Introduction
Hepatocellular carcinoma (HCC) ranks as the third
leading cause of cancer-associated mortality, and accounts for ~80%
of primary liver cancers (1,2). Despite the development of surgical
techniques and advances in molecularly targeted drugs, therapeutic
approaches for the treatment of HCC provide an unsatisfactory
5-year overall survival (OS) rate due to late detection as a result
of the lack of specific symptoms in the early stages of the disease
(3). Thus, the investigation of
potential predictive biomarkers and therapeutic targets is required
to improve the survival rate for patients with HCC.
Long non-coding RNAs (lncRNAs) are ~200 nt to 100 kb
long and have emerged as key regulators in the majority of
biological processes (4,5). Previous studies have demonstrated that
lncRNAs participate in a number of tumor processes and may be
suitable as therapeutic targets and biomarkers for predicting the
prognosis of patients (6,7). For example, the expression of PCAT-1 was
observed to be significantly increased in HCC tissues, and was
significantly associated with the OS time of patients with HCC
(8). ZFAS1 may function as an
oncogene in HCC progression, as it has been demonstrated to bind
miR-150 and abrogate its tumor suppressive function, thus promoting
the expression of ZEB1 and the matrix metalloproteinases MMP14 and
MMP16 (9). CARLo-5 has been
identified as an independent risk factor for OS and disease-free
survival (DFS) in HCC; it may promote the proliferation and
metastasis of HCC, and is a potential novel therapeutic target
(10). The decreased expression of
the lncRNA GAS5 indicates a relatively poor prognosis, and promotes
cell proliferation and invasion in HCC, via the regulation of
vimentin (11).
The association between lncRNA Sox2 overlapping
transcript (lncRNA Sox2ot; on human chromosome 3q26.33) expression
and the epithelial-mesenchymal transition (EMT) in HCC was not
previously examined. In the present study, it was determined that
the expression level of lncRNA Sox2ot was significantly higher in
HCC tissues compared with adjacent non-tumor tissues. Furthermore,
it was demonstrated that cell invasion was inhibited following the
knockdown of lncRNA Sox2ot in HCC cells. The knockdown of lncRNA
Sox2ot in the cells downregulated the expression levels of Twist1
and N-cadherin, but upregulated the E-cadherin expression level.
Thus, the results of the study indicated that Sox2ot could be a
novel biomarker and a potential therapeutic target for patients
with HCC.
Materials and methods
Human tissue samples
A total of 86 HCC tissue samples paired with
adjacent normal tissue samples were obtained from patients who
underwent surgical resection between November 2009 and March 2014
at the Shandong Provincial Hospital Affiliated to Shandong
University (Jinan, China). The fresh tissue samples were
immediately frozen in liquid nitrogen and stored at −80°C. The
clinical stage was assessed using the 2010 6th edition Tumor Node
Metastasis (TNM) system (International Union of Cancer
Control/American Joint Committee of Cancer (12). The present study was approved by the
Ethical Committee of Shandong Provincial Hospital Affiliated to
Shandong University, and written informed consent was obtained from
all patients.
Cell lines and culture
The MHCC97H and SMMC-7721 human HCC cell lines and
the LO2 normal liver cell line were purchased from the Cell Bank of
Type Culture Collection (Chinese Academy of Sciences, Shanghai,
China). All cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and 1% penicillin and streptomycin
in a humidified atmosphere containing 5% CO2 at
37°C.
Cell transfection and RNAi
The cells were transfected with siRNA against lncRNA
Sox2ot purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou,
China). The siRNA sequences were as follows: siR-Sox2ot,
5′-CAAAAUAGGUCAUAGCAAATT-3′; si-negative control,
5′-UUCUCCGAACGUGUCACGUTT-3′. Cells were seeded into 6-well plate
and cultured for 48 h, then transfected with
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) as
per the manufacturer's protocol. At 48 h post-transfection, the
efficiency of transfection was assessed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
RT-qPCR
Total RNA was extracted from tissues and cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. RNA were reverse
transcribed into cDNA using a reverse transcription reagent kit
(Takara Biotechnology Co., Ltd., Dalian, China). qPCR was performed
using the SYBR® Green PCR kit (Takara Biotechnology Co.,
Ltd.). The PCR reaction conditions were as follows: Preliminary
denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for
5 sec and 60°C for 20 sec. Primer sequences were as follows: lncRNA
Sox2ot forward, 5′-GCTCGTGGCTTAGGAGATTG-3′ and reverse
5′-CTGGCAAAGCATGAGGAACT-3′; GAPDH forward,
5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′. The relative quantification
(2−ΔΔCt) method was used for calculating fold change
(13).
