Introduction
Lymphoma may arise from a nodal or extra-nodal
origin, and the number of patients with extra-nodal non-Hodgkin's
lymphoma (NHL) is rapidly increasing (1,2). The exact
designation of primary extra-nodal NHL (PE-NHL) is controversial,
particularly when both nodal and extra-nodal sites are involved; a
number of studies have described PE-NHL as presenting only in
extra-nodal sites, with no visible lymphadenopathy on imaging
(3,4),
while others have used a broader definition, in which extra-nodal
disease with regional or distant involved lymph nodes is included
(1,5).
In the present study, the former definition was selected. The
incidence of PE-NHL varies between countries, accounting for 15–48%
of NHL cases. The most common pathological type of PE-NHL is
diffuse large B-cell lymphoma (DLBCL), representing 71–81.3% of
cases (6,7). Although lymphomas may involve almost all
extra-nodal organs, different organs are involved at different
frequencies. Primary extra-nodal DLBCLs (PE-DLBCLs) are common in
the gastrointestinal (GI) tract, and are relatively uncommon in the
central nervous system (CNS), thyroid, breast, female genital
system (FGS), testis, skin, adrenal gland, pancreas, bone or other
sites (6,8,9).
CNS relapse is nearly always fatal, and the overall
risk of CNS relapse of patients with DLBCL is ~5% (10). However, the incidence is much higher
in patients when breast, adrenal gland or testicular sites are
involved.
The diversity of clinical presentations suggests
that PE-DLBCLs are distinct entities, and efforts regarding the
risk factors and prevention methods for CNS recurrence are
inconclusive. Thus, the present study retrospectively analyzed the
clinical features, response to therapy, long-term outcomes and CNS
relapse of patients with PE-DLBCL at the Department of Hematology
(Peking Union Medical College Hospital, Beijing, China).
Patients and methods
Patients
A total of 677 patients (median age 58 years, age
range 12–78 years; male to female ratio 71:10) diagnosed with DLBCL
and treated at Peking Union Medical College Hospital between
December 2003 and December 2013, and 141 patients diagnosed with
PE-NHL were evaluated. The median age was 58 (range, 12–78), and
the sex ratio of male and female was 71:70. All biopsies were
classified according to the World Health Organization
classification system (11) and were
analyzed by immunohistochemistry. When lymphomas were contiguous
with neighboring organs, the site with the largest area of
involvement was defined as the dominant site. The Ann Arbor stage
for PE-NHL involving bilateral paired organs or diffuse lesions of
an organ remains a source of contention, but in the present study,
these situations were considered as stage IV. Patients presenting
with either systemic disease, primary nodal NHL with secondary
extra-nodal involvement, infection with human immunodeficiency
virus, hepatitis C or B, or recurrent lymphoma following previous
treatment were excluded from the study.
Measurements of complete blood count and biochemical
parameters, including serum lactate dehydrogenase (LDH), serum
total protein, serum albumin, creatinine, serum urea, uric acid,
liver enzymes and bilirubin, and bone marrow aspiration and
trephine biopsy, whole body computed tomography scan,
fluorodeoxyglucose positron emission tomography (FDG-PET) and
magnetic resonance imaging (MRI) of the brain were performed prior
to and following treatment. Patients were staged and evaluated
according to the Ann Arbor classification (12) and the International Prognostic Index
(IPI) score (13). Patients with
primary gastrointestinal DLBCL were evaluated by Lugano
classification (14).
All procedures were performed in accordance with the
ethical standards of the responsible Ethics Committee of Peking
Union Medical College Hospital (Beijing, China) on human
experimentation (institutional and national) and with the
Declaration of Helsinki (1975), as revised in 2000. Written
informed consent was obtained from all patients included in the
study.
Treatment and response
First-line therapy for patients with primary
CNS-DLBCL (PCNS-DLBCL) was high-dose methotrexate (HD-MTX)-based
combined chemotherapy (CT; 3 g/m2 over 4 h rapid
infusion time; the cycle length of HD-MTX transfusion was 28 days
and the number of cycles was 6–8.) and 14 patients received
radiotherapy (RT; 40–50 Gy) after CT. The first-line treatment for
the remaining PE-DLBCL patients was CHOP (cyclophosphamide 750
mg/m2 iv d1, doxorubicin 50 mg/m2 iv d1,
vindesine 1.4 mg/m2 iv d1, prednisone 100 mg, po d1-5)
or CHOP-like regimen combined with rituximab (R-CHOP. rituximab 375
mg/m2 d1). The regimen was given every 21 days for 6–8
cycles. However, 49 patients selected treatment without rituximab
due to its high cost. A total of 32 patients underwent surgery for
definitive diagnosis and 10 patients for the management of
complications.
The National Comprehensive Cancer Network (NCCN)
(15) guidelines divide patients into
low (score, 0–1), moderate (score, 2–3) and high-risk (score, 4–6)
CNS relapse groups based on the IPI (5 scores) and kidney or
adrenal involvement (1 score). Due to the retrospective nature of
the present study, scoring procedures were not standardized and not
all variables were available for each patient. In 2012, our center
began administering intravenous injections of HD-MTX for the
prevention of CNS recurrence. A total of 68 patients with a
moderate or high risk of CNS relapse received prophylaxis,
including 36 patients that only received intrathecal injection, 19
patients that only received the intravenous injection of HD-MTX (1
g/m2 for 4 cycles) and 13 patients that received
both.
The response to treatment was assessed, following
the completion of initial therapy, according to the International
Working Group criteria (16) as
complete remission (CR), partial remission (PR), stable disease
(SD) or progressive disease (PD).
Follow-up
Patients were followed up every 3 months for the
first 3 years after treatment and every 6 months thereafter.
Routine examinations were performed during the follow-up period,
including physical examination, laboratory tests, echocardiography,
and a whole-body computed tomography scan or FDG-PET. Lumbar
puncture head MRIs were performed on those with primary CNS
involvement or the clinical symptoms of CNS relapse. The final
follow-up date was November 31, 2016. Among all 141 patients, 16
were lost to follow-up.
Statistical analysis
Overall survival (OS) was calculated from the date
of diagnosis to the date of last follow-up or mortality from any
cause. Progression-free survival (PFS) was evaluated from the date
of diagnosis to the date of disease progression or relapse.
Statistical analysis was performed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA). The Kaplan-Meier method and the log-rank
test were used for univariate analysis and the generation of
survival curves. All factors with P-values <0.10 were included
in the multivariate analysis using the Cox proportional hazards
model. Differences were evaluated using a two-tailed test;
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinical characteristics
Patients with PE-DLBCL accounted for 20.8% of all
the patients with NHL during the study period. The characteristics
of the patients at diagnosis are summarized in Table I. The presence of B symptoms was less
common in patients with primary CNS, breast, thyroid gland or FGS
involvement than it was for other sites. The majority of patients
were classified as clinical stage I–II (87; 61.7%), whereas the
number of patients at stage III/IV was highest for patients with
primary adrenal gland (12/15; 80.0%) and bone (6/7; 85.7%)
involvement. The overall distribution of patients with PE-DLBCL is
presented in Table II.
 | Table I.Patient characteristics according to
the primary involved site. |
Table I.
