Introduction
Hemophagocytic syndrome (HPS) or hemophagocytic
lymphohistiocytosis (HLH), which was first reported as
virus-associated HPS by Risdall et al (1), represents an uncontrolled immune
response triggered by various stimuli. The excessive activation of
lymphocytes and macrophages produces a high level of inflammatory
cytokines, including interferon (IFN)-γ, interleukin (IL)-12, IL-18
and tumor necrosis factor (TNF)-α (2). The cytokine storm and infiltration of
activated macrophages are responsible for features, including
persistent fever, hepatosplenomegaly, pancytopenia and
hemophagocytosis in bone marrow, liver and other organs (2–4). HPS is
classified into primary and secondary forms. Primary HPS generally
occurs in infants or young children with a clear genetic or
familial inheritance (2). Due to a
variety of underlying conditions, secondary HPS development may be
triggered by infections, autoimmune disorders, malignancies and
immunosuppression (3,5).
Lymphoma is the most common underlying condition of
malignancy-associated HPS. HPS may occur as an initial presentation
of lymphoma, as well as a complication at the relapsed or advanced
stage of lymphoma (6). It was
reported that the most common type of lymphoma-associated HPS
(LAHS) was T/natural killer (NK)-cell lymphoma and there were fewer
cases derived from B-cell lymphomas (7,8).
Currently, LAHS is considered to be life-threatening. Few
systematic reports are available on LAHS (9). The median survival time for patients
with NK/T-cell and other types of T-cell lymphoma is 28 and 33
days, respectively (6). Han et
al (8) reviewed 29 patients with
LAHS and determined that the median survival time was only 36 days.
Furthermore, there are ongoing discussions in various aspects of
LAHS, including specific indicators for early diagnosis,
therapeutic regimens and hematopoietic stem cell transplantation
(HSCT) (9–11). Therefore, in the present study, the
clinical features, treatment and prognosis factors of 57 patients
with LAHS were analyzed. To the best of our knowledge, the present
study used the largest cohort of patients with LAHS. Furthermore,
the differences between B-cell and T/NK-cell LAHS were discussed in
order to improve the understanding of LAHS and attempt to find an
appropriate treatment for this disease.
Patients and methods
Patient selection
A total of 57 patients diagnosed with LAHS (34 males
and 23 females) were selected from the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China), who enrolled from December
2008 to March 2016. The median age was 36 years old with an age
range of 4–76 years old. All patients underwent laboratory tests,
including blood routine test, liver and kidney function, lactate
dehydrogenase, β2-microglobulin, serum ferritin, coagulation
function and polymerase chain reaction tests for Epstein-Barr virus
(EBV)-DNA. Bone marrow smears and biopsies were assessed.
Diagnosis of lymphoma was confirmed according to the
World Health Organization classification of hematopoietic and
lymphoid tumors in 2008 (12).
Diagnosis of HPS was based on the Histiocyte Society HLH-2004
pediatric diagnostic criteria (13).
NK cell activity and soluble CD25 levels were not evaluated. The
stage of lymphoma was evaluated by Ann Arbor staging system
(14) through computed tomography
(CT) scans or positron emission tomography (PET)/CT. Performance
status was assessed based on the Eastern Cooperative Oncology Group
(ECOG) scale (15).
Immuno-chemotherapy was based on HLH-2004 protocols (13). Etoposide-based regimens were followed,
including dexamethasone, cyclosporine A (CSA), intravenous
immunoglobulins and intrathecal therapy. The treatment response was
assessed according to Cheson et al (16).
Statistical analysis
The data was presented as the mean ± standard
deviation. The sample normality detection was assessed using a
Shapiro-Wilk test. Clinical and laboratory data of patients were
assessed using Pearson's χ2 test, Fisher's exact test,
Kruskal-Wallis test, independent-samples Student's t-test or Mann
Whitney U test. Overall survival (OS) time, measured as the period
from diagnosis to mortality or the last follow-up, was estimated by
the Kaplan-Meier method. Survival rates were compared by the
log-rank test. Univariate analysis using Cox proportional hazards
model was used to calculate hazard ratios of prognostic factors for
patients with LAHS. Multivariate analysis using Cox proportional
hazards model was used to identify the potential independent
effects of those factors. P<0.05 was considered to indicate a
statistically significant difference. The software package SPSS
21.0 (IBM Corp., Armonk, NY, USA) was used for statistical
analysis.
Results
Patient characteristics
Patient characteristics are summarized in Table I. There were 43 patients diagnosed
with T/NK-cell lymphoma, accounting for 75.44% of all patients with
LAHS. The most frequent histopathological type was extranodal
natural killer/T-cell lymphoma, nasal type (ENKL) (45.61%). The
other types included diffuse large B-cell lymphoma (24.56%),
anaplastic large cell lymphoma (10.53%), peripheral T cell
lymphoma, not otherwise specified (8.77%), progressive NK/T-cell
leukemia (PNKTL) (5.26%), subcutaneous panniculitis-like T-cell
lymphoma (3.51%) and hepatosplenic T-cell lymphoma (1.75%). The
majority of patients (92.98%) were classified into Ann Arbor III–IV
stage. Among all patients, 21 had a history of lymphoma (had been
previously diagnosed with lymphoma) and were diagnosed with HPS at
the advanced Ann Arbor stage of lymphoma. The most frequent symptom
was fever (100%), followed by splenomegaly (92.89%), multicavity
effusion (56.14%), hepatomegaly (43.86%), jaundice (31.58%) and
edema (31.58%). A total of 56 patients exhibited thrombocytopenia
(98.25%), 48 patients (84.21%) had a high level of aspartate
aminotransferase, 47 patients (82.46%) had an elevated level of
serum ferritin and 39 patients had hemophagocytosis in the bone
marrow (68.42%).
 | Table I.Characteristics of all patients with
lymphoma associated hemophagocytic syndrome. |
Table I.