Transwell invasion assay
Cell invasion was detected using Transwell chambers
(8-µm pore size; Corning, Inc., Corning, NY, USA) with Matrigel (BD
Biosciences, San Jose, CA, USA). At 48 h post-cell transfection,
1×105 cells MHCC97H or SMMC-7721 cells were added into
the upper chamber in 400 µl FBS-free culture medium. A total of 500
µl culture medium containing 10% FBS was added to the lower
chamber. Cells were cultured for 48 h in a humidified atmosphere
containing 5% CO2 at 37°C. The invasive cells on the
lower chamber were fixed with 100% ethanol for 15 min at room
temperature, then stained with 0.1% crystal violet for 15 min at
room temperature. Cells were counted under a light microscope in 5
random fields (magnification, ×200).
Western blot analysis
The total protein was extracted from cells using
radioimmunoprecipitation assay lysis buffer (Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). Protein concentration was measured
using a bicinchoninic acid protein assay kit (EMD Millipore,
Billerica, MA, USA). Equal quantities of protein (40 µg/lane) were
separated via 10% SDS-PAGE and then transferred onto a
polyvinylidene fluoride membrane (EMD Millipore). The membranes
were blocked using 5% non-fat milk at room temperature for 1 h. The
blotted membranes were incubated with antibodies against Twist1
(cat. no. sc-6269; dilution, 1:1,000), E-cadherin (cat. no.
sc-21791; dilution, 1:1,000), N-cadherin (cat. no. sc-31031;
dilution, 1:500) and GAPDH (cat. no. sc-69778; dilution, 1:2,000;
all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
antibodies and were incubated at 4°C overnight. The membrane was
incubated with a secondary goat anti-rabbit horseradish
peroxidase-conjugated antibody (cat no. CW0102S; dilution, 1:2,000;
Jiangsu Kangwei Century Biotechnology Co., Ltd., Beijing, China)
for 1 h at room temperature. The protein bands were detected using
an enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
All data were analyzed using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). All continuous variable values were represented
as the mean ± standard deviation from ≥3 independent experiments.
Differences between two groups were analyzed using Student's
t-test, and data from multiple groups were analyzed with a one-way
analysis of variance (ANOVA). The Student-Newman-Keuls test was
used as a post-hoc test following ANOVA. The associations between
lncRNA Sox2ot expression and clinicopathological factors were
analyzed by the χ2 test. The Kaplan-Meier method and a
log-rank test were used to analyze the association between lncRNA
Sox2ot expression and DFS or OS time. P<0.05 was considered to
indicate a statistically significant difference.
Results
lncRNA Sox2ot is upregulated in HCC
tissues and associated with a poor prognosis for patients with
HCC
To investigate whether lncRNA Sox2ot affected
hepatocarcinogenesis, the expression level of lncRNA Sox2ot was
measured in HCC tissues and adjacent normal tissues using RT-qPCR.
The results demonstrated that lncRNA Sox2ot was upregulated in HCC
tissues compared with the adjacent normal tissues (Fig. 1A; P<0.05). HCC tissue samples were
divided into higher- and lower-expression groups based on the mean
expression level for all the samples. The patients with the higher
expression of lncRNA Sox2ot had a significantly increased tumor
size (P=0.007), tumor number (P=0.036) and vein invasion rate
(P=0.011; Table I; Fig. 1B-D). There was no significant
association between the expression of lncRNA Sox2ot and other
clinicopathological features, including age, sex, hepatitis B
infection status, histological grade and serum α-fetoprotein level
(Table I). Furthermore, it was
demonstrated that higher lncRNA Sox2ot expression was predictive
for a relatively poor DFS and OS time (Fig. 1E and F; log-rank, 8.567, P<0.05 and
log-rank, 8.339, P<0.05, respectively) compared with lower
lncRNA Sox2ot expression.
 | Table I.The association between lncRNA Sox2ot
expression and clinicopathological features. |
Table I.
