Introduction
Lung cancer is one of the most common malignancies
globally, with non-small cell lung cancer (NSCLC) accounting for
the main type, exhibiting a high mortality rate and poor prognosis
(1). The management techniques for
NSCLC include surgery, radiotherapy and chemotherapy; however, they
exhibit limited effectiveness for NSCLC. Radical surgery has been
the standard treatment for early-stage NSCLC. However, patients
with similar stages and histological classifications have markedly
different survival outcomes (2). The
following resection remains poor due to the high rate of recurrence
and metastasis. The prognosis of NSCLC is predominantly based on
the Tumor-Node-Metastasis (TNM) staging system, but TNM staging is
not able to accurately predict clinical prognosis (3,4). Numerous
promising biomarkers have been evaluated as potential prognosis
predictors of NSCLC; however, none of them have been proven
effective for clinical use. Therefore, investigation of novel
biomarkers is clinically necessary to improve treatment
strategies.
Multidrug resistance (MDR)-associated protein 1
(MRP1) is a member of the ATP-binding cassette membrane transporter
family of proteins. MRP1 is primarily expressed in the basolateral
membrane of epithelial cells, and governs the absorption and
disposition of a wide variety of endogenous and exogenous
substrates (5,6). MRP1 is implicated in MDR and
accommodates the efflux of conventional cytotoxic anticancer agents
(7). Overexpression of MRP1 has been
associated with drug resistance and poor outcome in lung, breast
and gastric cancer cells and patients (8–13).
Platelets serve a multifaceted role in blood
clotting, inflammatory response, fibrinolysis and neoplasia.
Interactions between tumor cells and platelets are associated with
tumor aberrant angiogenesis, invasion and metastasis (14,15).
Previous studies have demonstrated that increased platelet count is
linked to a poor prognosis in various types of cancer, including
gastric, pancreatic, ovarian, colorectal and breast cancer
(16–20).
However, due to variance in study design and sample
size, these studies have reported inconsistent results. It is
therefore unknown whether MRP1 or platelet count is a suitable
prognostic indicator of NSCLC. In the present study, a
retrospective clinical analysis was designed to investigate the
prognostic impact of MRP1 and platelet count on the patients'
characteristics and survival in those with operable NSCLC.
Materials and methods
Patients and treatment
Between June 2007 and June 2011, 427 patients with
operable NSCLC were enrolled in the present study at Zhejiang
Cancer Hospital (Hangzhou, Zhejiang, China). Patients were newly
diagnosed and histologically confirmed, without any preoperative
anticancer therapy. The histological diagnosis was based on the
classification criteria for lung tumors of the World Health
Organization and International Association for the Study of Lung
Cancer (WHO/IASLC) (21). The tumor
stage was defined according to the seventh edition of the TNM
classification (4). The protocol of
present study was approved by the Institutional Review Board of
Zhejiang Cancer Hospital. All patients provided written informed
consent prior to surgery.
Blood samples (3 ml) were obtained from each patient
prior to surgery. Platelet count was measured using the XE2100
automatic hematology analyzer (Sysmex Co., Tokyo, Japan). A
platelet count of <300×109/l was regarded as normal,
while a count of >300×109/l was defined as
thrombocytosis, according to the manufacturer's protocols.
All patients underwent radical resection of
pulmonary carcinoma and radical mediastinal lymph node dissection.
All patients received standardized follow-up at a 3-month interval
until October 30, 2016. Among the 427 patients, 225 relapsed during
the follow-up, with 200 mortalities, while 15 patients had no
record of distal metastasis and 19 patients had no record of
overall survival.
Tissue microarray (TMA)
TMA blocks were obtained from 427 patients with
1.0-mm diameter representative regions used for each case.
Hematoxylin and eosin (H&E) slides (5-mm) were used to identify
and mark out representative areas of tumor tissue. The cores were
carefully selected from H&E stained sections and inserted into
new paraffin blocks using Tissue Arrayer Minicore (Alphelys,
Plaisir, France).
Immunohistochemistry (IHC)
Samples were fixed in 4% paraformaldehyde solution
at 4°C for 24 h, embedded in paraffin and cut into 15-µm serial
sections. Routine hematoxylin and eosin staining was performed
(hematoxylin staining for 5 min and eosin staining for 2 min, both
at room temperature). Then the sections were deparaffinized with
xylene, and washed using 100, 90 and 70% ethanol, then distilled
water. The sections (15 µm thick) were primed for antigen retrieval
in citrate buffer (pH 6.0) using microwave heating for a 5-min
cycle. Sections were incubated with primary antibody against MRP1
(dilution, 1:200; cat. no. 72202; Cell Signaling Technology, Inc.,
Danvers, MA, USA) overnight at 4°C, followed by incubation with
biotin labeled goat anti-mouse IgG and horseradish
peroxidase-conjugated streptavidin secondary antibody (dilution,
1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at
room temperature, stained with 5 mg/ml DAB (Dako Cytomation; cat
no. GK346810; Dako; Agilent Technologies, Inc., Sana Clara, CA,
USA) for 10 min at room temperature and counterstained with
hematoxylin for 2 min at room temperature according to the
manufacturer's protocol. The slides were scored according to
staining intensity as follows: 0, negative (−); 1, weak positive
(+); 2, positive (++), and 3, strong positive (+++). The percentage
of positive cells were scored from 0–3 as follows: 0, <5%; 1,
5–25%; 2, 25–50%, and 3, >50%. The final IHC score was a result
of multiplying the two scores, with a score of 0 indicating a
negative expression and a score of 1–3 indicating a positive
expression.