Patient characteristics according to
the primary involved site.
|
Characteristics | GI tract | CNS | Breast | Adrenal gland | FGS | Thyroid gland | Bone |
|---|
| Total | 42 | 38 | 19 | 15 | 12 | 8 | 7 |
| Age, years |
|
|
|
|
|
|
|
|
Median | 56 | 58 | 53 | 62 | 59 | 61 | 56 |
|
Range | 15–77 | 17–78 | 20–77 | 43–73 | 20–77 | 54–77 | 12–68 |
| Age
>60 years | 17 | 14 | 3 | 9 | 5 | 4 | 2 |
| Sex |
|
|
|
|
|
|
|
|
Male | 28 | 27 | 0 | 8 | 0 | 2 | 6 |
|
Female | 14 | 11 | 19 | 7 | 12 | 6 | 1 |
| B
symptoms | 24 | 4 | 2 | 7 | 3 | 0 | 5 |
|
Increased serum lactate
dehydrogenase | 18 | 13 | 3 | 10 | 4 | 4 | 3 |
| ECOG
performance status >1 | 13 | 22 | 1 | 7 | 0 | 4 | 3 |
| Ann Arbor
stage |
|
|
|
|
|
|
|
| I +
II | 25 | 34 | 10 | 3 | 6 | 8 | 1 |
| III +
IV | 17 | 4 | 9 | 12 | 6 | 0 | 6 |
| Lugano stage |
|
|
|
|
|
|
|
|
I–IIE | 33 | – | – | – | – | – | – |
| IV | 9 | – | – | – | – | – | – |
| International
prognostic index |
|
|
|
|
|
|
|
|
Low | 12 | 8 | 11 | 0 | 6 | 3 | 2 |
|
Low-intermediate | 8 | 14 | 4 | 4 | 1 | 1 | 2 |
|
High-intermediate | 11 | 5 | 4 | 2 | 5 | 1 | 2 |
|
High | 11 | 11 | 0 | 9 | 0 | 3 | 1 |
| Bone
marrow involvement | 2 | 0 | 2 | 1 | 1 | 0 | 1 |
| Hans
classification |
|
|
|
|
|
|
|
|
GCB | 9 | 3 | 5 | 0 | 2 | 3 | 1 |
|
Non-GCB | 11 | 14 | 6 | 5 | 3 | 2 | 3 |
| Treatment |
|
|
|
|
|
|
|
|
Chemotherapy without
rituximab | 7 | 22 | 7 | 4 | 6 | 0 | 3 |
|
Chemotherapy with
rituximab | 35 | 17 | 12 | 9 | 6 | 8 | 4 |
|
Chemotherapy with surgery | 23 | 5 | 5 | 0 | 8 | 4 | 0 |
| Risk of CNS
relapsea |
|
|
|
|
|
|
|
|
Low | 12 | – | 11 | 0 | 7 | 3 | 2 |
|
Moderate | 18 | – | 8 | 4 | 0 | 2 | 3 |
|
High | 12 | – | 0 | 11 | 5 | 3 | 2 |
| CNS
prophylaxis |
|
|
|
|
|
|
|
|
Intrathecal injection | 19 | – | 1 | 5 | 3 | 4 | 4 |
|
Intravenous injection of
methotrexate | 8 | – | 5 | 5 | 0 | 0 | 1 |
|
Combined prophylaxis | 3 | – | 5 | 2 | 2 | 1 | 0 |
| Median
follow-up time, months | 36 (1–108) | 29 (1–116) | 22 (3–60) | 14 (1–94) | 13 (6–84) | 24 (16–51) | 16 (9–20) |
| Loss to
follow-up | 0 | 7 | 5 | 0 | 1 | 0 | 3 |
 | Table II.Distribution of primary extra-nodal
diffuse large B-cell lymphoma cases. |
Table II.
Distribution of primary extra-nodal
diffuse large B-cell lymphoma cases.
| Extra-nodal
sites | No. of
patients |
|---|
| Gastrointestinal
tract | 42 |
| Stomach | 20 |
| Colon | 8 |
| Ileocecum | 7 |
| Small
intestine | 7 |
| Central nervous
system | 38 |
| Deep brain
tissue | 34 |
| Multiple
lesions | 20 |
| Breast | 19 |
| Unilaterally
involved | 19 |
| Adrenal gland | 15 |
| Bilaterally
involved | 8 |
| Female genital
system | 12 |
| Ovary | 6 |
| Cervix | 3 |
| Uterine body | 3 |
| Both cervix and
vagina | 2 |
| Thyroid gland | 8 |
| Bone | 7 |
| Axial skeleton | 5 |
| Skull | 2 |
| Pelvis and spinal
column | 2 |
| Pelvis | 1 |
| Limbs | 2 |
Response to treatment and
survival
The median OS and PFS times of patients with
PE-DLBCL were 28 months (range, 1–116 months) and 17 months (range,
1–108 months), respectively. The OS and PFS rates differed between
patients with the primary involvement of different sites (Table III). Although the prognosis was
improved for patients with primary thyroid DLBCL (PT-DLBCL),
compared with those primary adrenal gland DLBCL (PA-DLBCL), the
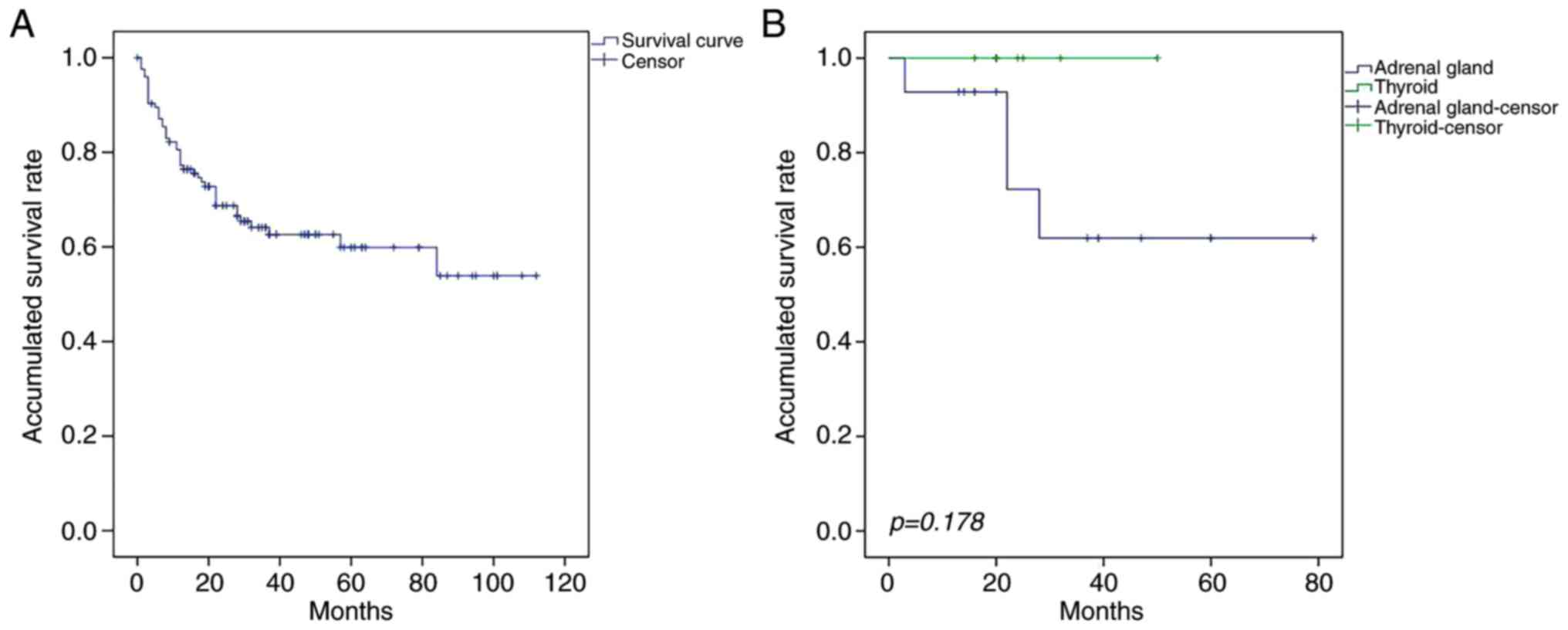
difference was not statistically significant (P=0.178; Fig. 1). The median OS times of patients with
primary GI tract, CNS, adrenal gland, breast, FGS, thyroid and bone
involvement were 36.5 months (range, 1–108), 29 months (range,
1–112), 14 months (range, 2–94), 25 months (range, 3–79), 20 months
(range, 3–60), 22 months (range, 16–50) and 18.5 months (range,
15–37), respectively, and the PFS times were 25.5 months (range,
1–108), 24 months (range, 1–85), 10 months (range, 1–50), 20 months
(range, 3–79), 11 months (range, 3–84), 22 months (range, 1–50) and
12.5 months (range, 9–20), respectively.