The association between lncRNA Sox2ot
expression and clinicopathological features.
|
|
| lncRNA Sox2ot
expression, n |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
features | All | Low | High | χ2
test | P-value |
|---|
| Total | 86 | 41 | 45 |
|
|
| Sex |
|
|
| 0.255a | 0.059 |
|
Female | 23 | 12 | 11 |
|
|
| Male | 63 | 29 | 34 |
|
|
| Age, years |
|
|
| 0.100a | 0.752 |
| ≤60 | 56 | 26 | 30 |
|
|
|
>60 | 30 | 15 | 15 |
|
|
| Tumor size, cm |
|
|
| 7.274a | 0.007b |
|
<5 | 50 | 30 | 20 |
|
|
| ≥5 | 36 | 11 | 25 |
|
|
| Hepatitis B
infection |
|
|
| 0.081a | 0.776 |
|
Positive | 60 | 28 | 32 |
|
|
|
Negative | 26 | 13 | 13 |
|
|
| Histological
grade |
|
|
| 1.325a | 0.250 |
| Well | 32 | 20 | 12 |
|
|
|
Moderate | 24 | 12 | 12 |
|
|
| Low | 30 | 9 | 21 |
|
|
| Tumor number |
|
|
| 2.628a | 0.036b |
|
Single | 49 | 26 | 23 |
|
|
|
Multiple | 37 | 15 | 22 |
|
|
| α-fetoprotein,
ng/ml |
|
|
| 2.098a | 0.148 |
|
<400 | 31 | 18 | 13 |
|
|
|
≥400 | 55 | 23 | 32 |
|
|
| Vein invasion |
|
|
| 6.539a | 0.011b |
|
Negative | 34 | 22 | 12 |
|
|
|
Positive | 52 | 19 | 33 |
|
|
| AJCC stage |
|
|
| 0.078a | 0.780 |
|
I–II | 49 | 24 | 25 |
|
|
|
III–IV | 37 | 17 | 20 |
|
|
lncRNA Sox2ot promotes cell invasion
in MHCC97H and SMCC-7721 cells
It was determined that lncRNA Sox2ot expression was
higher in MHCC97H and SMCC-7721 HCC cells compared with LO2
non-cancer cells (Fig. 2A). lncRNA
Sox2ot was knocked down with siRNA in MHCC97H and SMCC-7721 cells.
It was verified that the expression of lncRNA Sox2ot was reduced by
the transfection with the lncRNA Sox2ot siRNA compared with a