Characteristics of all patients with
lymphoma associated hemophagocytic syndrome.
|
Characteristics | Patients, n
(%) |
|---|
| Sex (male) |
|
|
Male | 34 (59.65) |
|
Female | 23 (40.35) |
| B-cell
lymphoma | 14 (24.56) |
|
DLBCL | 14 (24.56) |
| T/NK/-cell
lymphoma | 43 (75.44) |
|
ENKL | 26 (45.61) |
|
PNKL | 3 (5.26) |
| PTCL,
NOS | 5 (8.77) |
|
ALCL | 6 (10.53) |
|
SPTL | 2 (3.51) |
|
HSTL | 1 (1.75) |
| Ann Arbor Stage
I–II | 4 (7.02) |
| Ann Arbor Stage
III–IV | 53 (92.98) |
| Previous lymphoma
history | 21 (36.84) |
| Symptoms and
signs |
|
|
Fever | 57 (100) |
|
Splenomegaly | 53 (92.89) |
|
Hepatomegaly | 25 (43.86) |
|
Multicavity effusion | 32 (56.14) |
|
Jaundice | 18 (31.58) |
|
Edema | 18 (31.58) |
| Laboratory
data |
|
| ANC
<1.5×109/l | 41 (71.93) |
| Hb
<90 g/l | 36 (63.16) |
| PLT
<100×109/l | 56 (98.25) |
| ALT
>40 U/l | 46 (80.70) |
| AST
>40 U/l | 48 (84.21) |
| ALB
<30 g/l | 38 (66.67) |
| TBIL
>25 µmol/l | 23 (40.35) |
| LDH
>500 U/l | 40 (70.18) |
| FIB
<1.5 g/l | 40 (70.18) |
| TG
>3.0 mmol/l | 22 (38.60) |
|
Ferritin >1,000 µg/l | 47 (82.46) |
| EBV DNA
copies >102 | 39 (68.42) |
| BM
hemophagocytosis | 39 (68.42) |
The comparison of clinical features and laboratory
data between patients with B-cell and T/NK-cell LAHS are listed in
Tables II and III. Compared with patients with T/NK-cell
LAHS, patients with B-cell LAHS were older (P<0.001), had a
higher level of triglycerides (P=0.012), and a lower level of serum
ferritin (P=0.014) and the number of copies of EBV DNA
(P<0.001). The differences in the remaining features were not
statistically significant.
 | Table II.Clinical features of patients with
B-cell LAHS and T/NK-cell LAHS. |
Table II.
Clinical features of patients with
B-cell LAHS and T/NK-cell LAHS.
| Patients'
Characteristics | B-cell lymphoma
(n=14) | T/NK-cell lymphoma
(n=43) | P-value |
|---|
| Sex |
|
| 0.397a |
|
Male | 7 | 27 |
|
|
Female | 7 | 16 |
|
| Age (years); mean ±
SE | 51.1±4.0 | 33.1±2.2 |
<0.001c |
| IPI score |
|
| 0.720d |
|
0–1 | 0 | 2 |
|
|
2–3 | 8 | 19 |
|
|
4–5 | 6 | 22 |
|
| ECOG |
|
| 0.822a |
|
0–2 | 4 | 9 |
|
|
3–5 | 10 | 34 |
|
| Splenomegaly |
|
| 1.000b |
|
Yes | 13 | 41 |
|
| No | 1 | 2 |
|
| Hepatomegaly |
|
| 0.479a |
|
Yes | 5 | 20 |
|
| No | 9 | 23 |
|
| Multicavity
effusion |
|
| 0.931a |
|
Yes | 8 | 24 |
|
| No | 6 | 19 |
|
| Jaundice |
|
| 0.958a |
|
Yes | 5 | 13 |
|
| No | 9 | 30 |
|
| Edema |
|
| 0.958a |
|
Yes | 5 | 13 |
|
| No | 9 | 30 |
|
| Previous lymphoma
history |
|
| 0.169a |
|
Yes | 3 | 18 |
|
| No | 11 | 25 |
|
| Diagnosis time
(days); median (range) | 22.5
(6.0–42.0) | 20.0
(5.0–90.0) | 0.993e |
 | Table III.Laboratory data of patients with
B-cell LAHS and T/NK-cell LAHS. |
Table III.
Laboratory data of patients with
B-cell LAHS and T/NK-cell LAHS.
| Features median
(range) | B-cell lymphoma
(n=14) | T/NK-cell lymphoma
(n=43) | P-value |
|---|
| ANC
(×109/l) | 1.3 (0.0–3.0) | 0.8 (0.0–5.3) | 0.082a |
| Hb (g/l); mean ±
SE | 84.5±6.1 | 83.4±3.0 | 0.858b |
| PLT
(×109/l) | 26.5
(7.0–96.0) | 22.0
(2.0–129.0) | 0.475a |
| Liver function |
| ALT
(U/l) | 81.5
(12.0–755.0) | 108.0
(5.0–578.0) | 0.404a |
| AST
(U/l) | 140.0
(15.0–1227.0) | 160.0
(6.0–1294.0) | 0.904a |
| ALB
(g/l); mean ± SE | 26.5±1.1 | 28.9±0.8 | 0.128b |
| TBIL
(µmol/l) | 21.1
(5.7–356.3) | 18.0
(6.7–500.5) | 0.753a |
| IBIL
(µmol/l) | 8.5
(2.5–106.5) | 7.6
(0.7–108.0) | 0.948a |
| DBIL
(µmol/l) | 12.3
(2.3–294.2) | 11.4
(3.1–451.5) | 0.867a |
| Coagulation
function |
| FIB
(g/l) | 1.9 (0.3–4.8) | 1.1 (0.5–3.9) | 0.093a |
| LDH
(U/l) | 942.5
(227.0–2597.0) | 854.0
(131.0–14851.0) | 0.838a |
| β2-MG
(mg/l); mean ± SE | 7.5±1.0 | 6.6±0.6 | 0.467b |
| TG
(mmol/l) | 3.7 (0.9–11.7) | 2.6 (0.8–8.2) | 0.012a |
|
Ferritin (µg/l) | 1335.7
(316.0–2129.0) | 2000.0
(760.9–11816.0) | 0.014a |
| BM
hemophagocytosis |
| Yes | 10 | 29 | 1.000c |
| No | 4 | 14 |
|
| EBV DNA copies |
|
>102 | 3 | 33 |
<0.001c |
|
<102 | 11 | 10 |
|
Treatment and survival
Following a median follow-up of 33 days (range,
5–1,133 days), 52/57 patients (91.23%) had succumbed. The median
survival time of all patients was 43 days (range, 5–1,133 days).