Statistical analysis
A one-way analysis of variance and χ2
test were performed to evaluate the association between the
clinicopathological variables and MRP1 expression. DFS and OS were
analyzed by Kaplan-Meier curves and log-rank test. All the
statistical calculations were performed using SPSS 15.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients
Among the 427 patients, there were 333 men and 94
women (age range, 38–78 years; mean, 59.7±8.8 years). According to
the WHO/IASLC classification criteria for lung tumors, there were
197 cases of adenocarcinoma and 230 cases of squamous cell
carcinoma. According to the IASLC staging system, there were 163
cases of stage I (25 cases of stage Ia and 138 cases of stage Ib),
120 cases of stage II (2 cases of stage IIa and 118 cases of stage
IIb) and 143 cases of stage III (116 cases of stage IIIa and 27
cases of stage IIIb). Only 1 case had no staging information.
Association between MRP1 expression
and patient characteristics
The expression of MRP1 protein was localized in the
cytoplasm (Fig. 1). Among the 427
patients, there were 208 (48.7%) patients with positive or
strong-positive MRP1 expression and 219 (51.3%) patients with
weak-positive or negative MRP1 expression. MRP1 expression was
significantly associated with histological type and tumor
differentiation, and there was a statistically significant
difference between the expression of MRP and the count of PTL
(Table I). However, sex, stage, lymph
node metastasis, family history, smoking and alcohol intake history
did not have significant association with MRP1 expression (Tables I and II).
 | Table I.Multidrug resistance-associated
protein 1 protein expression with multivariate analysis of
prognosis in patients with non-small cell lung cancer. |
Table I.
Multidrug resistance-associated
protein 1 protein expression with multivariate analysis of
prognosis in patients with non-small cell lung cancer.
|
|
|
|
|
|
| Exp (B) of 95%
confidence interval |
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | B | Standard error | Wald | P-value | Exp (B) | Lower limit | Upper limit |
|---|
| Sex |
|
|
|
|
|
|
|
|
Male | 0.595 | 0.435 | 1.869 | 0.172 | 1.813 | 0.773 | 4.253 |
|
Female |
|
|
|
|
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
<65 | −0.096 | 0.224 | 0.185 | 0.667 | 0.908 | 0.585 | 1.409 |
|
≥65 |
|
|
|
|
|
|
|
| Family history |
|
|
|
|
|
|
|
| No | 0.108 | 0.263 | 0.167 | 0.683 | 1.114 | 0.665 | 1.864 |
|
Yes |
|
|
|
|
|
|
|
| Smoking |
|
|
|
|
|
|
|
|
Never | 0.381 | 0.404 | 0.887 | 0.346 | 1.463 | 0.663 | 3.230 |
|
Ever/current |
|
|
|
|
|
|
|
| Alcohol |
|
|
|
|
|
|
|
|
Never | 0.302 | 1.455 | 0.043 | 0.836 | 1.353 | 0.078 | 23.408 |
|
Ever/current |
|
|
|
|
|
|
|
| Histological
type |
|
|
|
|
|
|
|
|
Squamous cell carcinoma | 0.905 | 0.241 | 14.100 |
<0.001a | 2.472 | 1.541 | 3.964 |
|
Adenocarcinoma |
|
|
|
|
|
|
|
| Grade |
|
|
|
|
|
|
|
|
High-middle | −0.734 | 0.219 | 11.182 | 0.001a | 0.480 | 0.312 | 0.738 |
|
Middle-low |
|
|
|
|
|
|
|
| Clinical stage |
|
|
|
|
|
|
|
|
I–II | 0.058 | 0.219 | 0.040 | 0.791 | 1.060 | 0.690 | 1.626 |
|
III |
|
|
|
|
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
| No | 0.238 | 0.254 | 0.877 | 0.349 | 1.268 | 0.771 | 2.085 |
|
Yes |
|
|
|
|
|
|
|
| PLT |
|
|
|
|
|
|
|
|
<300×109/l | 0.852 | 0.252 | 9.854 | 0.001 | 1.985 | 1.456 | 2.989 |
|
≥300×109/l |
|
|
|
|
|
|
|
 | Table II.Association of MRP1 protein
expression with clinicopathological factors in patients with
non-small cell lung cancer. |
Table II.