control oligonucleotide (Fig. 2B and
C). Transwell cell invasion assays demonstrated that the cell
invasion ability was inhibited following the knockdown of lncRNA
Sox2ot in MHCC97H and SMCC-7721 cells (Fig. 2D-F). Thus, the results indicated that
lncRNA Sox2ot promoted invasion by HCC cells.
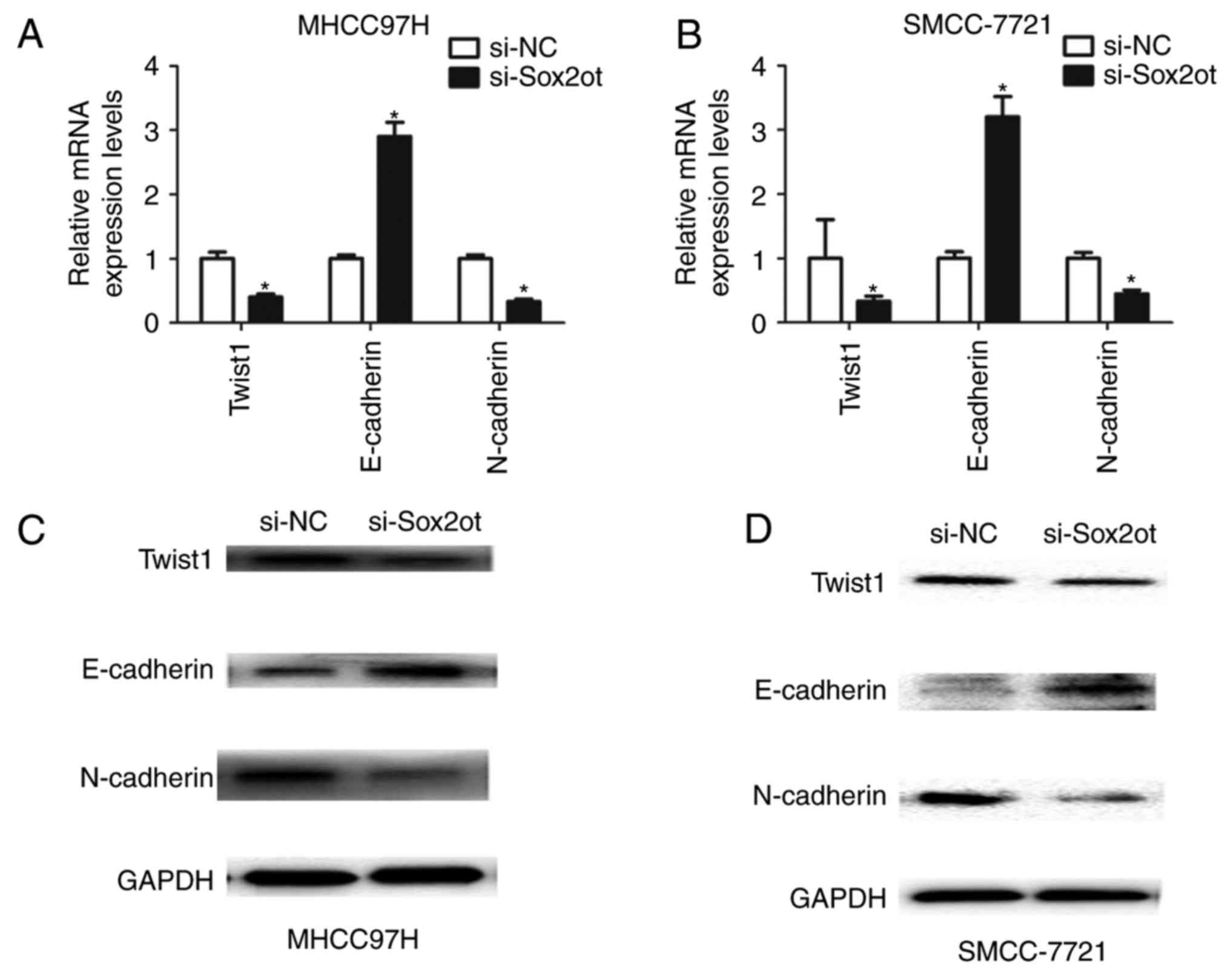
lncRNA Sox2ot promotes cell EMT in
MHCC97H and SMCC-7721 cells
Furthermore, to evaluate the association between
lncRNA Sox2ot expression and the EMT process, the expression of
EMT-associated factors was assessed. It was identified that the
mRNA level of E-cadherin was upregulated, and those of Twist1 and
N-cadherin downregulated following the knockdown of lncRNA Sox2ot
in MHCC97H or SMCC-7721 cells (Fig. 3A
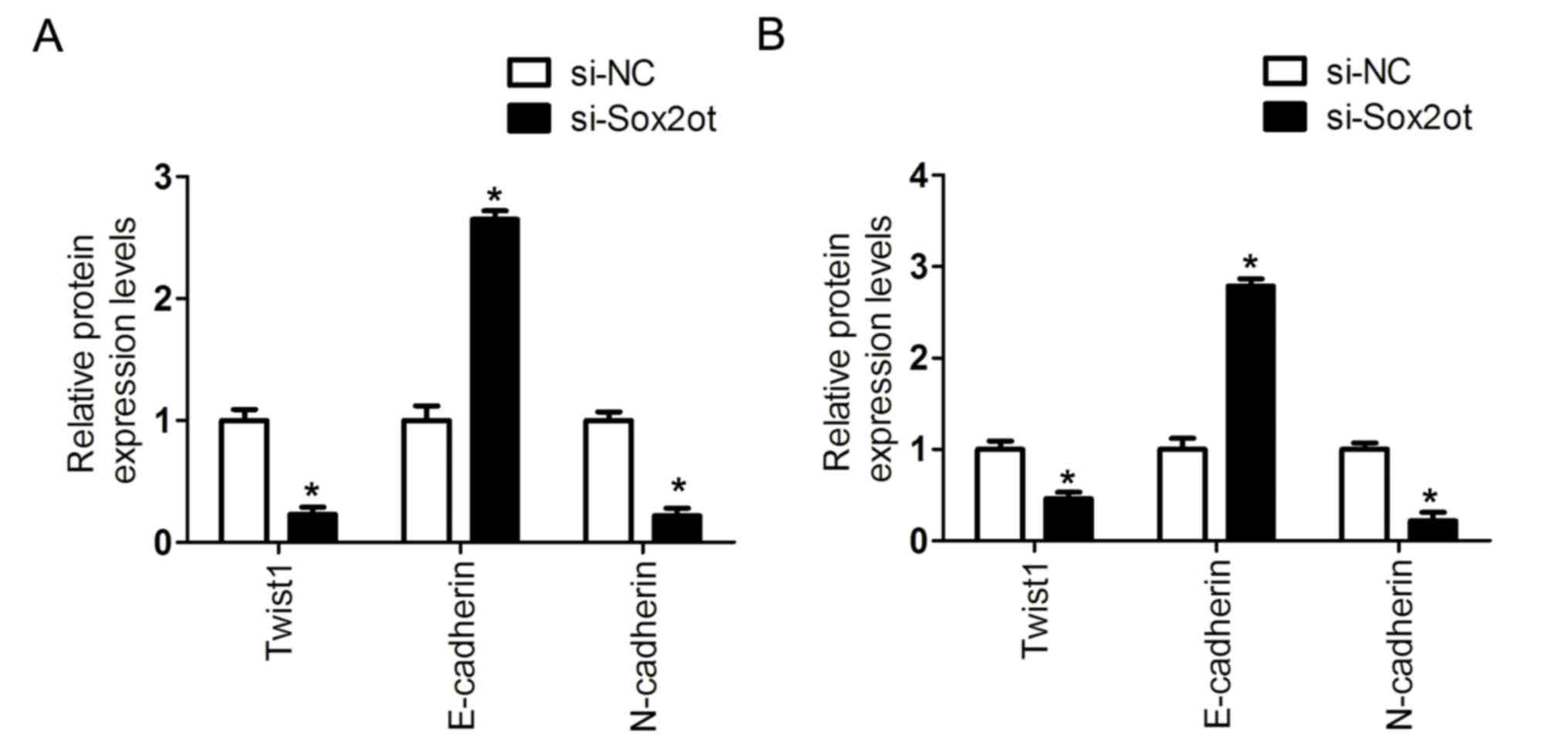
and B). In addition, the protein expression was detected
following the knockdown of lncRNA Sox2ot in MHCC97H or SMCC-7721
cells. It was demonstrated that the protein level of E-cadherin was
upregulated, whereas Twist1 and N-cadherin were downregulated
(Figs. 3C, D and 4). Thus, these results indicate that lncRNA
Sox2ot may promote EMT in HCC cells.
Discussion
lncRNAs participate in the progression of a range of
tumor types and may be suitable as biomarkers for predicting
prognosis (14). For example,
HOTAIRM1 may function as a tumor suppressor, and is a potential
biomarker for the diagnosis of colorectal cancer (15). The high expression of lncRNA HULC is a
predictor of a relatively poor prognosis and promotes cell
proliferation in glioma (16). The
high expression of lncRNA Sox2ot is associated with the aggressive