Survival curves are depicted in Fig.
1. The median survival time of patients with B-cell LAHS and
T/NK-cell LAHS was 55 (range, 11–1,133 days) days and 40 days
(range, 5–809 days), respectively (P=0.797). The 0.5, 1 and 2-year
OS rates for patients with B-cell LAHS were 16.0, 16.0 and 8.0%,
respectively. The rates for OS for patients with T/NK-cell LAHS
were 26.0, 17.0 and 13.0%, respectively. Compared with 41 patients
who were treated with the HLH-2004 regimen combined with multidrug
chemotherapy (median survival time, 55 days), those who only
received the HLH-2004 regimen (and did not receive chemotherapy for
lymphoma) had a significantly reduced prognosis (median survival
time, 25 days) (P=0.002; Fig. 2A). Of
the 57 patients, five underwent autologous or allogeneic HSCT
following chemotherapy and had a significantly improved OS (median
survival time, 1,110 days) compared with the 52 remaining patients
without HSCT (median survival time, 36 days) (P=0.001; Fig. 2B).
 | Figure 1.Kaplan-Meier survival analysis of
patients with B-cell and T/NK-cell LAHS. The median survival time
of B-cell LAHS was 55 days, 0.5, 1 and 2-year OS rates were 16.0,
16.0 and 8.0%, respectively. The median survival time of T/NK-cell
LAHS was 40 days, and 0.5, 1 and 2-year OS rates were 26.0, 17.0
and 13.0%, respectively. NK, natural killer; LAHS, lymphoma
associated hemophagocytic syndrome; OS, overall survival. |
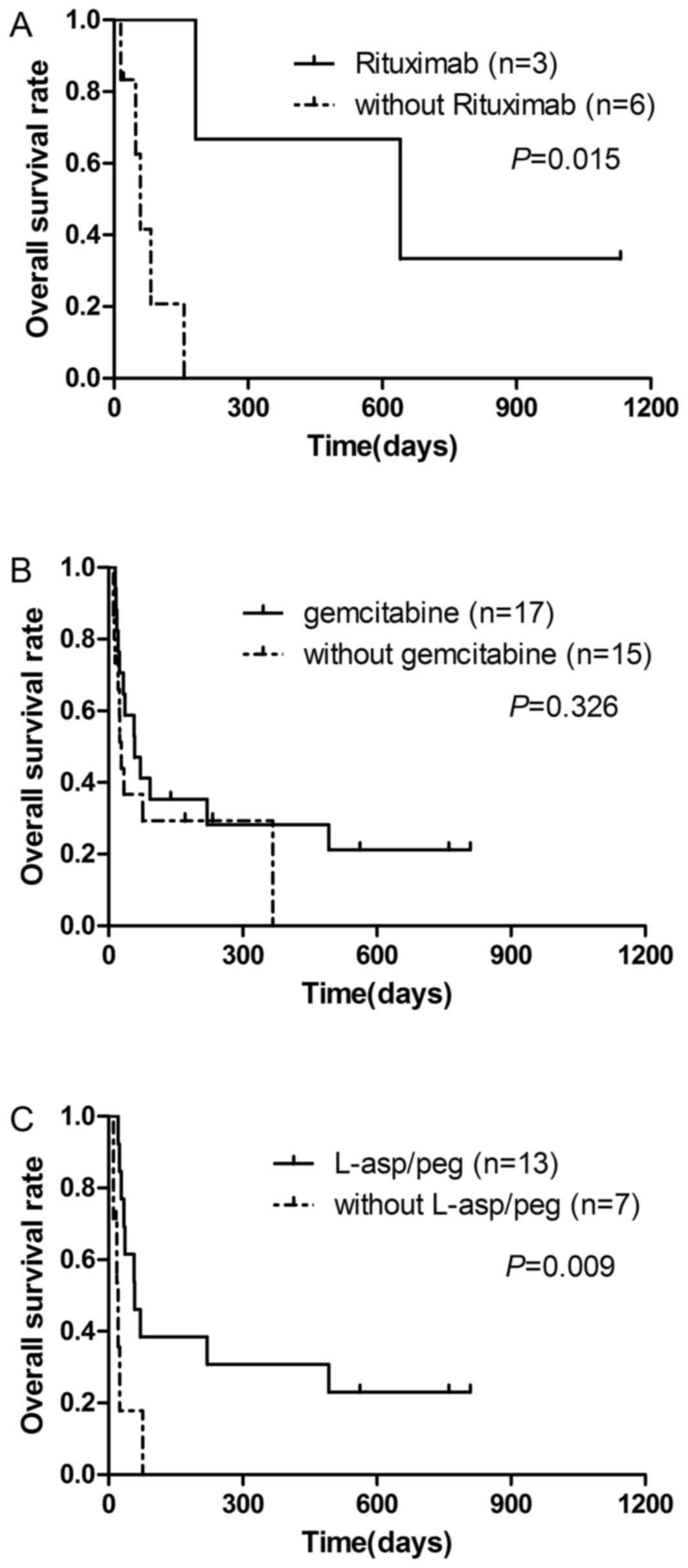
Of 14 patients with B-cell LAHS, five were treated
with CSA or dexamethasone and four patients faced rapid disease
progression in a short time. A total of 3 patients with B-cell LAHS
received chemotherapy plus rituximab, and two patients exhibited
complete remission (CR). The survival time of the 3 patients who
received chemotherapy plus rituximab (median, 645 days) was
significantly longer compared with 6 patients who did not receive
rituximab (median, 53 days) (P=0.015; Fig. 3A). For patients with T/NK-cell LAHS,
17 patients underwent chemotherapy with gemcitabine, and they did
not exhibit a significantly improved OS (median, 56 days), compared
with 15 patients not treated with gemcitabine (median, 27 days)
(P=0.326; Fig. 3B). For patients with
ENKL, 13 patients received chemotherapy regimens with
L-asparaginase (L-asp) or pegaspargase (peg), and they had an
improved prognosis (median survival time, 56 days) compared with 7
patients not treated with these drugs (median survival time, 20
days) (P=0.009; Fig. 3C).