Association of MRP1 protein
expression with clinicopathological factors in patients with
non-small cell lung cancer.
|
|
| MRP1
expression |
|
|---|
|
|
|
|
|
|---|
| Factors | Patients, n
(%) | Negative, n
(%) | Positive, n
(%) | P-value |
|---|
| Sex |
|
|
| 0.017 |
|
Male | 333 (78.0) | 181 (42.4) | 152 (35.6) |
|
|
Female | 94 (22.0) | 38 (8.9) | 56 (13.1) |
|
| Age, years |
|
|
| 0.704 |
|
<65 | 296 (69.3) | 150 (35.1) | 146 (34.2) |
|
|
≥65 | 131 (30.7) | 69 (16.2) | 62 (14.5) |
|
| Family history |
|
|
| 0.375 |
| No | 328 (76.8) | 176 (41.2) | 152 (35.6) |
|
|
Yes | 77 (18.0) | 37 (8.7) | 40 (9.4) |
|
| Smoking |
|
|
| 0.086 |
|
Never | 114 (26.7) | 52 (12.2) | 62 (14.5) |
|
|
Ever/current | 293 (68.6) | 162 (37.9) | 131 (30.7) |
|
| Alcohol |
|
|
| 0.655 |
|
Never | 197 (46.1) | 99 (23.2) | 98 (23.0) |
|
|
Ever/current | 208 (48.7) | 114 (26.7) | 94 (22.0) |
|
| Histological
type |
|
|
|
<0.001a |
|
Squamous cell carcinoma | 230 (53.9) | 136 (31.9) | 94 (22.0) |
|
|
Adenocarcinoma | 197 (46.1) | 83 (19.4) | 114 (26.7) |
|
| Grade |
|
|
| 0.014a |
|
High-middle | 210 (49.2) | 95 (22.2) | 115 (26.9) |
|
|
Middle-low | 217 (50.8) | 124 (29.0) | 93 (21.8) |
|
| Clinical stage |
|
|
| 0.866 |
|
I–II | 283 (66.3) | 114 (26.7) | 139 (32.6) |
|
|
III | 143 (33.5) | 74 (17.3) | 69 (16.2) |
|
| Lymph node
metastasis |
|
|
| 0.664 |
| No | 193 (45.2) | 101 (23.7) | 92 (21.5) |
|
|
Yes | 231 (54.1) | 116 (27.2) | 115 (26.9) |
|
Association between platelet count and
patient characteristics
The platelet count in all 427 patients range from 67
to 704×109/l. A total of 375 patients (87.8%) had a
normal platelet count (<300×109/l) and 62 (14.5%) had
thrombocytosis (>300×109/l). The number of platelets
was analyzed using a T-test, and patients with platelet numbers
higher compared with the normal value were grouped into the high
group, and those with lower platelet numbers compared with the
normal value were grouped into the low group. A χ2 test
was performed with pathological data. Platelet count was
significantly associated with smoking behavior (P=0.041),
histological type (P=0.039), clinical stage (P=0.007) and lymph
node metastasis (P=0.003) (Table
III). Platelet count was significantly higher in patients of
stage III compared with those of stage I–II (P=0.021), in patients
with lymph node metastasis than in those with no lymph node
metastasis (P=0.014) and in smokers than in non-smokers (P=0.017).
Other factors, including age, sex, family history, alcohol intake
history and tumor differentiation, did not have a significant
association with platelet count (Table
IV). Furthermore, platelet count was significantly higher in
patients with negative MRP1 expression than in those with positive
MRP1 expression (Fig. 2).
 | Table III.Association of PLT level with
clinicopathological factors in patients with non-small cell lung
cancer. |
Table III.
Association of PLT level with
clinicopathological factors in patients with non-small cell lung
cancer.