 | Table III.Univariate and multivariate analysis
of the survival of patients with primary extra-nodal diffuse large
B-cell lymphoma. |
Table III.
Univariate and multivariate analysis
of the survival of patients with primary extra-nodal diffuse large
B-cell lymphoma.
|
| 3-year overall
survival | 3-year
progression-free survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | Cases, n | OS rate (%) | P-value | HR (95% CI) | P-value | PFS rate (%) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
| 0.899 |
|
|
| 0.853 |
|
|
|
Male | 62 | 62.8 |
|
|
| 64.1 |
|
|
|
|
Female | 63 | 67.3 |
|
|
| 77.2 | 0.634 |
|
|
| Age |
|
| 0.799 |
|
|
|
|
|
|
| ≤60
years | 75 | 61.6 |
|
|
| 64.1 |
|
|
|
| >60
years | 50 | 66.2 |
|
|
| 64.3 |
|
|
|
| LDH |
|
| 0.001 |
|
|
| 0.084 |
|
|
|
Normal | 74 | 76.2 |
|
|
| 68.1 |
|
|
|
|
Elevated | 51 | 45.6 |
|
|
| 57.9 |
|
|
|
| B symptoms |
|
| 0.009 |
|
|
| 0.703 |
|
|
| No | 78 | 78.4 |
|
|
| 69.5 |
|
|
|
|
Yes | 47 | 49.5 |
|
|
| 59.4 |
|
|
|
| ECOG performance
status |
|
| 0.096 |
|
|
| 0.536 |
|
|
| ≤1 | 76 | 72.4 |
|
|
| 65 |
|
|
|
|
>1 | 49 | 56.4 |
|
|
| 63 |
|
|
|
| Ann Arbor
stage |
|
| <0.001 |
|
|
| 0.022 |
|
|
| I +
II | 75 | 85.8 |
|
|
| 75.6 |
|
|
|
| III +
IV | 50 | 54.3 |
|
|
| 60 |
|
|
|
| International
prognostic index |
|
| 0.023 |
|
|
| 0.901 |
|
|
|
0–2 | 66 | 74.1 |
| 1.864
(1.002–3.468) | 0.049 | 66.9 |
|
|
|
|
3–5 | 59 | 58 |
|
|
| 60 |
|
|
|
| Rituximab |
|
| <0.001 | 0.259
(0.138–0.483) | <0.001 |
| <0.001 | 0.297
(0.151–0.584) | <0.001 |
| No | 41 | 42.1 |
|
|
| 44.3 |
|
|
|
|
Yes | 83 | 76.1 |
|
|
| 74.4 |
|
|
|
| CR following
first-line treatment |
|
| <0.001 | 0.134
(0.042–0.430) | 0.001 |
| 0.001 | 0.417
(0.211–0.825) | 0.012 |
| No | 51 | 52.8 |
|
|
| 34.5 |
|
|
|
|
Yes | 73 | 87.7 |
|
|
| 75.9 |
|
|
|
| Pathology |
|
| 0.213 |
|
|
| 0.237 |
|
|
|
Non-GCB | 26 | 67 |
|
|
| 56.9 |
|
|
|
|
GCB | 32 | 95.2 |
|
|
| 78.6 |
|
|
|
| Bone marrow
involvement |
|
| 0.134 |
|
|
| 0.396 |
|
|
| No | 118 | 64.8 |
|
|
| 65.4 |
|
|
|
|
Yes | 7 | 41.7 |
|
|
| 41.7 |
|
|
|
| Primary organ |
|
| 0.442 |
|
|
| 0.451 |
|
|
|
Gastrointestinal tract | 42 | 62.6 |
|
|
| 77.1 |
|
|
|
| Central
nervous system | 31 | 60.4 |
|
|
| 57.4 |
|
|
|
| Adrenal
gland | 15 | 48.1 |
|
|
| 48.4 |
|
|
|
|
Breast | 14 | 61.9 |
|
|
| 50.3 |
|
|
|
| Female
genital system | 11 | 75 |
|
|
| 49.9 |
|
|
|
|
Thyroid | 8 | 100 |
|
|
| 100 |
|
|
|
|
Bone | 4 | 75 |
|
|
| 75 |
|
|
|
A total of 59 patients with PE-DLBCL achieved CR
(47.5%) and 15 achieved PR (12.1%), resulting in a total response
rate (RR) of 59.6%. The RR for patients with PT-DLBCL was 100%,
while that of those with primary bone, breast, FGS, CNS, GI tract,
and adrenal gland involvement was 75, 64.3, 63.6, 58.1, 57.1 and
33.3%, respectively. Patients treated with rituximab exhibited a
better RR than those not treated with rituximab (68.2 vs.
34.1%).