Univariate and multivariate analysis
for prognostic factors
The results of the univariate analysis of patients
with LAHS are presented in Table IV.
Among the patients with B-cell LAHS, it was determined that OS was
significantly associated with serum ferritin level (P=0.036).
However, the following factors predicted poor OS for patients with
T/NK-cell LAHS and all patients with LAHS: Long diagnosis time
(P<0.001 for both); high ECOG scores (P=0.024 and P=0.005,
respectively); low hemoglobin (P=0.023 and P=0.005, respectively);
and high EBV DNA copies (P=0.011 and P=0.004, respectively).
Furthermore, low level of fibrinogen was also a negative prognostic
factor (P=0.036) for all patients with LAHS. Other baseline
characteristics were not significantly associated with
prognosis.
 | Table IV.Prognostic factors by univariate
analysis. |
Table IV.
Prognostic factors by univariate
analysis.
|
| B-cell
lymphoma | T/NK-cell
lymphoma | All patients |
|---|
|
|
|
|
|
|---|
| Risk factor | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Sex
(Male/Female) | 3.822 | 0.972–15.030 | 0.055 | 0.524 | 0.260–1.054 | 0.070 | 0.844 | 0.468–1.524 | 0.574 |
| Lymphoma history
(No/Yes) | 0.313 | 0.069–1.416 | 0.131 | 1.578 | 0.774–3.217 | 0.210 | 1.174 | 0.639–2.154 | 0.605 |
| Diagnosis time
(>20 days/≤20 days) | 1.208 | 0.385–3.793 | 0.746 | 5.594 | 2.392–13.082 | 0.000 | 3.198 | 1.732–5.906 | 0.000 |
| ECOG (3–5/0-2) | 5.929 | 0.728–48.299 | 0.096 | 2.914 | 1.151–7.375 | 0.024 | 3.258 | 1.437–7.389 | 0.005 |
| IPI score
(4–5/0-3) | 1.334 | 0.403–4.418 | 0.637 | 1.049 | .0529–2.082 | 0.890 | 1.109 | 0.616–1.997 | 0.731 |
| Multicavity
effusion (Yes/No) | 3.311 | 0.829–13.222 | 0.090 | 1.583 | 0.780–3.212 | 0.203 | 1.715 | 0.939–3.132 | 0.079 |
| ANC
(≤1.0×109/l/>1.0×109/l) | 0.557 | 0.164–1.894 | 0.348 | 0.640 | 0.300–1.365 | 0.249 | 0.964 | 0.496–1.874 | 0.914 |
| Hb (≤90 g/l/>90
g/l) | 3.668 | 0.741–18.155 | 0.111 | 2.566 | 1.141–5.767 | 0.023 | 2.745 | 1.359–5.545 | 0.005 |
| PLT
(≤25×109/l/>25×109/l) | 1.85 | 0.531–6.449 | 0.199 | 1.625 | 0.775–3.406 | 0.199 | 1.714 | 0.919–3.194 | 0.090 |
| ALT (>80 U/l/≤80
U/l) | 0.818 | 0.258–2.596 | 0.733 | 0.906 | 0.442–1.859 | 0.788 | 0.925 | 0.508–1.685 | 0.798 |
| AST (>80 U/l/≤80
U/l) | 1.069 | 0.318–3.590 | 0.914 | 0.701 | 0.341–1.441 | 0.334 | 0.799 | 0.433–1.476 | 0.474 |
| TBIL (>25
µmol/l/≤25 µmol/l) | 1.385 | 0.550–2.196 | 0.790 | 1.385 | 0.444–4.325 | 0.575 | 1.152 | 0.637–2.082 | 0.640 |
| DBIL (>10
µmol/l/≤10 µmol/l) | 1.797 | 0.479–6.736 | 0.385 | 1.377 | 0.686–2.764 | 0.368 | 1.499 | 0.817–2.752 | 0.192 |
| IBIL (>14
µmol/l/≤14 µmol/l) | 2.601 | 0.615–11.010 | 0.194 | 0.733 | 0.298–1.803 | 0.499 | 0.898 | 0.429–1.878 | 0.775 |
| β2-MG (>5
mg/l/≤5 mg/l) | 1.578 | 0.412–6.038 | 0.505 | 0.916 | 0.451–1.862 | 0.809 | 1.090 | 0.589–2.019 | 0.784 |
| FIB (<1.5
g/l/≥1.5 g/l) | 1.951 | 0.559–6.817 | 0.295 | 2.830 | 0.985–8.134 | 0.053 | 2.090 | 1.051–4.157 | 0.036 |
| TG (≥3 mmol/l/<3
mmol/l) | 2.609 | 0.566–12.019 | 0.219 | 1.559 | 0.742–3.276 | 0.241 | 1.610 | 0.890–2.911 | 0.115 |
| Ferritin (≥2,000
µg/l/<2,000 µg/l) | 3.911 | 1.092–14.010 | 0.036 | 0.637 | 0.319–1.269 | 0.199 | 0.966 | 0.536–1.741 | 0.908 |
| EBV DNA
(≥105/<105) | 6.161 | 0.558–67.968 | 0.138 | 2.834 | 1.274–6.304 | 0.011 | 2.508 | 1.336–4.706 | 0.004 |
| BM hemophagocytosis
(Yes/No) | 0.648 | 0.182–2.312 | 0.504 | 0.608 | 0.291–1.271 | 0.186 | 0.625 | 0.332–1.177 | 0.145 |
As presented in Table
V, multivariate analysis was performed using the Cox
proportional hazards model to assess the potential independent
prognostic factors. Results demonstrated that diagnosis time
(P=0.021) and ECOG scores (P=0.022) were independent predictors of
all patients with LAHS. Furthermore, diagnosis time (P=0.003) was
an independent predictor of patients with T/NK-cell LAHS. The
median survival time of patients with long diagnosis time (>20
days) and high ECOG score (3–5 scores) was 25.3 and 30.9 days,
respectively. However, the median survival time of patients with a
short diagnostic time (≤20 days) and low ECOG score (0–2 scores)
was 85.0 and 383.8 days, respectively. For patients with T/NK-cell,
the median survival time of patients with long and short diagnosis
time were 23.7 and 213.1 days, respectively.
 | Table V.Prognostic factors by multivariate
analysis. |
Table V.