|
|
| PLT
(×109/l) |
| PLT |
|
|---|
|
|
| PLT
(×109/l) |
|
|
|
|---|
| Factors | Patients, n
(%) | Median (mean,
5–95th) | P-value | Low expression, n
(%) | High expression, n
(%) | P-value |
|---|
| Sex |
|
| 0.095 |
|
| 0.028a |
|
Male | 333 (78.0) | 214.0 (226.4,
217.2–235.5) |
| 278 (65.1) | 55 (12.9) |
|
|
Female | 94 (22.0) | 200.0 (210.6,
197.4–223.7) |
| 87 (20.4) | 7 (1.6) |
|
| Age, years |
|
| 0.575 |
|
| 0.135 |
|
<65 | 296 (69.3) | 213.0 (224.3,
215.2–233.5) |
| 248 (58.1) | 48 (11.2) |
|
|
≥65 | 131 (30.7) | 202.0 (219.6,
205.1–234.0) |
| 117 (27.4) | 14 (3.3) |
|
| Family history |
|
| 0.169 |
|
| 0.426 |
| No | 328 (76.8) | 215.0 (225.1,
216.5–233.6) |
| 128 (30.0) | 50 (11.7) |
|
|
Yes | 77 (18.0) | 185.0 (213.7,
193.1–234.2) |
| 68 (15.9) | 9 (2.1) |
|
| Smoking |
|
| 0.447 |
|
| 0.041a |
|
Never | 114 (26.7) | 202.5 (213.8,
201.6–226.0 |
| 104 (24.4) | 10 (2.3) |
|
|
Ever/current | 293 (68.6) | 213.0 (226.1,
216.2–236.0) |
| 244 (57.1) | 49 (11.5) |
|
| Alcohol |
|
| 0.805 |
|
| 0.840 |
|
Never | 197 (46.1) | 208.0 (223.8,
212.4–235.1) |
| 168 (39.3) | 29 (6.8) |
|
|
Ever/current | 208 (48.7) | 210.0 (221.8,
210.6–233.0) |
| 178 (41.7) | 30 (7.0) |
|
| Histological
type |
|
| 0.039a |
|
| 0.473 |
|
Squamous cell carcinoma | 230 (53.9) | 222.0 (229.5,
218.7–240.2) |
| 194 (45.4) | 36 (8.4) |
|
|
Adenocarcinoma | 197 (46.1) | 199.0 (215.2,
204.2–226.2) |
| 171 (40.0) | 26 (6.1) |
|
| Grade |
|
| 0.971 |
|
| 0.491 |
|
High-middle | 210 (49.2) | 222.0 (229.5,
218.7–240.2) |
| 177 (41.5) | 33 (7.7) |
|
|
Middle-low | 217 (50.8) | 199.0 (215.2,
204.2–226.2) |
| 188 (44.0) | 29 (6.8) |
|
| Clinical stage |
|
| 0.007a |
|
| 0.003a |
|
I–II | 283 (66.3) | 206.0 (215.5,
206.7–224.3) |
| 252 (59.0) | 31 (7.3) |
|
|
III | 143 (33.5) | 226.0 (237.8,
223.0–252.6) |
| 112 (26.2) | 31 (7.3) |
|
| Lymph node
metastasis |
|
| 0.030 |
|
| 0.046a |
| No | 193 (45.2) | 201.0 (213.9,
203.7–224.1) |
| 172 (40.3) | 21 (4.9) |
|
|
Yes | 231 (54.1) | 221.0 (231.0,
219.7–242.3) |
| 190 (44.5) | 41 (9.6) |
|
| MRP1 |
|
| 0.014a |
|
| 0.002a |
|
Negative (−/+) | 219 (51.3) | 221.0 (232.3,
221.2–243.5) |
| 176 (41.2) | 43 (10.1) |
|
|
Positive (++/+++) | 208 (48.7) | 206.5 (213.0,
202.4–223.6) |
| 189 (44.3) | 19 (4.4) |
|
 | Table IV.Platelet level with multivariate
analysis of prognosis in patients with non-small cell lung
cancer. |
Table IV.
Platelet level with multivariate
analysis of prognosis in patients with non-small cell lung
cancer.
|
|
|
|
|
|
| Exp (B) of 95%
confidence interval |
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | B | Standard error | Wald | P-value | Exp (B) | Lower limit | Upper limit |
|---|
| Sex |
|
|
|
|
|
|
|
|
Male | −0.311 | 0.434 | 0.514 | 0.473 | 0.732 | 0.313 | 1.715 |
|
Female |
|
|
|
|
|
|
|
| Age, years |
|
|
|
|
|
|
|
|
<65 | −0.363 | 0.224 | 2.619 | 0.106 | 0.695 | 0.448 | 1.080 |
|
≥65 |
|
|
|
|
|
|
|
| Family history |
|
|
|
|
|
|
|
| No | 0.105 | 0.398 | 0.070 | 0.791 | 1.111 | 0.509 | 2.425 |
|
Yes |
|
|
|
|
|
|
|
| Smokings |
|
|
|
|
|
|
|
|
Never | −0.641 | 0.269 | 5.663 | 0.017a | 0.527 | 0.311 | 0.893 |
|
Ever/current |
|
|
|
|
|
|
|
| Alcohol |
|
|
|
|
|
|
|
|
Never | −0.418 | 0.310 | 1.816 | 0.178 | 0.659 | 0.545 | 1.209 |
|
Ever/current |
|
|
|
|
|
|
|
| Histological
type |
|
|
|
|
|
|
|
|
Squamous cell carcinoma | −0.581 | 0.241 | 5.791 | 0.016a | 0.560 | 0.349 | 0.898 |
|
Adenocarcinoma |
|
|
|
|
|
|
|
| Grade |
|
|
|
|
|
|
|
|
High-middle | 0.216 | 0.217 | 0.983 | 0.321 | 1.241 | 0.810 | 1.900 |
|
Middle-low |
|
|
|
|
|
|
|
| Clinical stage |
|
|
|
|
|
|
|
|
I–II | 0.508 | 0.220 | 5.324 | 0.021a | 1.661 | 1.079 | 2.557 |
|
III |
|
|
|
|
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
| No | 0.599 | 0.243 | 6.060 | 0.014 | 0.550 | 0.341 | 0.855 |
|
Yes |
|
|
|
|
|
|
|
| MRP1 |
|
|
|
|
|
|
|
|
Negative (−/+) | −0.590 | 0.213 | 5.794 | 0.001a | 0.526 | 1.245 | 2.255 |
|
Positive (++/+++) |
|
|
|
|
|
|
|
Association of MRP1 expression with
survival
There was no association between MRP1expression and
OS (P=0.441) or DFS (P=0.656) according to Kaplan-Meier analysis
and log-rank test (Fig. 3A and D).
