SUV for NL with multiple lesions ranges from 4.9
to 13.0 in multiple peripheral nerves (31). Therefore, in NL including CEL, the SUV
of the lesion may not be as high as in general DLBCL. In this case,
the reason for the low SUV max of CEL may reflect good prognostic
biological characteristics, as this patient with CEL maintained no
evidence of disease for 6 years after treatment. The maximum SUV on
FDG-PET/CT for newly diagnosed patients with DLBCL is an important
predictor of disease progression (30). FDG-PET/CT examination in CEL is also
useful for evaluating lesion spread, staging of lymphoma, and
response to therapy (25). However,
further information on the maximum SUV for NL including CEL is
required.
Treatment for PCNSLs includes radiotherapy alone,
chemotherapy alone, and radiation therapy combined with
chemotherapy. The reported treatments for primary CEL include three
cases treated with radiation alone, seven with chemotherapy alone,
and 10 with radiation combined with chemotherapy. The most
effective chemotherapeutic regimens for PCNSLs are high-dose
methotrexate and multimodal therapy such as adding other
chemotherapeutic agents with or without radiation (32). Ferreri et al reported that the
addition of high-dose cytarabine to high-dose methotrexate and
radiation improves the overall response rate from 40 to 69% and
prolongs progression-free survival from 3 to 18 months (33). In our case, the patient was treated
with combination therapy that included high-dose methotrexate and
high-dose cytarabine, and he has been living long-term for over 6
years with no evidence of disease. Major toxic effects of
chemotherapy with high-dose cytarabine plus methotrexate include
neutropenia, thrombocytopenia, and anemia, at a frequency of 90% or
more (32). In our case, the patient
showed neutropenia and Grade 3–4 anemia. Survival data for primary
CEL, including our case, were available for 21 of the 23 cases.
Thirteen patients were alive at the last follow-up observation
(follow-up period was 3 to 81 months).
In summary, primary CEL is a rare tumor among NL.
This is the first summary of the 23 reported cases, including our
present additional case, of primary CEL in terms of the clinical
characteristics, laboratory data, analysis of CSF, and features of
MR imaging and FDG-PET/CT. Clinical symptoms of CEL are more common
in cases of cauda equina syndrome, but are rarely seen in cases of
radiculopathy. The typical features seen on MR imaging are
enlargement of the cauda equina with iso- or low intensity relative
to the spinal cord signal on both T1- and T2-weighted imaging and
enhancement of the cauda equina on contrast MR images. Although a
few reports of FDG-PET/CT findings are available, FDG accumulation
in CEL appears to be increased, but to a lesser extent than in
general DLBCL. For the early adjunct diagnosis of CEL, measuring
sIL-2R in the CSF, CSF cytology, and immunotyping of CSF are
useful, but histopathological analysis following a biopsy of the
cauda equina is necessary for definitive diagnosis. The standard
treatment for CEL is chemotherapy using high-dose methotrexate, and
prognosis is expected to be better when starting treatment
early.
Acknowledgements
The authors would like to thank Dr Hirofumi Konishi,
Department of Neurology, University of Toyama (Toyama, Japan) and
Dr Jun Murakami, The Third Department of Internal Medicine,
University of Toyama for providing specialized discussion on
diagnosis and therapy.
Funding
This study was supported by the Japan Society for
the Promotion of Science (JSPS), grant no. JP17K16681.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KS and TY made substantial contributions to
conception and design. KS and TH contributed to analysis and
interpretation of data. MK and TK assisted in the data analysis. YK
was involved in the surgical treatment. TY and YK were involved in
drafting the manuscript or revising it critically for important
intellectual content. KS made a critical revision of the article
for important intellectual content. All authors approved the final
version of the manuscript.
Ethics approval and consent to
participate
This report was approved by the Ethics Committee,
University of Toyama (Toyama, Japan) and clinical research (no.
21-22) was granted.