Prognostic factors by multivariate
analysis.
|
| T/NK-cell
lymphoma | All patients |
|---|
|
|
|
|
|---|
| Risk Factor | Hazard ratio | 95%CI | P-value | Hazard ratio | 95%CI | P-value |
|---|
| Diagnosis time
(>20 days/≤20 days) | 3.901 | 1.586–9.597 | 0.003 | 2.182 | 1.123–4.230 | 0.021 |
| ECOG (3–5/0-2) | 2.318 | 0.867–6.541 | 0.092 | 2.814 | 1.164–6.803 | 0.022 |
| Hb (≤90 g/l/>90
g/l) | 1.826 | 0.761–4.382 | 0.177 | 1.950 | 0.915–4.157 | 0.084 |
| FIB (<1.5
g/l/≥1.5 g/l) | 2.747 | 0.902–8.369 | 0.075 | 1.448 | 0.700–2.994 | 0.318 |
| EBV DNA
(≥105/<105) | 1.525 | 0.619–3.756 | 0.359 | 1.834 | 0.947–3.551 | 0.072 |
Discussion
To the best of our knowledge, this is one of the
largest cohort of patients with LAHS in a study. The present study
demonstrated that LAHS, a subtype of secondary HPS, has specific
clinical features, prognostic factors and outcomes. A total of 57
patients with LAHS were retrospectively reviewed in the present
study. A total of 43 patients were diagnosed with T/NK-cell LAHS.
Patients with ENKL and PNKTL accounted for half of all patients
with LAHS. Although a number of reports stated that there were an
increasing number of B-LAHS cases (17,18), the
majority of reported cases of LAHS remained as T/NK-cell lymphoma
(8,9,19), which
was consistent with the present study.
In the present study, patients with B-cell LAHS and
T/NK-cell LAHS shared similar clinical features and laboratory
data; however, patients with B-cell LAHS were older, which was
similar to the previous studies by Han et al (8) and Sano et al (9). Patients with T/NK-cell LAHS also
presented a higher level of serum ferritin compared with patients
with B-cell LAHS. In a study conducted by Yu et al (18), serum ferritin level was significantly
higher in patients with T-cell LAHS, which was consistent with the
present result. They also considered that a higher ferritin level
may be associated with reduced survival outcome in patients with
T-cell LAHS, as hyperferritinemia may indicate elevated cytokine
activation and result in activating hepatic proinflammatory
mediators through the nuclear factor-κB signaling pathway (18). Allen et al (20) reported that a ferritin level
>10,000 µg/l was 90% sensitive and 96% specific for HPS;
however, a high level of ferritin alone is just indicative, and the
diagnosis of HPS may not be confirmed.
In the present study, plasma EBV DNA was determined
to be higher in patients with T/NK-cell LAHS compared with patients
with B-cell LAHS, whereas Yu et al (18) indicated that there was no significant
difference between patients with T-cell and B-cell lymphoma in the
presence of an EBV infection. Opposing results may be due to
numerous reasons. Firstly, the constituent ratio of the underlying
disease was different. In the present study a total of 2/3 of the
T/NK-cell lymphoma accounted for ENKL, whereas ENKL accounted for
<6% according to Yu et al (18). Furthermore, in the study by Yu et
al, patients were only checked if they were EBV-positive,
whereas in the present study overall plasma EBV DNA copies were
detected. EBV serves an important role in T/NK-cell lymphoma as
well as EBV-associated HPS, and it may cause oncogenesis or occur
in tumor-associated lymphocytes as a function of immune
dysregulation (21). A number of
previous studies have confirmed that plasma EBV-DNA was a
prognostic marker for ENKL (22,23).
EBV-infected T cells selectively upregulate TNF-α expression, which
may activate macrophages in combination with IFN-γ and other
cytokines (24,25). The elevated levels of cytokines
secreted by EBV-infected cells cause a series of clinical
manifestations (25); however, Ohno
et al (24) demonstrated that
EBV involvement was not detected in patients with B-cell LAHS,
which indicated that EBV infection was not involved in the onset of
B-cell LAHS. Instead, numerous reactive CD3+ T cells were detected
in the bone marrow of all patients with B-cell LAHS, and these
reactive T cells were functionally activated, thus indicating that
they may be responsible for cytokine production in B-cell LAHS
(24).
In the present study, the OS of patients with LAHS
was poor. The median survival time was 43 days. Previous studies
also reported an inferior OS of patients with LAHS. Barba et
al (26) demonstrated that
lymphoma was one of the factors associated with increased mortality
in patients with HPS. Tong et al (19) conducted a study of 28 patients with
aggressive T-cell LAHS and indicated that the median survival time
of the patients was 40 days. However, Yu et al (18) reviewed 30 patients with LAHS and
reported that the median survival time was 231 days. They indicated
that the improved survival may be due to rituximab treatment and
HSCT in patients with B-cell LAHS and T-cell LAHS,
respectively.
In the present study, although patients with B-cell
LAHS exhibited a longer survival time compared with patients with
T/NK-cell LAHS, this difference was not statistically significant
(P=0.797). These results were confirmed by Yu et al
(18). However, a number of studies
indicated that B-cell lymphoma was associated with a better
prognosis compared with T/NK-cell lymphoma (9,27). The
potential reasons for the inconsistencies between the results of
these previous studies and the present study was that there were
five patients with B-cell LAHS who only received CSA or
dexamethasone in the present study.