However, in the patients with no lymph node metastasis, the OS time
was significantly longer in patients with positive MRP1 expression
than in those with negative expression (P=0.009) (Fig. 3B). Notably, in the patients with lymph
node metastasis, the DFS time was significantly shorter in patients
with positive MRP1 expression than in those with negative
expression (P=0.022) (Fig. 3F)
(Table V).
 | Table V.Association between the expression of
MRP1 protein and the prognosis of patients with lymph node
metastasis. |
Table V.
Association between the expression of
MRP1 protein and the prognosis of patients with lymph node
metastasis.
| A, No lymph node
metastasis |
|---|
|
|---|
| Factor | Crude HR | 95% CI | P-value | Adjusted HR | 95% CI | P-value |
|---|
| Disease-free
survival |
|
MRP1-negative | 1.000 |
|
| 1.000 |
|
|
|
MRP1-positive | 1.453 | 0.960–2.199 | 0.077 | 0.726 | 0.443–1.190 | 0.204 |
| Overall
survival |
|
MRP1-negative | 1.000 |
|
| 1.000 |
|
|
|
MRP1-positive | 0.521 | 0.317–0.857 |
0.010e | 0.554 | 0.333–0.923 | 0.023 |
|
| B, Lymph node
metastasis |
|
| Factor | Crude
HR | 95% CI | P-value | Adjusted
HR | 95% CI | P-value |
|
| Disease-free
survival |
|
MRP1-negative | 1.000 |
|
| 1.000 |
|
|
|
MRP1-positive | 0.710 | 0.439–1.147 | 0.162 | 1.400 | 0.921–2.127 | 0.115 |
| Overall
survival |
|
MRP1-negative | 1.000 |
|
| 1.000 |
|
|
|
MRP1-positive | 1.277 | 0.870–1.731 | 0.244 | 1.223 | 0.862–1.733 | 0.259 |
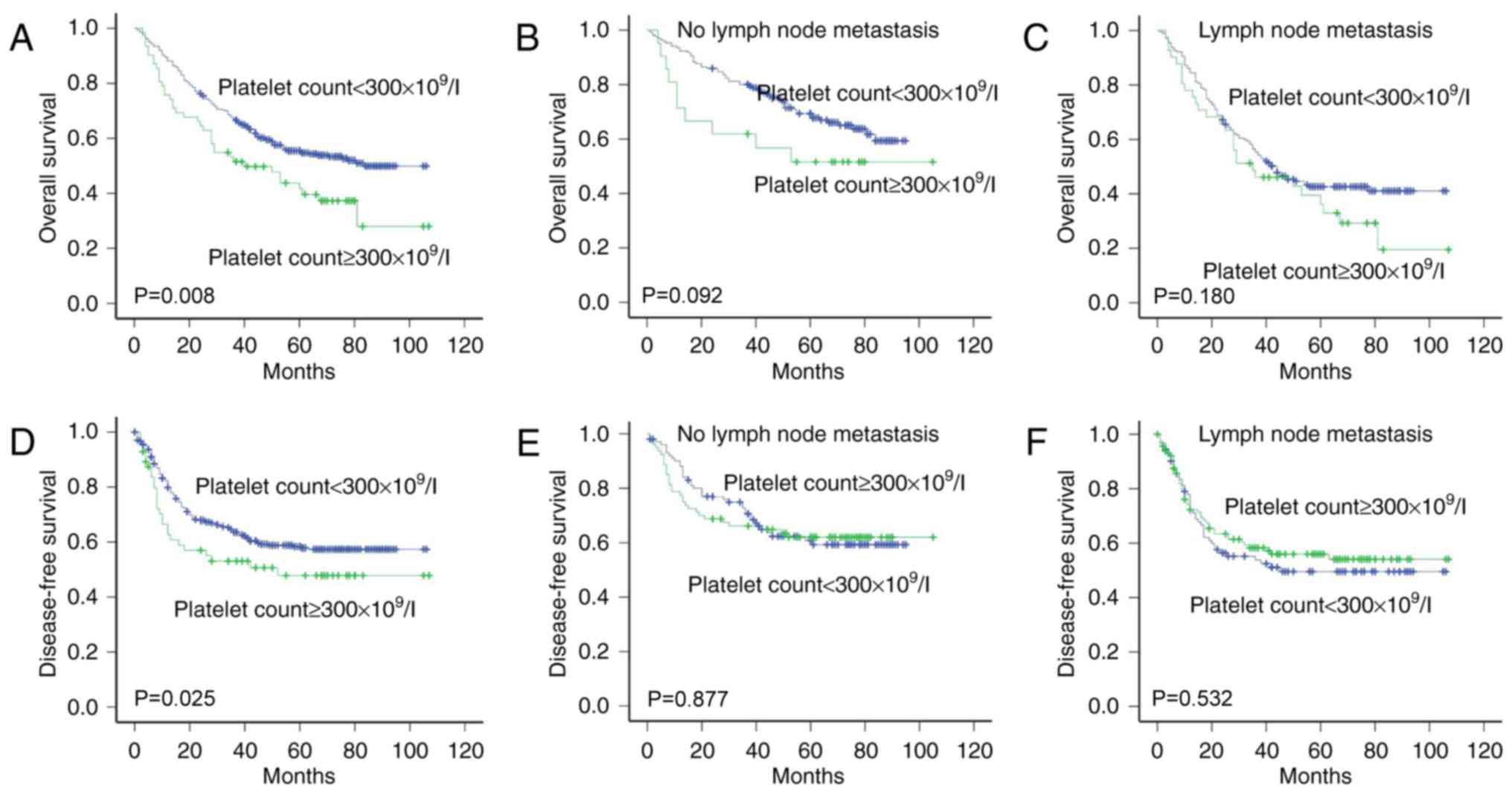
Association of platelet count with
survival
There was an association between the platelet count
and OS (P=0.008) or DFS (P=0.025) according to Kaplan-Meier
analysis and log-rank test (Fig. 4A and
D). The DFS and OS times were significantly longer in patients
with a normal platelet count (<300×109/l) than in
those with thrombocytosis (>300×109/l). There was no
association between the platelet count and survival for the
presence and absence of lymph node metastasis (Fig. 4B, C, E and F).
Discussion
MRP1 has previously been evaluated and is known to
serve an important role in MDR in vitro (22–24). MRP1
has also been associated with a poor outcome in NSCLC patients
(25,26). Preclinical studies showed that MRP1
protein levels correlated with the resistance to chemotherapeutic
agents in NSCLC cell lines, including SK-MES-1, A549, Calu-1,
Calu-6, SW-900, SK-LU-1, SK-Luci-6, and SW-1573 (23,24).
Although high levels of MRP1 expression are frequently observed in
the specimens of NSCLC patients, the predictive value of MRP1
expression remains a controversial issue (25–29).
Certain previous studies reported that patients with NSCLC with
high MRP1 expression had a poorer prognosis than those with low
MRP1 expression (25,27). However, another study indicated that
high MRP1 expression contributed to longer OS times in NSCLC
patients (27). It was also reported
that there was no association between MRP1 expression and survival
in advanced-stage NSCLC patients following platinum-based