Prognostic factors
The survival rate was not significantly influenced
by age, sex, Eastern Cooperative Oncology Group performance status
(17), primary site or bone marrow
involvement. Univariate analysis demonstrated that elevated serum
LDH, B symptoms, Ann Arbor stage, inclusion of rituximab, CR
following first-line therapy and IPI significantly affected
survival (Fig. 2). In the
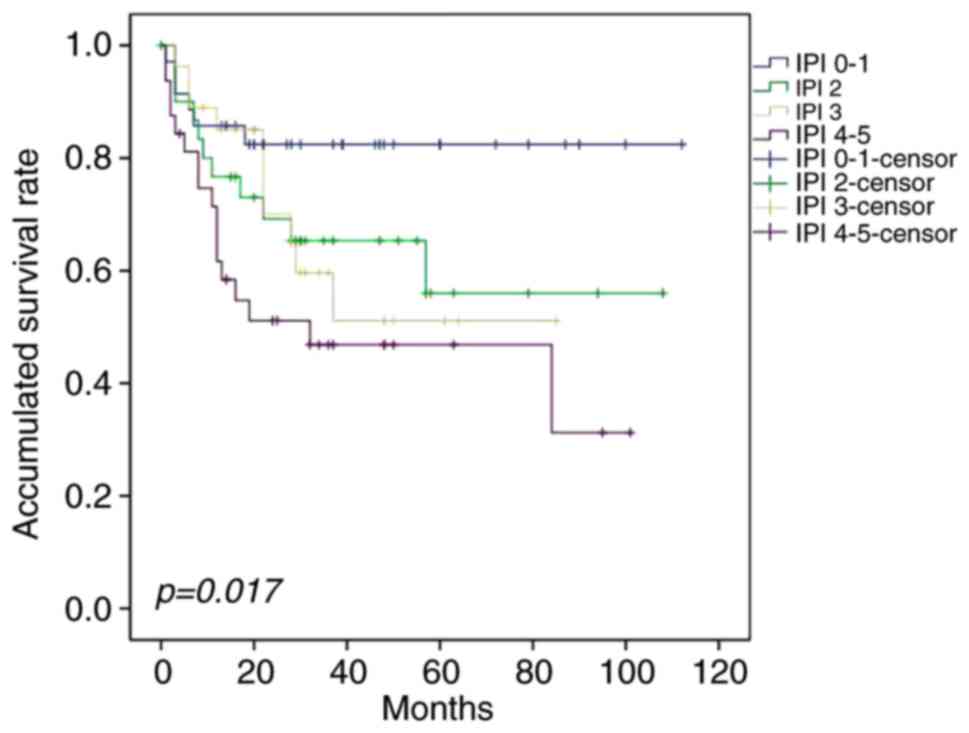
multivariate analysis, IPI ≤2, CR following first-line treatment
and combination with rituximab were independent predictive factors
for OS in patients with PE-DLBCL; the latter two were also
significantly associated with PFS (Table III). Patients could be divided into
four groups with different prognoses on the basis of IPI (Fig. 3).
Of the patients with primary GI DLBCL (PGI-DLBCL),
23 received surgery combined with CT, 20 of which were Lugano stage
I–IIE and 8 (40.0%) of which succumbed to the primary disease
during follow-up. A total of 8 patients with primary FGS-DLBCL
(PFGS-DLBCL) and 5 patients with primary breast DLBCL (PB-DLBCL)
underwent surgery. However, no statistically significant
differences were observed in the 3-year OS and PFS rates between
patients who had and had not undergone surgery (Table IV).
 | Table IV.Survival analysis of patients with
primary extra-nodal diffuse large B-cell lymphoma treated with
chemotherapy combined with surgery. |
Table IV.
Survival analysis of patients with
primary extra-nodal diffuse large B-cell lymphoma treated with
chemotherapy combined with surgery.
|
| 3-year overall
survival rate (%) | 3-year progression
free survival rate (%) |
|---|
|
|
|
|
|---|
| Primary organ | Chemotherapy | Chemotherapy +
surgery | P-value | Chemotherapy | Chemotherapy +
surgery | P-value |
|---|
| Gastrointestinal
tract | 77.9 | 68.4 | 0.622 | 90 | 100 | 0.254 |
| Female genital
system | 66.7 | 87.5 | 0.592 | 66.7 | 50 | 0.955 |
| Breast | 53.3 | 75 | 0.479 | 25 | 75 | 0.257 |
A total of 22 patients with PCNS-DLBCL received CT
alone, and 9 were treated with CT followed by RT. The 3-year OS
rates of the two groups were 57.8 and 66.7% respectively, and the
3-year PFS rates were 56.8 and 62.5% respectively, but no
significant differences were observed between the two groups
(P=0.592 vs. P=0.703).
Analysis of CNS relapse
A higher rate of CNS relapse was observed in
patients with primary FGS (6/11, 54.5%) and adrenal gland (3/15,
20.0%) involvement compared with other sites, and the difference
was statistically significant (Table
V). A significant trend towards a prolonged time to CNS relapse
following intravenous rituximab treatment was observed (P=0.003;
Fig. 4), but neither intravenous
HD-MTX nor intrathecal injection reduced the incidence of CNS
recurrence (P=0.689 and P=0.876, respectively).
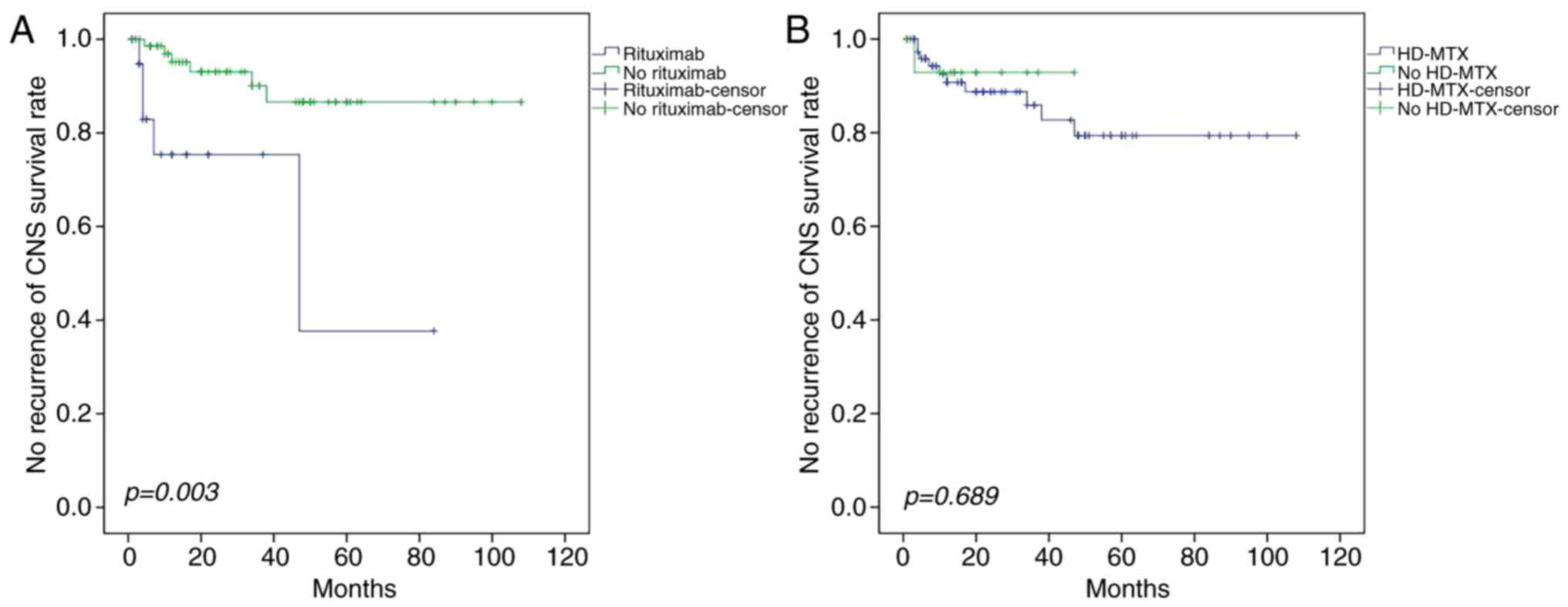 | Figure 4.Effect of rituximab and HD-MTX on
survival rate. (A) Patients receiving the R-CHOP regimen exhibited
a lower incidence of CNS recurrence than those receiving the CHOP
regimen. (B) Intravenous infusion of HD-MTX combined with CHOP
yielded no significant difference in the CNS recurrence rate
compared with those who did not receive HD-MTX. HD-MTX, high-dose
methotrexate; R-CHOP, rituximab, cyclophosphamide, doxorubicin,
vindesine, and prednisone; CNS, central nervous system; CHOP,
cyclophosphamide, doxorubicin, vindesine and prednisone. |
 | Table V.Univariate and multivariate analysis
of risk factors for CNS relapse. |
Table V.