progression of gastric cancer, and poor OS and DFS times (17). The increased expression of lncRNA
Sox2ot has been demonstrated to promote cell proliferation and
motility in colorectal cancer via promoting Cyclin B1 and CDC25C
expression (18).
In the present study, it was determined that lncRNA
Sox2ot was upregulated in HCC tissues, and that higher lncRNA
Sox2ot expression was associated with the tumor size, tumor number
and vein invasion. The patients with a higher level of lncRNA
Sox2ot expression had a reduced DFS and OS time. A previous
meta-analysis of the prognostic value of various abnormally
expressed lncRNAs in HCC demonstrated that the transcription level
of various lncRNAs was significantly associated with the tumor
size, microvascular invasion and portal vein tumor thrombus, and
may serve in the prognostic evaluation of patients with HCC
(6). In another previous study,
lncRNA Sox2ot expression was associated with T stage, distant
metastasis, differentiation and a poorer OS and DFS time in breast
cancer (17). Tang et al
(19) reported that lncRNA Sox2ot was
overexpressed in breast cancer tissues, and that a higher
expression of lncRNA Sox2ot increased the risk of breast cancer for
Chinese women. Shi et al (20)
determined that the high expression of lncRNA Sox2ot was associated
with the histological grade, the Tumor-Node-Metastasis stage, vein
invasion and a relatively poor 5-year OS time in HCC, which was
consistent with the results from the present study. Thus, lncRNA
Sox2ot may be a prognostic biomarker for patients with HCC.