The median diagnosis time in the present study was
22 days. Univariate and multivariate analysis identified that a
long diagnosis time was a poor prognostic factor for patients with
LAHS. Numerous factors may influence the diagnosis. For patients
with lymphoma suspected of having HPS, misdiagnosis often occurs as
fever and pancytopenia may also be caused by severe infection or
myelosuppression following chemotherapy. For patients without
lymphoma, once the diagnosis of HPS was established, the underlying
diseases were difficult to identify, due to a number of patients
being too weak to receive biopsies.
Numerous attempts have been made for an early
diagnosis of LAHS. It is reported that PET-CT may act as a
significant tool to assess patients with LAHS, as it is highly
sensitive in detecting neoplasms of the majority of histologic
subtypes of lymphoma, and also demonstrates extensive
18-fluorodeoxyglucose (FDG) uptake in tumor tissues (28). It was also reported that the maximum
standardized uptake values of patients with malignancy-associated
HPS, particularly lymphoma, was statistically higher compared with
those with an infection or rheumatosis-associated HPS. Therefore,
PET-CT may serve an important role in differential diagnosis of
secondary HPS (29). Furthermore, FDG
uptake may reflect the level of cytokine storm to a certain extent
and be a prognostic factor for patients with LAHS (30). Tabata et al (10) reviewed 57 LAHS cases and 53 benign
disease-associated HPS cases, and indicated that the serum soluble
IL-2 receptor (sIL-2R) level and the sIL-2R/ferritin ratio may act
as useful markers for distinguishing underlying lymphoma from other
causes in patients with HPS. Maruoka et al (31) identified that IFN-inducible protein 10
(IP-10) and monokine induced by IFN-γ (MIG) were useful markers for
early diagnosis of LAHS. The sensitivity and specificity for the
diagnosis were 100 and 95%, respectively. The serum level of IP-10
and MIG in T or NK/T-cell LAHS were higher compared with those in
B-cell LAHS.
HLH-1994 (32) or
HLH-2004 protocols are validated treatments for primary HPS.
However, the efficacy of these treatment protocols for LAHS is
poorly understood. It is generally considered that the most
important treatments for LAHS are combined chemotherapy regimens
that target malignancy lymphomas (6,18). In the
present study, patients who were treated according to the HLH-2004
protocol and with multidrug chemotherapy exhibited improved
outcomes compared with those who did not receive chemotherapy,
which demonstrates that it is equally important to treat primary
diseases as well as treating HPS. Furthermore, the survival time of
three patients with B-cell LAHS who received regimens containing
rituximab was significantly longer compared with those who did not
receive rituximab. This survival benefit may be due to introducing
rituximab. To date, gemcitabine-based combination chemotherapy has
been demonstrated to be highly successful in improving the
treatment outcome of T/NK-cell lymphoma (33–35).
However, in the present study patients with T/NK-cell LAHS who were
treated with gemcitabine did not exhibit survival advantage.
L-asp/peg-based regimens were analyzed in the ENKL group and led to
an improved prognosis. L-asp/peg-containing regimens have been
indicated to be highly effective for patients with NK/T-cell
lymphoma (35). The prior studies
also demonstrated that the significant efficacy and safety profile
of the peg-based regimen in the treatment of newly diagnosed and
relapsed/refractory ENKL (35–37). The
anticancer effect of L-asp is not affected by multidrug resistance
gene due to its unique mechanism (37). Since ENKL cells cannot synthesize
asparagine themselves, tumor cell proliferation is suppressed under
the effect of hydrolyzing asparagine by L-asp (38).
It was reported that patients with primary HPS may
achieve long-term survival following a treatment regime of
immunochemotherapy combined with HSCT (11). A large prospective study of 249
patients with familial, refractory or recurrent HPS indicated that
the 5-year survival rate of 124 patients who underwent HSCT was
66±8% (39). A study comparing
reduced intensity conditioning (RIC) regimens with myeloablative
conditioning (MAC) regimens demonstrated that the overall 3-year
survival following HSCT was 43% for patients with MAC and 92% for
patients with RIC (40). However,
HSCT has rarely been reported as a treatment of LAHS, and the
efficacy remains unknown. It was reported that a number of patients
with LAHS achieved CR and obtained long-term survival from
allogeneic or autologous HSCT (8,18,41,42). The
present study indicated that HSCT may improve the outcome of
patients with LAHS. However, further research is required to
investigate the role of HSCT in LAHS treatment.
The present study has several limitations. The study
was retrospectively conducted from a single center, and the number
of patients involved was relatively small. Additionally, NK cell
activity and soluble CD25 levels were not analyzed.
In conclusion, LAHS is relatively common but has the
worst prognosis of secondary HPS types, and it poses a challenge to
clinicians (43). The results of the
present study demonstrated that the survival time did not differ
between patients with B-cell and T/NK-cell LAHS. Early diagnosis
and immunochemotherapy plus HSCT may lead to better outcomes.
Treatment of the underlying lymphoma in patients with LAHS ought to
be treated at the same time as the implementation of
countermeasures for the suppression of the extreme inflammation
triggered by HPS. The outcome of patients with B-cell LAHS may be
significantly improved following treatment with rituximab.
L-asp/peg-containing regimens are promising treatments if NK/T-cell
lymphomas are recognized as an underlying disease. Prospective
multicenter studies with larger sample sizes are required for an
optimal treatment for LAHS.
Acknowledgements
The authors would like to thank Yingjun Wang and Li
Tian from Lymphoma Diagnosis and Treatment Centre of Henan Province
for providing clinical data.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81570203).
Availability of data and materials
All data analyzed during this study are included in
this published article.
Authors' contributions
YC, GS and MZ designed the study, interpreted the
results and wrote the manuscript. MC, XF, LH and LZ performed the
data analysis and statistical analysis. LL, XL, ZS, JW, XZ, ZL, FN
and JY performed literature research and the clinical data
acquisition.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
the First Affiliated Hospital of Zhengzhou University. Written
informed consent for the collection of medical information was
obtained from all patients.
Consent for publication
Informed consent for the collection and publication
of medical information was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Risdall RJ, McKenna RW, Nesbit ME, Krivit
W, Balfour HJ, Simmons RL and Brunning RD: Virus-associated
hemophagocytic syndrome: A benign histiocytic proliferation
distinct from malignant histiocytosis. Cancer. 3:993–1002. 1979.