Univariate and multivariate analysis
of risk factors for CNS relapse.
|
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variables | Total cases | CNS relapse, cases
(%) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
| 0.054 | 1.549
(0.255–9.431) | 0.635 |
|
Male | 40 | 2 (4.9) |
|
|
|
|
Female | 54 | 9 (16.7) |
|
|
|
| Age |
|
| 0.980 |
|
|
| ≤60
years | 56 | 7 (12.3) |
|
|
|
| >60
years | 38 | 4 (10.5) |
|
|
|
| Lactate
dehydrogenase |
|
| 0.354 |
|
|
|
Normal | 52 | 5 (9.4) |
|
|
|
|
Elevated | 42 | 6 (14.3) |
|
|
|
| B symptoms |
|
| 0.345 |
|
|
| No | 54 | 8 (14.5) |
|
|
|
|
Yes | 40 | 3 (7.5) |
|
|
|
| ECOG performance
status |
|
| 0.506 |
|
|
|
0–1 | 68 | 9 (13.2) |
|
|
|
|
>1 | 26 | 2 (7.0) |
|
|
|
| International
prognostic index |
|
| 0.202 |
|
|
|
0–2 | 49 | 8 (16.3) |
|
|
|
|
3–5 | 45 | 3 (6.7) |
|
|
|
| Ann Arbor
stage |
|
| 0.321 |
|
|
| I +
II | 53 | 5 (9.4) |
|
|
|
| III +
IV | 41 | 6 (14.6) |
|
|
|
| Treatment
regimen |
|
| 0.003 | 0.160
(0.045–0.569) | 0.005 |
|
CHOP | 24 | 5 (20.8) |
|
|
|
|
R-CHOP | 70 | 6 (8.4) |
|
|
|
| High-dose
methotrexate |
|
| 0.689 |
|
|
| No | 79 | 10 |
|
|
|
|
Yes | 15 | 1 |
|
|
|
| Bone marrow
involvement |
|
| 0.363 |
|
|
| No | 87 | 11 (12.6) |
|
|
|
|
Yes | 7 | 0 (0.0) |
|
|
|
| Risk of CNS
relapse |
|
| 0.174 |
|
|
|
Low | 31 | 6 (19.4) |
|
|
|
|
Mediate | 31 | 1 (3.2) |
|
|
|
|
High | 32 | 4 (12.5) |
|
|
|
| Intrathecal
injection |
|
| 0.876 |
|
|
| No | 63 | 8 (12.7) |
|
|
|
|
Yes | 31 | 3 (9.7) |
|
|
|
| Primary organ |
|
|
|
|
|
| Female
genital system | 11 | 6 (54.5) | <0.001 | 14.839
(2.249–97.896) | 0.005 |
| Adrenal
gland | 15 | 3 (20.0) | 0.101 | 10.452
(1.737–62.908) | 0.010 |
|
Breast | 14 | 1 (7.1) | 0.585 |
|
|
|
Bone | 4 | 1 (25.0) | 0.288 |
|
|
|
Gastrointestinal tract | 42 | 0 (0.0) | 0.001 | 0.000
(0–12.51×10114) | 0.928 |
| Thyroid
gland | 8 | 0 (0.0) | 0.300 |
|
|
Discussion
PE-DLBCL is a heterogeneous disease with various
clinical manifestations and molecular alterations at different
anatomical sites. The majority of patients with primary nodal DLBCL
are classified as clinical stage III–IV (18,19),
whereas only 38.3% of patients in the present study exhibited stage
III–IV disease, a difference that may be the consequence of the
variation in the definition of primary extra-nodal involvement, or
differences in staging criteria. When lymphomas presented at
extra-nodal organs with distant lymph node, spleen or thymus
involvement, they were categorized as primary nodal NHL, leading to
more patients being classified as stage I–II.
For PE-DLBCL patients with localized lesions,
neither surgery nor radiotherapy alone is preferred, and the choice
of treatment strategies should be adjusted according to the
stratification of anatomic location and disease stage. Several
studies have demonstrated improved OS in the groups of patients
with PGI-DLBCL who underwent surgery combined with CT, particularly
in those with early disease stages (20–23).
However, certain centers suggest that patients with stage I–II
PGFS-DLBCL should be treated with systemic CT and localized RT or
surgery to optimize chances of remission (24–26). In
previous studies, mastectomy was reported to be non-beneficial for
patients with PB-DLBCL (27–29) or PT-DLBCL (30–32), and
the International Extra-Nodal Lymphoma Study Group (IELSG) revealed
that radical mastectomy is an adverse factor for cause-specific
survival in multivariate analysis (29). In the present study, mastectomy
combined with CT was not associated with improved outcomes in
patients with primary GI tract, FGS, breast or thyroid involvement.
Surgery was performed on 15 patients with PGI-DLBCL who required
pathological diagnosis, and 8 who were experiencing bleeding,
perforation or other acute complications, delaying the commencement
of CT, with a detrimental outcome. Limited by the small number of
patients with primary breast, FGS or thyroid involvement, and due
to the study's retrospective nature and lack of randomized
comparisons, the present study has not reliably assessed the effect
of surgery on survival.
HD-MTX is considered to be the most effective agent
for treating PCNS-DLBCL (33), and
the addition of high-dose cytarabine (HD-Ara-C) has been
demonstrated to significantly improve the RR and failure-free
survival (34,35). The efficacy of adding of whole brain
RT (WBRT) to HD-MTX-based CT as consolidative therapy is debatable.