Various lncRNAs were previously determined to serve
crucial roles in cancer invasion and metastasis via regulating
critical biological events, particularly the EMT. Increased
expression of lncRNA ZFAS1 is associated with EMT in gastric cancer
progression (21). lncRNA HULC may
promote the EMT, tumorigenesis and metastasis of HCC via the
mediation of the miR-200a-3p/ZEB1 signaling pathway (22). lncRNA CPS1-IT1 may inhibit HCC
invasion and metastasis via regulating HIF-1α activity and
suppressing EMT (23). The
upregulation of H19 may indicate a poorer prognosis in gallbladder
carcinoma and promote EMT via upregulating Twist1 expression
(24). Overexpression of prostate
lncRNA-1 significantly increases cell proliferation, migration and
invasion via enhancing EMT signaling (25). In the present study, it was determined
that the knockdown of lncRNA Sox2ot inhibited the cell invasion
ability and the EMT process via upregulating E-cadherin expression
and downregulating Twist1 and N-cadherin expression. In the EMT
process, activating Twist upregulates N-cadherin expression and
downregulates E-cadherin expression, which are considered hallmarks
of EMT (26). Thus, lncRNA Sox2ot may
have promoted cell invasion and the EMT process via promoting
Twist1 and N-cadherin expression. As the underlying molecular
regulatory mechanisms between lncRNA Sox2ot and Twist or N-cadherin
are unknown, further investigation is required.
In conclusion, the results of the present study
demonstrated that lncRNA Sox2ot expression was increased in HCC
tissue, and higher lncRNA Sox2ot expression was predictive of a
poorer prognosis in patients with HCC. Furthermore, it was
determined that the knockdown of lncRNA Sox2ot inhibited cell
invasion and the EMT; thus, lncRNA Sox2ot may be a novel biomarker
of HCC prognosis and a potential target for HCC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used in the present study are available
from the corresponding author on reasonable request.
Author's contributions
JS and XW conceived and designed the study. JS, XW
and LX performed the experiments. JS analyzed and interpreted the
data. LX wrote the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Committee of Shandong Provincial Hospital Affiliated to Shandong
University.
Consent for publication
Written informed consent was obtained from all
patients in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gramantieri L, Fornari F, Callegari E,
Sabbioni S, Lanza G, Croce CM, Bolondi L and Negrini M: MicroRNA
involvement in hepatocellular carcinoma. J Cell Mol Med.
12:2189–2204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rich N and Singal AG: Hepatocellular
carcinoma tumour markers: Current role and expectations. Best Pract
Res Clin Gastroenterol. 28:843–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang X, Xie X, Xiao YF, Xie R, Hu CJ, Tang
B, Li BS and Yang SM: The emergence of long non-coding RNAs in the
tumorigenesis of hepatocellular carcinoma. Cancer Lett.
360:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li C, Chen J, Zhang K, Feng B, Wang R and
Chen L: Progress and prospects of long noncoding RNAs (lncRNAs) in
hepatocellular carcinoma. Cell Physiol Biochem. 36:423–434. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qu Z, Yuan CH, Yin CQ, Guan Q, Chen H and
Wang FB: Meta-analysis of the prognostic value of abnormally
expressed lncRNAs in hepatocellular carcinoma. Onco Targets Ther.
9:5143–5152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi L, Peng F, Tao Y, Fan X and Li N:
Roles of long noncoding RNAs in hepatocellular carcinoma. Virus
Res. 223:131–139. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan TH, Yang H, Jiang JH, Lu SW, Peng CX,
Que HX, Lu WL and Mao JF: Prognostic significance of long
non-coding RNA PCAT-1 expression in human hepatocellular carcinoma.