View Article : Google Scholar
|
|
2
|
Szyper-Kravitz M: The hemophagocytic
syndrome/macrophage activation syndrome: A final common pathway of
a cytokine storm. Isr Med Assoc J. 10:633–634. 2009.
|
|
3
|
Janka GE and Lehmberg K: Hemophagocytic
lymphohistiocytosis: Pathogenesis and treatment. Hematology Am Soc
Hematol Educ Program. 2013:605–611. 2013.PubMed/NCBI
|
|
4
|
Rouphael NG, Talati NJ, Vaughan C,
Cunningham K, Moreira R and Gould C: Infections associated with
haemophagocytic syndrome. Lancet Infect Dis. 12:814–822. 2007.
View Article : Google Scholar
|
|
5
|
Janka GE and Lehmberg K: Hemophagocytic
syndromes-an update. Blood Rev. 4:135–142. 2014. View Article : Google Scholar
|
|
6
|
Han L, Li L, Wu J, Li X, Zhang L, Wang X,
Fu X, Ma W, Sun Z, Zhang X, et al: Clinical features and treatment
of natural killer/T cell lymphoma associated with hemophagocytic
syndrome: Comparison with other T cell lymphoma associated with
hemophagocytic syndrome. Leuk Lymphoma. 9:2048–2055. 2014.
View Article : Google Scholar
|
|
7
|
Parikh SA, Kapoor P, Letendre L, Kumar S
and Wolanskyj AP: Prognostic factors and outcomes of adults with
hemophagocytic lymphohistiocytosis. Mayo Clin Proc. 89:484–492.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han AR, Lee HR, Park BB, Hwang IG, Park S,
Lee SC, Kim K, Lim HY, Ko YH, Kim SH and Kim WS:
Lymphoma-associated hemophagocytic syndrome: Clinical features and
treatment outcome. Ann Hematol. 86:493–498. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sano H, Kobayashi R, Tanaka J, Hashino S,
Ota S, Torimoto Y, Kakinoki Y, Yamamoto S, Kurosawa M, Hatakeyama
N, et al: Risk factor analysis of non-Hodgkin lymphoma-associated
haemophagocytic syndromes: A multicentre study. Br J Haematol.
165:786–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tabata C and Tabata R: Possible prediction
of underlying lymphoma by high sIL-2R/ferritin ratio in
hemophagocytic syndrome. Ann Hematol. 91:63–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seo JJ: Hematopoietic cell transplantation
for hemophagocytic lymphohistiocytosis: Recent advances and
controversies. Blood Res. 50:131–139. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sabattini E, Bacci F, Sagramoso C and
Pileri SA: WHO classification of tumours of haematopoietic and
lymphoid tissues in 2008: An overview. Pathologica. 102:83–87.
2010.PubMed/NCBI
|
|
13
|
Henter JI, Horne A, Arico M, Egeler RM,
Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski
J and Janka G: HLH-2004: Diagnostic and therapeutic guidelines for
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer.
48:124–131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olweny CL: Cotswolds modification of the
Ann Arbor staging system for Hodgkin's disease. J Clin Oncol.
9:15981990.
|
|
15
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al: Revised response criteria for malignant lymphoma. J Clin
Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takahashi N, Chubachi A, Miura I, Nakamura
S and Miura AB: Lymphoma-associated hemophagocytic syndrome in
Japan. Rinsho Ketsueki. 40:542–549. 1999.(In Japanese). PubMed/NCBI
|
|
18
|
Yu JT, Wang CY, Yang Y, Wang RC, Chang KH,
Hwang WL and Teng CL: Lymphoma-associated hemophagocytic
lymphohistiocytosis: Experience in adults from a single
institution. Ann Hematol. 11:1529–1536. 2013. View Article : Google Scholar
|
|
19
|
Tong H, Ren Y, Liu H, Xiao F, Mai W, Meng
H, Qian W, Huang J, Mao L, Tong Y, et al: Clinical characteristics
of T-cell lymphoma associated with hemophagocytic syndrome:
Comparison of T-cell lymphoma with and without hemophagocytic
syndrome. Leuk Lymphoma. 49:81–87. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allen CE, Yu X, Kozinetz CA and McClain
KL: Highly elevated ferritin levels and the diagnosis of
hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer.
50:1227–1235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen YP, Jones D, Chen TY and Chang KC:
Epstein-Barr virus present in T cells or B cells shows differential
effects on hemophagocytic symptoms associated with outcome in
T-cell lymphomas. Leuk Lymphoma. 55:2038–2047. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwong YL, Pang AW, Leung AY, Chim CS and
Tse E: Quantification of circulating Epstein-Barr virus DNA in
NK/T-cell lymphoma treated with the SMILE protocol: Diagnostic and
prognostic significance. Leukemia. 28:865–870. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suzuki R, Yamaguchi M, Izutsu K, Yamamoto
G, Takada K, Harabuchi Y, Isobe Y, Gomyo H, Koike T, Okamoto M, et
al: Prospective measurement of Epstein-Barr virus-DNA in plasma and
peripheral blood mononuclear cells of extranodal NK/T-cell
lymphoma, nasal type. Blood. 118:6018–6022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ohno T, Ueda Y, Nagai K, Takahashi T,
Konaka Y, Takamatsu T, Suzuki T, Sasada M and Uchiyama T; Kyoto
University Hematology/Oncology Study Group: The serum cytokine
profiles of lymphoma-associated hemophagocytic syndrome: A
comparative analysis of B-cell and T-cell/natural killer cell
lymphomas. Int J Hematol. 77:286–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chuang HC, Lay JD, Hsieh WC and Su IJ:
Pathogenesis and mechanism of disease progression from
hemophagocytic lymphohistiocytosis to Epstein-Barr virus-associated
T-cell lymphoma: Nuclear factor-kappa B pathway as a potential
therapeutic target. Cancer Sci. 98:1281–1287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barba T, Maucort-Boulch D, Iwaz J, Bohé J,
Ninet J, Hot A, Lega JC, Guérin C, Argaud L, Broussolle C, et al:
Hemophagocytic Lymphohistiocytosis in intensive care unit: A
71-case strobe-compliant retrospective study. Medicine (Baltimore).