Doses of 30–36 Gy to the whole brain are currently used (36–39), but
certain studies do not report a clear benefit of WBRT in prolonging
OS (40–42). In the present study, WBRT with CT
improved the 3-year OS rate, but the difference was not
statistically significant, potentially due to the fact that 5 of
the 9 patients who underwent CT combined with WBRT selected RT as
salvage therapy, and 3 patients began RT prior to achieving CR.
Nonetheless, there is a general agreement that WBRT is associated
with delayed treatment-associated neurotoxicity and that it may
hinder the benefits of disease control (40–42).
Recently, Ibrutinib (43) and
Nivolumab (44) were reported to be
active in relapsed/refractory PCNS lymphoma, as previous studies
have demonstrated that PCNS-DLBCL is characterized by a high
expression of myeloid differentiation primary response 88 (MYD88)
(45–47), and programmed death (PD)-1/PD-ligand 1
is immunohistochemically and genetically detectable (48,49). These
studies outline potential future treatments for PCNS-DLBCL.
In the present study, three main prognostic factors
were identified that influenced survival-rituximab, IPI and
achieving CR following first-line therapy. The wide application of
rituximab, R-CHOP or R-CHOP-like regimens have achieved significant
therapeutic effects in DLBCL (50).
At present, systemic CT is accepted as the cornerstone of PE-DLBCL
treatment, but a general consensus regarding the therapeutic effect
of rituximab in PE-DLBCL has not been reached. A single-center
study retrospectively analyzed 48 PE-NHL patients and confirmed
that combined rituximab therapy did not improve OS (P=0.361),
likely influenced by the selection bias for patients whose primary
sites were adrenal, ovarian or pancreatic; other commonly involved
organs, including the GI tract, were excluded (7). However, other studies have demonstrated
that rituximab may improve the OS and PFS of patients with DLBCL
with primary GI tract, adrenal and breast involvement (51–55). The
present study demonstrated that the addition of rituximab to CT
significantly improved the OS and PFS rates of patients with
PE-DLBCL. However, as it was a non-randomized comparison, future
prospective studies are required to confirm this observation.
IPI and its variants are the main prognostic tools
used in patients with DLBCL and, in the present study, the 3-year
OS rates were 82.1, 61.2, 50.0 and 46.1% for patients in low,
low-intermediate, high-intermediate and high-risk groups,
respectively. The difference was statistically significant, and the
multivariate analysis also indicated that IPI ≥2 was an independent
risk factor for PE-DLBCL. Therefore, IPI was also suitable for
assessing the prognosis of patients with PE-DLBCL (P=0.017).
Recent studies have demonstrated that patients with
DLBCL may be divided into two groups of different prognoses using
the Hans classification; in addition, the germinal center B-cell
(GCB) type is associated with a better prognosis than the non-GCB
type (56,57). Furthermore, it has been reported that
the majority of patients with primary breast, adrenal and CNS DLBCL
are of the non-GCB type (52,58–63). In
the present study, a total 44/67 patients were non-GCB type, and
primary CNS (14/17) and adrenal gland (5/5) accounted for the
majority of these cases. The 3-year OS rate for GCB was not
significantly higher than non-GCB, perhaps because the origin of
the cells or the genetic causes of PE-DLBCL were different from
those of primary intra-nodal DLBCL, as studies have demonstrated
that PCNS-DLBCL is primarily non-GCB, but sequencing suggests that
the cell source of original nodal non-GCB DLBCL and PCNS-DLBCL are
different (64).
There are discrepancies in the prognosis of PE-DLBCL
originating from different sites. Primary thyroid lymphoma (PTL) is
associated with a relatively favorable prognosis (32,65,66), and
in a study considering 108 cases of PTL, there was no mortality in
the follow-up period for patients with stage I disease (65). The reason for this may be that ~90% of
these patients were diagnosed at an early stage (32,65).
However, the prognosis for PA-DLBCL is relatively poor (52,67), and
the event-free survival for elderly patients with PA-DLBCL was only
12–18 months (68,69). In the present study, the 3-year OS
rates for PT-DLBCL and PA-DLBCL were 100 and 48.1%, respectively.
However, since the number of PT-DLBCL cases was small, the
difference was not statistically significant.
A number of centers have reported that involvement
of the breast, renal, adrenal or female reproductive system is
associated with a high risk of CNS recurrence (7,70–75), but the mechanism of this remains
unclear. L265P mutations of MYD88 are common in PCNS-DLBCLs
(38–75%) (45,76,77), and
these mutations were predominantly present in primary testicle and
breast DLBCLs, whereas the mutation rate in PGI-DLBCL was
relatively low (76,78). These observations indicate that the
mutation of MYD88 may be associated with the preferential
dissemination to CNS. In the present study, PFGS-DLBCL and PA-DLBCL
were risk factors, as determined by multivariate analysis; our
previous study demonstrated that patients with PFGS-DLBCL have a
high frequency of MYD88 mutations (61). However, we have not yet sequenced the
specimens from patients with PA-DLBCL, and the reason patients with
PA-DLBCLs are more likely to experience CNS relapse remains
unclear. It is possible that PE-DLBCL involves organs with
preferential dissemination to the CNS, representing a distinct
cohort of DLBCL driven by equivalent oncogenic mutations. However,
a large cohort study is required to confirm this hypothesis, and
further molecular analysis may elucidate the specific nature of
extra-nodal DLBCLs with preferential dissemination to CNS.
Patients with the recurrence of NHL in the CNS
exhibit a poor prognosis, and solving this problem is urgent.
Intrathecal MTX is used to prevent CNS relapse, but the present
study revealed that it did not provide sufficient prevention
(P=0.876), consistent with previous reports (66,79). The
reason for this may be that CNS recurrence is more frequent in the
parenchyma than the meninges and thus, the typical intrathecal
injection is less effective. A multicenter retrospective analysis
revealed that the continuous intravenous infusion of HD-MTX (1–3
g/m2) over 24 h may reduce the likelihood of CNS
recurrence (80). In the present
study, the CNS RFS for patients who received HD-MTX (1
g/m2) was not significantly higher than that in
untreated patients. This may be because there were too few cases
for the data to reach significance, or because the concentration of
MTX in the cerebrospinal fluid (CSF) may not have reached 0.5
mmol/l, which is the concentration required to kill tumor cells
(81). Therefore, the detection of
the concentration of MTX in the CSF requires further
investigation.
The addition of rituximab has improved the outcomes
in DLBCL, but there is no general consensus regarding the impact of
rituximab in preventing CNS recurrence. Certain studies have
indicated that rituximab may reduce CNS recurrence (82–85), while
others have suggested that adding rituximab did not lower the
incidence of CNS recurrence (86,87). The
present study demonstrated that CT combined with rituximab may
effectively prevent CNS recurrence. Considering that only 1% of the
rituximab dose can cross the blood-brain barrier, the present study
revealed that it significantly improved the OS and PFS of patients
with PE-DLBCL, indicating that rituximab may lower the risk of
recurrent CNS by reducing the tumor burden.