chemotherapy (28), and another study
confirmed that no significant association between MRP1 expression
and OS time was observed in completely resected NSCLC patients
(29). In the present study, it was
found that MRP1 expression was significantly associated with sex,
histological type and tumor differentiation. There was no
association between MRP1 expression and DFS. However, in the
patients with no lymph node metastasis, the OS time was
significantly longer in patients with positive MRP1 expression than
in those with negative expression (P=0.009). Notably, in the
patients with lymph node metastasis, the DFS time was significantly
shorter in patients with positive MRP1 expression than in those
with negative expression. The differences between the present study
and previous studies may be due to different experimental detective
methodologies and sample types. The present results suggest that
MRP1 is a predictive factor for the survival of NSCLC patients.
Platelets serve a critical role in tumor progression
and metastasis. Studies have confirmed the association between
platelets and tumor biology. Platelets act as an important
regulator in physiological processes; however, angiogenesis is
associated with tumor growth and metastasis (14,30,31).
Platelets promote the hematogenous metastasis process by arresting
tumor cells within the organ vasculature (32,33).
Meanwhile, tumor cells have the ability to aggregate platelets,
leading to tumor cell-induced platelet aggregation that allows
tumor cells to evade immune surveillance (34). It has been indicated that
anti-platelet drugs, including heparin or warfarin, exert antitumor
effects in vivo and in vitro (35). Clinical studies have shown that the
risk of venous thrombosis in NSCLC patients is higher than that in
tumor-free patients (36) and that
high platelet count is correlated to a worse prognosis in NSCLC
patients (37). In the present study,
there was an association between the platelet count and DFS or OS
time according to Kaplan-Meier analysis and log-rank test. The DFS
and OS times were significantly longer in patients with a normal
platelet count than in those with thrombocytosis. Usually,
lymphatic metastasis is a strong prognostic factor for NSCLC. The
present study showed that there was no association between the
platelet count and survival whether the lymph node metastasis was
present or not. Therefore, platelet count may not be a useful
biomarker for predicting lymph node status.
There are several limitations to the present study,
which should be taken into consideration. The study was
retrospective and information on post-treatment recurrence was
insufficient. However, the major positive factor of the study was
the large population of NSCLC samples, which assisted in avoiding
bias and offsetting the heterogeneity. A prospective study is also
required to determine the prognostic value of MRP1 expression and
platelet count.