Consent for publication
Written informed consents were obtained from the
patient for publication of this report and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NL
|
neurolymphomatosis
|
|
CEL
|
cauda equina lymphoma
|
|
FDG-PET/CT
|
2-fluoro-2-deoxy-glucose positron
emission tomography/computed tomography
|
|
WBC
|
white blood cell
|
|
sIL-2R
|
soluble interleukin 2 receptor
|
|
MR
|
magnetic resonance
|
|
SUV
|
standardized uptake value
|
|
CSF
|
cerebrospinal fluid
|
|
CD
|
cluster of differentiation
|
|
BCL2
|
B-cell lymphoma 2
|
|
MUM-1
|
multiple myeloma oncogene 1
|
|
DLBCL
|
diffuse large B-cell lymphoma
|
|
PCNSL
|
primary central nervous system
lymphoma
|
References
|
1
|
Grisariu S, Avni B, Batchelor TT, van den
Bent MJ, Bokstein F, Schiff D, Kuittinen O, Chamberlain MC, Roth P,
Nemets A, et al: Neurolymphomatosis: An international primary CNS
lymphoma collaborative group report. Blood. 115:5005–5011. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khong P, Pitham T and Owler B: Isolated
neurolymphomatosis of the cauda equina and filum terminale: Case
report. Spine (Phila Pa 1976). 33:E807–E811. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baehring JM, Damek D, Martin EC, Betensky
RA and Hochberg FH: Neurolymphomatosis. Neuro Oncol. 5:104–115.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lagarde S, Tabouret E, Matta M, Franques
J, Attarian S, Pouget J, De Paula Maues A, Figarella-Branger D,
Dory-Lautrec P, Chinot O and Barrié M: Primary neurolymphomatosis
diagnosis and treatment: A retrospective study. J Neurol Sci.
342:178–181. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deckert M, Paulus W, Kluin PM and Ferry
JA: Diffuse large B-cell lymphoma of the CNSLouis DN, Ohgaki H,
Wiestler OD and Cavenee WK: WHO Classification of Tumours of the
Central Nervous System. WHO/IARC Classification of Tumours,
Revised. 4th edition. 1. IAR Press; Lyon: pp. 272–275. 2016
|
|
6
|
Mauney M and Sciotto CG: Primary malignant
lymphoma of the cauda equina. Am J Surg Pathol. 7:185–190. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toner GC, Holmes R, Sinclair RA, Tang SK
and Schwarz MA: Central nervous system lymphoma: Primary lumbar
nerve root infiltration. Acta Haematol. 81:44–47. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klein P, Zientek G, VandenBerg SR and
Lothman E: Primary CNS lymphoma: Lymphomatous meningitis presenting
as a cauda equina lesion in an AIDS patient. Can J Neurol Sci.
17:329–331. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knopp EA, Chynn KY and Hughes J: Primary
lymphoma of the cauda equina: Myelographic, CT myelographic, and MR
appearance. AJNR Am J Neuroradiol. 15:1187–1189. 1994.PubMed/NCBI
|
|
10
|
Ooi GC, Peh WC and Fung CF: Case report:
Magnetic resonance imaging of primary lymphoma of the cauda equina.
Br J Radiol. 69:1057–1060. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giobbia M, Carniato A, Scotton PG, Vaglia
A and Marchiori GC: Primary EBV-associated cauda equina lymphoma. J
Neurol. 246:739–740. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zagami AS and Granot R: Non-Hodgkin's
lymphoma involving the cauda equina and ocular cranial nerves: Case
reports and literature review. J Clin Neurosci. 10:696–699. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar N and Dyck PJ: Hypertrophy of the
nerve roots of the cauda equina as a paraneoplastic manifestation
of lymphoma. Arch Neurol. 62:1776–1777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tajima Y, Sudo K and Matumoto A: Malignant
lymphoma originating in the cauda equina mimicking the inflammatory
polyradiculoneuropathy. Intern Med. 46:1029–1032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morita M, Osawa M, Naruse H and Nakamura
H: Primary NK/T-cell lymphoma of the cauda equina: A case report
and literature review. Spine (Phila Pa 1976). 34:E882–E885. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beitzke M, Enzinger C, Beitzke D,
Neureiter D, Ladurner G and Fazekas F: Primary leptomeningeal
lymphoma of the cauda equina: A rare cause of radiculopathy. J
Neurol. 257:1734–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Teo MK, Mathieson C, Carruthers R, Stewart
W and Alakandy L: Cauda equina lymphoma-a rare presentation of
primary central nervous system lymphoma: Case report and literature
review. Br J Neurosurg. 26:868–871. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cugati G, Singh M, Symss NP, Pande A,
Vasudevan MC and Ramamurthi R: Primary spinal intradural
extramedullary lymphoma causing cauda equina syndrome. J
Craniovertebr Junction Spine. 3:58–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwasaki M, Hida K, Yano S, Aoyama T,
Kaneko S and Iwasaki Y: Primary cauda equina lymphoma treated with
high-dose methotrexate. Neurol Med Chir (Tokyo). 52:679–683. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nishida H, Hori M and Obara K: Primary
B-cell lymphoma of the cauda equina, successfully treated with
high-dose methotrexate plus high-dose cytarabine: A case report
with MRI findings. Neurol Sci. 33:403–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakashima H, Imagama S, Ito Z, Ando K,
Kobayashi K, Ukai J, Muramoto A, Shinjyo R, Matsumoto T, Yamauchi
I, et al: Primary cauda equina lymphoma: Case report and literature
review. Nagoya J Med Sci. 76:349–354. 2014.PubMed/NCBI
|
|
22
|
Broen M, Draak T, Riedl RG and Weber WE:
Diffuse large B-cell lymphoma of the cauda equina. BMJ Case Rep.