In summary, the overall prognosis of patients with
PE-DLBCL was analyzed, and it was revealed that CT, whether
combined with surgery or RT, did not improve the prognosis of
patients. Therefore, it is advisable that surgery is used for
diagnosing and treating acute complications. However, the
implications of the present study are limited by the number of
patients; a study with a larger cohort is required. Preventing CNS
relapse is urgent; and patients with primary FGS and adrenal gland
involvement were identified as exhibiting an increased risk.
Treatment with rituximab was demonstrated to be effective for CNS
relapse prevention. Additionally, the intravenous infusion of
HD-MTX lowered the rate of CNS relapse, although the effect was not
statistically significant. Therefore, future studies with larger
sample sizes are required to fully elucidate the efficacy of
rituximab.
Acknowledgements
Not applicable.
Funding
CAMS Innovation Fund for Medical Sciences (CIFMS)
2016–12M-1-001 Capital Foundation of Medical Developments (CFMD)
016-2-4016 Pumch Science Fund for Junior Faculty 2016–1.19. This
funding provided the help of data analysis.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
HS analyzed and interpreted the patient data and was
a major contributor in writing the manuscript. DZ and ZW conceived
and designed the work that led to the submission, acquired data and
had an important role in interpreting the results. YZ, XH, WW, LZ,
CY and JF participated in the diagnosis and treatment of patients
and provided the clinical data, they also contributed significantly
to acquisition and analysis of data, drafting the manuscript and
revising it critically. JF gave final approval of the version to be
published. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures followed were in accordance with the
ethical standards of the responsible committee on human
experimentation (institutional and national) and with the Helsinki
Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all
patients for being included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Economopoulos T, Asprou N, Stathakis N,
Papageorgiou E, Dervenoulas J, Xanthaki K and Raptis S: Primary
extranodal non-Hodgkin's lymphoma in adults: Clinicopathological
and survival characteristics. Leuk Lymphoma. 21:131–136. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vitolo U, Seymour JF, Martelli M,
Illerhaus G, Illidge T, Zucca E, Campo E, Ladetto M and
ESMOGuidelines Committee: Extranodal diffuse large B-cell lymphoma
(DLBCL) and primary mediastinal B-cell lymphoma: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 27(Suppl 5): v91–v102. 2016.PubMed/NCBI
|
|
3
|
Rudders RA, Ross ME and DeLellis RA:
Primary extranodal lymphoma: Response to treatment and factors
influencing prognosis. Cancer. 42:406–416. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paryani S, Hoppe RT, Burke JS, Sneed P,
Dawley D, Cox RS, Rosenberg SA and Kaplan HS: Extralymphatic
involvement in diffuse non-Hodgkin's lymphoma. J Clin Oncol.
1:682–688. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'amore F, Christensen BE, Brincker H,
Pedersen NT, Thorling K, Hastrup J, Pedersen M, Jensen MK, Johansen
P and Andersen E: Clinicopathological features and prognostic
factors in extranodal non-hodgkin lymphomas. Danish LYFO study
group. Eur J Cancer. 27:1201–1208. 1991. View Article : Google Scholar
|
|
6
|
AlShemmari SH, Ameen RM and Sajnani KP:
Extranodal lymphoma: A comparative study. Hematology. 13:163–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yun J, Kim SJ, Won JH, Choi CW, Eom HS,
Kim JS, Kim MK, Kwak JY, Kim WS and Suh C: Clinical features and
prognostic relevance of ovarian involvement in non-Hodgkin's
lymphoma: A Consortium for Improving Survival of Lymphoma (CISL)
report. Leuk Res. 34:1175–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freeman C, Berg JW and Cutler SJ:
Occurrence and prognosis of extranodal lymphomas. Cancer.
29:252–260. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reddy S, Pellettiere E, Saxena V and
Hendrickson FR: Extranodal non-Hodgkin's lymphoma. Cancer.
46:1925–1931. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Chen B and Xu X: Impact of
rituximab on incidence of and risk factors for central nervous
system relapse in patients with diffuse large B-cell lymphoma: A
systematic review and meta-analysis. Leuk Lymphoma. 55:509–514.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaffe ES: The 2008 WHO classification of
lymphomas: Implications for clinical practice and translational
research. Hematology Am Soc Hematol Educ Program. 523–531:2009.
|
|
12
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the committee on hodgkin's
disease staging classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
13
|
International Non-Hodgkin's Lymphoma
Prognostic Factors Project: A predictive model for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 329:987–994. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E and Lister TA: Recommendations for
initial evaluation, staging, and response assessment of Hodgkin and
non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol.
32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zelenetz AD, Gordon LI, Wierda WG,
Abramson JS, Advani RH, Andreadis CB, Bartlett N, Bellam N, Byrd
JC, Czuczman MS, et al: Non-Hodgkin's lymphomas, version 2.2014. J
Natl Compr Canc Netw. 12:916–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cheson BD, Pfistner B, Juweid ME, Gascoyne
RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca
E, et al: Revised response criteria for malignant lymphoma. J Clin
Oncol. 25:579–586. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
A clinical evaluation of the international
lymphoma study group classification of non-hodgkin's lymphoma: The
non-hodgkin's lymphoma classification project. Blood. 89:3909–3918.
1997.PubMed/NCBI
|
|
19
|
Armitage JO and Weisenburger DD: New
approach to classifying non-Hodgkin's lymphomas: Clinical features
of the major histologic subtypes. Non-hodgkin's lymphoma
classification project. J Clin Oncol. 16:2780–2795. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khosla D, Kumar R, Kapoor R, Kumar N, Bera
A and Sharma SC: A retrospective analysis of clinicopathological
characteristics, treatment, and outcome of 27 patients of primary
intestinal lymphomas. J Gastrointest Cancer. 44:417–421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gou H, Zang J, Jiang M, Yang Y, Cao D and
Chen X: Clinical prognostic analysis of 116 patients with primary
intestinal non-hodgkin lymphoma. Med Oncol. 29:227–234. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SJ, Choi CW, Mun Y, Oh SY, Kang HJ,
Lee SI, Won JH, Kim MK, Kwon JH, Kim JS, et al: Multicenter
retrospective analysis of 581 patients with primary intestinal
non-hodgkin lymphoma from the Consortium for Improving Survival of
Lymphoma (CISL). BMC Cancer. 11:3212011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SJ, Kang HJ, Kim JS, Oh SY, Choi CW,
Lee SI, Won JH, Kim MK, Kwon JH, Mun Y, et al: Comparison of
treatment strategies for patients with intestinal diffuse large
B-cell lymphoma: Surgical resection followed by chemotherapy versus
chemotherapy alone. Blood. 117:1958–1965. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Frey NV, Svoboda J, Andreadis C, Tsai DE,
Schuster SJ, Elstrom R, Rubin SC and Nasta SD: Primary lymphomas of
the cervix and uterus: The University of Pennsylvania's experience
and a review of the literature. Leuk Lymphoma. 47:1894–1901. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller TP, Dahlberg S, Cassady JR,
Adelstein DJ, Spier CM, Grogan TM, LeBlanc M, Carlin S, Chase E and
Fisher RI: Chemotherapy alone compared with chemotherapy plus
radiotherapy for localized intermediate- and high-grade
non-Hodgkin's lymphoma. N Engl J Med. 339:21–26. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng H, Tang X, Cheng J, Zhang B, Zhang
YL, Wang WQ and Teng P: Pathologic character and diagnosis of
female primary genital system diffuse large B cell lymphoma. Eur
Rev Med Pharmacol Sci. 21:1471–1476. 2017.PubMed/NCBI
|
|
27
|
Aviv A, Tadmor T and Polliack A: Primary
diffuse large B-cell lymphoma of the breast: Looking at
pathogenesis, clinical issues and therapeutic options. Ann Oncol.