In conclusion, MRP1 expression and platelet count
are valuable independent prognostic biomarkers for survival in
patients with operable NSCLC, and they should be assessed in
patients with NSCLC in future studies to confirm their prognostic
significance. Large prospective studies are required to validate
these findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. LY13H160028) and
the Zhejiang Provincial Medicine and Health Science Fund (grant
nos. 2013KYB048, 2015KYA035, 2017KY238 and 2017KY243).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JF contributed to study conception and design. LF,
HS and DW analyzed and interpreted patient data. CZ, RJ and XS
performed the experiments and collected data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving human participants were
approved by the Institutional Review Board of Zhejiang Cancer
Hospital (Zhejiang, China). Informed written consent was obtained
from all individual participants included in the present study.
Consent for publication
The patient provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Varella-Garcia M, Bunn PA Jr,
Di Maria MV, Veve R, Bremmes RM, Barón AE, Zeng C and Franklin WA:
Epidermal growth factor receptor in non-small-cell lung carcinomas:
Correlation between gene copy number and protein expression and
impact on prognosis. J Clin Oncol. 21:3798–3807. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reck M, Popat S, Reinmuth N, de Ruysscher
D, Kerr KM and Peters S; ESMO Guidelines Working Group: Metastatic
non-small-cell lung cancer (NSCLC): ESMO Clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol.
25(Suppl 3): iii27–iii39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vallières E, Shepherd FA, Crowley J, van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P: International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac. 4:1049–1059. 2009.
View Article : Google Scholar
|
|
5
|
Flens MJ, Zaman GJ, van der Valk P,
Izquierdo MA, Schroeijers AB, Scheffer GL, van der Groep P, de Haas
M, Meijer CJ and Scheper RJ: Tissue distribution of the multidrug
resistance protein. Am J Pathol. 148:1237–1247. 1996.PubMed/NCBI
|
|
6
|
Schinkel AH and Jonker JW: Mammalian drug
efflux transporters of the ATP binding cassette (ABC) family: An
overview. Adv Drug Deliv Rev. 55:3–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cole SP: Targeting multidrug resistance
protein 1 (MRP1, ABCC1): Past, present, and future. Ann Rev
Pharmacol Toxicol. 54:95–117. 2014. View Article : Google Scholar
|
|
8
|
Li A, Song J, Lai Q, Liu B, Wang H, Xu Y,
Feng X, Sun X and Du Z: Hypermethylation of ATP-binding cassette B1
(ABCB1) multidrug resistance 1 (MDR1) is associated with cisplatin
resistance in the A549 lung adenocarcinoma cell line. Int J Exp
Pathol. 97:412–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen JJ, Liu SP, Zhao J, Wang SC, Liu TJ
and Li X: Effects of a novel photoactivated photosensitizer on MDR1
over-expressing human breast cancer cells. J Photochem Photobiol B
Biol. 171:67–74. 2017. View Article : Google Scholar
|
|
10
|
Melguizo C, Prados J, Luque R, Ortiz R,
Caba O, Alvarez PJ, Gonzalez B and Aranega A: Modulation of MDR1
and MRP3 gene expression in lung cancer cells after paclitaxel and
carboplatin exposure. Int J Mol Sci. 13:16624–16635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luque R, Gonzalez Flores E, Delgado JR,
Melguizo C, Prados JC, Gonzalez Astorga B, Ortiz R, Sánchez Toro C,
Valdivia J and Aránega A: MDR1 gene expression in peripheral blood
as a marker of treatment response in lung cancer. J Clin Oncol.
30:962012. View Article : Google Scholar
|
|
12
|
Wu DD, Zhang JX, Li J and Dong WG: Lack of
association of the MDR1 C3435T polymorphism with susceptibility to
gastric cancer and peptic ulcer: A systemic review and
meta-analysis. Asian Pac J Cancer Prev. 15:3021–3027. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiao W, Wang T, Zhang L, Tang Q, Wang D
and Sun H: Association between single genetic polymorphisms of MDR1
gene and gastric cancer susceptibility in Chinese. Med Oncol.
30:6432013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bambace NM and Holmes CE: The platelet
contribution to cancer progression. J Thromb Haemost. 9:237–249.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goubran HA, Stakiw J, Radosevic M and
Burnouf T: Platelet-cancer interactions. Semin Thromb Hemost.
40:296–305. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li FX, Wei LJ, Zhang H, Li SX and Liu JT:
Significance of thrombocytosis in clinicopathologic characteristics
and prognosis of gastric cancer. Asian Pac J Cancer Prev.
15:6511–6517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chadha AS, Kocak-Uzel E, Das P, Minsky BD,
Delclos ME, Mahmood U, Guha S, Ahmad M, Varadhachary GR and Javle
M: et alParaneoplastic thrombocytosis independently predicts
poor prognosis in patients with locally advanced pancreatic cancer.