Int J Clin Exp Pathol. 8:4126–4131. 2015.PubMed/NCBI
|
|
9
|
Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z,
Deng X, Chen H, Shen B, Peng C, et al: Amplification of long
noncoding RNA ZFAS1 promotes metastasis in hepatocellular
carcinoma. Cancer Res. 75:3181–3191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang F, Xie C, Zhao W, Deng Z, Yang H and
Fang Q: Long non-coding RNA CARLo-5 expression is associated with
disease progression and predicts outcome in hepatocellular
carcinoma patients. Clin Exp Med. 17:33–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang L, Li C, Lan T, Wu L, Yuan Y, Liu Q
and Liu Z: Decreased expression of long non-coding RNA GAS5
indicates a poor prognosis and promotes cell proliferation and
invasion in hepatocellular carcinoma by regulating vimentin. Mol
Med Rep. 13:1541–1550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestive
systemFourth edition. Lyon: World Health Organization; pp. 205–227.
2010
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan L, Kong J, Tang J, Wu Y, Xu E, Lai M
and Zhang H: HOTAIRM1 as a potential biomarker for diagnosis of
colorectal cancer functions the role in the tumour suppressor. J
Cell Mol Med. 20:2036–2044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan H, Tian R, Zhang M, Wu J, Ding M and
He J: High expression of long noncoding RNA HULC is a poor
predictor of prognosis and regulates cell proliferation in glioma.
Onco Targets Ther. 10:113–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou JH, Li CY, Bao J and Zheng GQ: High
expression of long noncoding RNA Sox2ot is associated with the
aggressive progression and poor outcome of gastric cancer. Eur Rev
Med Pharmacol Sci. 20:4482–4486. 2016.PubMed/NCBI
|
|
18
|
Liu S, Xu B and Yan D: Enhanced expression
of long non-coding RNA Sox2ot promoted cell proliferation and
motility in colorectal cancer. Minerva Med. 107:279–286.
2016.PubMed/NCBI
|
|
19
|
Tang X, Gao Y, Yu L, Lu Y, Zhou G, Cheng
L, Sun K, Zhu B, Xu M and Liu J: Correlations between lncRNA-SOX2OT
polymorphism and susceptibility to breast cancer in a Chinese
population. Biomark Med. 11:277–284. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi XM and Teng F: Up-regulation of long
non-coding RNA Sox2ot promotes hepatocellular carcinoma cell
metastasis and correlates with poor prognosis. Int J Clin Exp
Pathol. 8:4008–4014. 2015.PubMed/NCBI
|
|
21
|
Zhou H, Wang F, Chen H, Tan Q, Qiu S, Chen
S, Jing W, Yu M, Liang C, Ye S and Tu J: Increased expression of
long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal
transition of gastric cancer. Aging (Albany NY). 8:2023–2038. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li SP, Xu HX, Yu Y, He JD, Wang Z, Xu YJ,
Wang CY, Zhang HM, Zhang RX, Zhang JJ, et al: LncRNA HULC enhances
epithelial-mesenchymal transition to promote tumorigenesis and
metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1
signaling pathway. Oncotarget. 7:42431–42446. 2016.PubMed/NCBI
|
|
23
|
Wang TH, Yu CC, Lin YS, Chen TC, Yeh CT,
Liang KH, Shieh TM, Chen CY and Hsueh C: Long noncoding RNA
CPS1-IT1 suppresses the metastasis of hepatocellular carcinoma by
regulating HIF-1α activity and inhibiting epithelial-mesenchymal
transition. Oncotarget. 7:43588–43603. 2016.PubMed/NCBI
|
|
24
|
Wang SH, Wu XC, Zhang MD, Weng MZ, Zhou D
and Quan ZW: Upregulation of H19 indicates a poor prognosis in
gallbladder carcinoma and promotes epithelial-mesenchymal
transition. Am J Cancer Res. 6:15–26. 2015.PubMed/NCBI
|
|
25
|
Dong L, Ni J, Hu W, Yu C and Li H:
Upregulation of long non-coding RNA PlncRNA-1 promotes metastasis
and induces epithelial-mesenchymal transition in hepatocellular
carcinoma. Cell Physiol Biochem. 38:836–846. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan MA, Chen HC, Zhang D and Fu J: Twist:
A molecular target in cancer therapeutics. Tumour Biol.
34:2497–2506. 2013. View Article : Google Scholar : PubMed/NCBI
|