94:e23182015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cattaneo C, Oberti M, Skert C, Passi A,
Farina M, Re A, Tozzi P, Borlenghi E and Rossi G: Adult onset
hemophagocytic lymphohistiocytosis prognosis is affected by
underlying disease and coexisting viral infection: Analysis of a
single institution series of 35 patients. Hematol Oncol.
35:828–834. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yiu CR, Kao YH, Phipps C and Tan D:
Positron emission tomography findings in patients with
lymphoma-associated haemophagocytic syndrome. Singapore Med J.
7:e156–e159. 2011.
|
|
29
|
Zhang LJ, Xu J, Liu P, Ding CY, Li JY, Qiu
HX and Zhang SJ: The significance of 18F-FDG PET/CT in secondary
hemophagocytic lymphohistiocytosis. J Hematol Oncol. 5:402012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang YQ, Ding CY, Xu J, Fan L, Wang L,
Tian T, Li TN, Li JY and Xu W: Exploring the role of bone marrow
increased FDG uptake on PET/CT in patients with lymphoma-associated
hemophagocytic lymphohistiocytosis: A reflection of bone marrow
involvement or cytokine storm? Leuk Lymphoma: Jun 19, 1–8, 2015
(Epub ahead of print).
|
|
31
|
Maruoka H, Inoue D, Takiuchi Y, Nagano S,
Arima H, Tabata S, Matsushita A, Ishikawa T, Oita T and Takahashi
T: IP-10/CXCL10 and MIG/CXCL9 as novel markers for the diagnosis of
lymphoma-associated hemophagocytic syndrome. Ann Hematol.
3:393–401. 2014. View Article : Google Scholar
|
|
32
|
Henter JI, Arico M, Egeler RM, Elinder G,
Favara BE, Filipovich AH, Gadner H, Imashuku S, Janka-Schaub G,
Komp D, et al: HLH-94: A treatment protocol for hemophagocytic
lymphohistiocytosis. HLH study group of the histiocyte society. Med
Pediatr Oncol. 28:342–347. 1997. View Article : Google Scholar
|
|
33
|
Park BB, Kim WS, Suh C, Shin DY, Kim JA,
Kim HG and Lee WS: Salvage chemotherapy of gemcitabine,
dexamethasone, and cisplatin (GDP) for patients with relapsed or
refractory peripheral T-cell lymphomas: A consortium for improving
survival of lymphoma (CISL) trial. Ann Hematol. 94:1845–1851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pellegrini C, Dodero A, Chiappella A,
Monaco F, Degl'Innocenti D, Salvi F, Vitolo U, Argnani L, Corradini
P and Zinzani PL; Italian Lymphoma Foundation (Fondazione Italiana
Linfomi Onlus, FIL): A phase II study on the role of gemcitabine
plus romidepsin (GEMRO regimen) in the treatment of
relapsed/refractory peripheral T-cell lymphoma patients. J Hematol
Oncol. 9:382016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Cui Y, Sun Z, Zhang L, Li L, Wang X,
Wu J, Fu X, Ma W, Zhang X, et al: DDGP versus SMILE in newly
diagnosed advanced natural Killer/T cell lymphoma: A randomized
controlled, multicenter, Open-label study in China. Clin Cancer
Res. 22:5223–5228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li L, Zhang C, Zhang L, Li X, Wu JJ, Sun
ZC, Fu XR, Wang XH, Chang Y, Wang R, et al: Efficacy of a
pegaspargase-based regimen in the treatment of newly-diagnosed
extranodal natural killer/T-cell lymphoma. Neoplasma. 61:225–232.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Z, Li X, Chen C, Li X, Zhang L, Li L,
Wang X, Ma W, Fu X, Wu J, et al: Effectiveness of gemcitabine,
pegaspargase, cisplatin, and dexamethasone (DDGP) combination
chemotherapy in the treatment of relapsed/refractory extranodal
NK/T cell lymphoma: A retrospective study of 17 patients. Ann
Hematol. 93:1889–1894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T,
Tse E, Leung AY and Chim CS: SMILE for natural killer/T-cell
lymphoma: Analysis of safety and efficacy from the Asia lymphoma
study group. Blood. 120:2973–2980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trottestam H, Horne A, Arico M, Egeler RM,
Filipovich AH, Gadner H, Imashuku S, Ladisch S, Webb D, Janka G, et
al: Chemoimmunotherapy for hemophagocytic lymphohistiocytosis:
Long-term results of the HLH-94 treatment protocol. Blood.
118:4577–4584. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marsh RA, Vaughn G, Kim MO, Li D, Jodele
S, Joshi S, Mehta PA, Davies SM, Jordan MB, Bleesing JJ and
Filipovich AH: Reduced-intensity conditioning significantly
improves survival of patients with hemophagocytic
lymphohistiocytosis undergoing allogeneic hematopoietic cell
transplantation. Blood. 116:5824–5831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Machaczka M, Nahi H, Karbach H, Klimkowska
M and Hagglund H: Successful treatment of recurrent
malignancy-associated hemophagocytic lymphohistiocytosis with a
modified HLH-94 immunochemotherapy and allogeneic stem cell
transplantation. Med Oncol. 29:1231–1236. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Inoue D, Nagai Y, Takiuchi Y, Nagano S,
Arima H, Kimura T, Shimoji S, Mori M, Togami K, Tabata S, et al:
Successful treatment of extranodal natural killer/T-cell lymphoma,
nasal type, complicated by severe hemophagocytic syndrome, with
dexamethasone, methotrexate, ifosfamide, L-asparaginase, and
etoposide chemotherapy followed by autologous stem cell transplant.
Leuk Lymphoma. 51:720–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
George MR: Hemophagocytic
lymphohistiocytosis: Review of etiologies and management. J Blood
Med. 5:69–86. 2014. View Article : Google Scholar : PubMed/NCBI
|