2014:pii: bcr2014205950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shin HK, Oh SK, Woo CG, Huh JR, Suh CW and
Jeon SR: Cauda equina lymphoma mimicking non-neoplastic
hypertrophic neuropathy of the cauda equina: A case report. Br J
Neurosurg. 30:678–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Belcastro V, Bellcocchi S, Patriarca C,
Gini G, Piola M, Barca S and Arnaboldi M: Cauda equina syndrome due
to large B-cell lymphoma: A case report. Neurol Sci. 37:825–827.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang G, Liu Y and He F: Primary lymphoma
involving cranial nerves and cauda equina detected by (18)F-FDG
PET/CT and MRI. Nuklearmedizin. 55:N46–N48. 2016.PubMed/NCBI
|
|
26
|
Hochberg FH and Miller DC: Primary central
nervous system lymphoma. J Neurosurg. 68:835–853. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Goto N, Tsurumi H, Goto H, Shimomura YI,
Kasahara S, Hara T, Yasuda I, Shimizu M, Murakami N, Yoshikawa T,
et al: Serum soluble interleukin-2 receptor (sIL-2R) level is
associated with the outcome of patients with diffuse large B cell
lymphoma treated with R-CHOP regimens. Ann Hematol. 91:705–714.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kitai R, Sasaki H, Matsuda K, Tsunetoshi
K, Yamauchi T, Neishi H, Matsumura K, Tsunoda A, Takeuchi H, Sato K
and Kikuta K: Measurement and cellular sources of the soluble
interleukin-2 receptor in primary central nervous system lymphoma.
Brain Tumor Pathol. 30:34–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boukhris S, Magy L, Kabore R, Mabrouk T,
Li Y, Sindou P, Tabaraud F and Vallat JM: Atypical
electrophysiologic findings in chronic inflammatory demyelinating
polyneuropathy (CIDP)-diagnosis confirmed by nerve biopsy.
Neurophysiol Clin. 34:71–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyazaki Y, Nawa Y, Miyagawa M, Kohashi S,
Nakase K, Yasukawa M and Hara M: Maximum standard uptake value of
18F-fluorodeoxyglucose positron emission tomography is a prognostic
factor for progression-free survival of newly diagnosed patients
with diffuse large B cell lymphoma. Ann Hematol. 92:239–244. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hong CM, Lee SW, Lee HJ, Song BI, Kim HW,
Kang S, Jeong SY, Ahn BC, Lee J and Chae YS: Neurolymphomatosis on
F-18 FDG PET/CT and MRI findings: A case report. Nucl Med Mol
Imaging. 45:76–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grommes C and DeAngelis LM: Primary CNS
lymphoma. J Clin Oncol. 35:2410–2418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ferreri AJ, Reni M, Foppoli M, Martelli M,
Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G,
Ilariucci F, et al: High-dose cytarabine plus high-dose
methotrexate versus high-dose methotrexate alone in patients with
primary CNS lymphoma: A randomised phase 2 trial. Lancet.
374:1512–1520. 2009. View Article : Google Scholar : PubMed/NCBI
|