Acta Oncol. 54:971–978. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng Z, Wen H, Bi R, Duan Y, Yang W and Wu
X: Thrombocytosis and hyperfibrinogenemia are predictive factors of
clinical outcomes in high-grade serous ovarian cancer patients. BMC
Cancer. 16:432016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Josa V, Krzystanek M, Eklund AC, Salamon
F, Zarand A, Szallasi Z and Baranyai Z: Relationship of
postoperative thrombocytosis and survival of patients with
colorectal cancer. Int J Surg. 18:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Taucher S, Salat A, Gnant M, Kwasny W,
Mlineritsch B, Menzel RC, Schmid M, Smola MG, Stierer M and Tausch
C: et alImpact of pretreatment thrombocytosis on survival in
primary breast cancer. Thromb Haemost. 89:1098–1106. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ
and van Schil PE: et alInternational association for the
study of lung cancer/american thoracic society/european respiratory
society international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berger W, Elbling L, Hauptmann E and
Micksche M: Expression of the multidrug resistanceassociated
protein (MRP) and chemoresistance of human non-small-cell lung
cancer cells. Int J Cancer 1997:. 73:84–93. 1997.
|
|
23
|
Young LC, Campling BG, Voskoglou-Nomikos
T, Cole SP, Deeley RG and Gerlach JH: Expression of multidrug
resistance protein-related genes in lung cancer: Correlation with
drug response. Clin Cancer Res. 5:673–680. 1999.PubMed/NCBI
|
|
24
|
Young LC, Campling BG, Cole SP, Deeley RG
and Gerlach JH: Multidrug resistance proteins MRP3, MRP1, and MRP2
in lung cancer: Correlation of protein levels with drug response
and messenger RNA levels. Clin Cancer Res. 7:1798–1804.
2001.PubMed/NCBI
|
|
25
|
Ota E, Abe Y, Oshika Y, Ozeki Y, Iwasaki
M, Inoue H, Yamazaki H, Ueyama Y, Takagi K and Ogata T: et
alExpression of the multidrug resistance-associated protein
(MRP) gene in non-small-cell lung cancer. Br J Cancer. 72:550–554.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Oshika Y, Nakamura M, Tokunaga T,
Fukushima Y, Abe Y, Ozeki Y, Yamazaki H, Tamaoki N and Ueyama Y:
Multidrug resistance-associated protein and mutant p53 protein
expression in non-small cell lung cancer. Mod Pathol. 11:1059–1063.
1998.PubMed/NCBI
|
|
27
|
Berger W, Setinek U, Hollaus P, Zidek T,
Steiner E, Elbling L, Cantonati H, Attems J, Gsur A and Micksche M:
Multidrug resistance markers P-glycoprotein, multidrug resistance
protein 1, and lung resistance protein in non-small cell lung
cancer: Prognostic implications. J Cancer Res Clin Oncol.
131:355–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yoh K, Ishii G, Yokose T, Minegishi Y,
Tsuta K, Goto K, Nishiwaki Y, Kodama T, Suga M and Ochiai A: Breast
cancer resistance protein impacts clinical outcome in
platinum-based chemotherapy for advanced non-small cell lung
cancer. Clin Cancer Res. 10:1691–1697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Filipits M, Haddad V, Schmid K, Huynh A,
Dunant A, André F, Brambilla E, Stahel R, Pignon JP and Soria JC:
et alMultidrug resistance proteins do not predict benefit of
adjuvant chemotherapy in patients with completely resected
non-small cell lung cancer: International Adjuvant Lung Cancer
Trial Biologic Program. Clin Cancer Res. 13:3892–3898. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dvorak HF, Brown LF, Detmar M and Dvorak
AM: Vascular permeability factor/vascular endothelial growth
factor, microvascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
31
|
Ma L, Perini R, McKnight W, Dicay M, Klein
A, Hollenberg MD and Wallace JL: Proteinase-activated receptors 1
and 4 counter-regulate endostatin and VEGF release from human
platelets. Proc Natl Acad Sci USA. 102:216–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lewalle JM, Castronovo V, Goffinet G and
Foidart JM: Malignant cell attachment to endothelium of ex vivo
perfused human umbilical vein. Modulation by platelets, plasma and
fibronectin. Thromb Res. 62:287–298. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shim M, Song C, Park S, Choi SK, Cho YM,
Kim CS and Ahn H: Prognostic significance of platelet-derived
growth factor receptor-β expression in localized clear cell renal
cell carcinoma. J Cancer Res Clin Oncol. 141:2213–2220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jurasz P, Alonso-Escolano D and Radomski
MW: Platelet-cancer interactions: Mechanisms and pharmacology of
tumour cell-induced platelet aggregation. Br J Pharmacol.
143:819–826. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bobek V and Kovarík J: Antitumor and
antimetastatic effect of warfarin and heparins. Biomed
Pharmacother. 58:213–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blom JW, Osanto S and Rosendaal FR: The
risk of a venous thrombotic event in lung cancer patients: Higher
risk for adenocarcinoma than squamous cell carcinoma. J Thromb
Haemost. 2:1760–1765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barcala JG, Portal JA, Carmona MJ and
González CM: Exposure to environmental contaminants and respiratory
disease. Spotlight on the year 2009. Arch Bronconeumol. 46(Suppl
1): S17–S20. 2010.(In Spanish).
|