24:2236–2244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jennings WC, Baker RS, Murray SS, Howard
CA, Parker DE, Peabody LF, Vice HM, Sheehan WW and Broughan TA:
Primary breast lymphoma: The role of mastectomy and the importance
of lymph node status. Ann Surg. 245:784–789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ryan G, Martinelli G, Kuper-Hommel M,
Tsang R, Pruneri G, Yuen K, Roos D, Lennard A, Devizzi L, Crabb S,
et al: Primary diffuse large b-cell lymphoma of the breast:
Prognostic factors and outcomes of a study by the international
extranodal lymphoma study group. Ann Oncol. 19:233–241. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ha CS, Shadle KM, Medeiros LJ, Wilder RB,
Hess MA, Cabanillas F and Cox JD: Localized non-Hodgkin lymphoma
involving the thyroid gland. Cancer. 91:629–635. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pyke CM, Grant CS, Habermann TM, Kurtin
PJ, van Heerden JA, Bergstralh EJ, Kunselman A and Hay ID:
Non-Hodgkin's lymphoma of the thyroid: Is more than biopsy
necessary? World J Surg. 16:604–609; discussion 609-10. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meyer-Rochow GY, Sywak MS, Reeve TS,
Delbridge LW and Sidhu SB: Surgical trends in the management of
thyroid lymphoma. Eur J Surg Oncol. 34:576–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Abrey LE, Ben-Porat L, Panageas KS,
Yahalom J, Berkey B, Curran W, Schultz C, Leibel S, Nelson D, Mehta
M and DeAngelis LM: Primary central nervous system lymphoma: The
memorial sloan-kettering cancer center prognostic model. J Clin
Oncol. 24:5711–5715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ferreri AJ, Reni M, Foppoli M, Martelli M,
Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G,
Ilariucci F, et al: High-dose cytarabine plus high-dose
methotrexate versus high-dose methotrexate alone in patients with
primary CNS lymphoma: A randomised phase 2 trial. Lancet.
374:1512–1520. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ferreri AJ, Cwynarski K, Pulczynski E,
Ponzoni M, Deckert M, Politi LS, Torri V, Fox CP, Rosee PL, Schorb
E, et al: Chemoimmunotherapy with methotrexate, cytarabine,
thiotepa, and rituximab (matrix regimen) in patients with primary
cns lymphoma: Results of the first randomisation of the
international extranodal lymphoma study group-32 (ielsg32) phase 2
trial. Lancet Haematol. 3:e217–e227. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hoang-Xuan K, Bessell E, Bromberg J,
Hottinger AF, Preusser M, Ruda R, Schlegel U, Siegal T, Soussain C,
Abacioglu U, et al: Diagnosis and treatment of primary CNS lymphoma
in immunocompetent patients: Guidelines from the european
association for neuro-oncology. Lancet Oncol. 16:e322–e332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morris PG, Correa DD, Yahalom J, Raizer
JJ, Schiff D, Grant B, Grimm S, Lai RK, Reiner AS, Panageas K, et
al: Rituximab, methotrexate, procarbazine, and vincristine followed
by consolidation reduced-dose whole-brain radiotherapy and
cytarabine in newly diagnosed primary CNS lymphoma: Final results
and long-term outcome. J Clin Oncol. 31:3971–3979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pels H and Schlegel U: Primary central
nervous system lymphoma. Curr Treat Options Neurol. 8:346–357.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shah GD, Yahalom J, Correa DD, Lai RK,
Raizer JJ, Schiff D, LaRocca R, Grant B, DeAngelis LM and Abrey LE:
Combined immunochemotherapy with reduced whole-brain radiotherapy
for newly diagnosed primary CNS lymphoma. J Clin Oncol.
25:4730–4735. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thiel E, Korfel A, Martus P, Kanz L,
Griesinger F, Rauch M, Roth A, Hertenstein B, von Toll T,
Hundsberger T, et al: High-dose methotrexate with or without whole
brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase
3, randomised, non-inferiority trial. Lancet Oncol. 11:1036–1047.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Herrlinger U, Schafer N, Fimmers R,
Griesinger F, Rauch M, Kirchen H, Roth P, Glas M, Bamberg M, Martus
P, et al: Early whole brain radiotherapy in primary CNS lymphoma:
Negative impact on quality of life in the randomized G-PCNSL-SG1
trial. J Cancer Res Clin Oncol. 143:1815–1821. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
DeAngelis LM, Seiferheld W, Schold SC,
Fisher B and Schultz CJ; Radiation Therapy Oncology Group Study
93–10: Combination chemotherapy and radiotherapy for primary
central nervous system lymphoma: Radiation therapy oncology group
study 93-10. J Clin Oncol. 20:4643–4648. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Grommes C, Pastore A, Palaskas N, Tang SS,
Campos C, Schartz D, Codega P, Nichol D, Clark O, Hsieh W, et al:
Ibrutinib unmasks critical role of bruton tyrosine kinase in
primary cns lymphoma. Cancer Discov. 7:1018–1029. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nayak L, Iwamoto FM, LaCasce A, Mukundan
S, Roemer MGM, Chapuy B, Armand P, Rodig SJ and Shipp MA: PD-1
blockade with nivolumab in relapsed/refractory primary central
nervous system and testicular lymphoma. Blood. 129:3071–3073. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Montesinos-Rongen M, Godlewska E, Brunn A,
Wiestler OD, Siebert R and Deckert M: Activating L265P mutations of
the MYD88 gene are common in primary central nervous system
lymphoma. Acta Neuropathol. 122:791–792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim Y, Ju H, Kim DH, Yoo HY, Kim SJ, Kim
WS and Ko YH: CD79B and MYD88 mutations in diffuse large B-cell
lymphoma. Hum Pathol. 45:556–564. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng M, Perry AM, Bierman P, Loberiza F
Jr, Nasr MR, Szwajcer D, Del Bigio MR, Smith LM, Zhang W and
Greiner TC: Frequency of MYD88 and CD79B mutations, and MGMT
methylation in primary central nervous system diffuse large B-cell
lymphoma. Neuropathology. 37:509–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Berghoff AS, Ricken G, Widhalm G, Rajky O,
Hainfellner JA, Birner P, Raderer M and Preusser M: PD1 (CD279) and
PD-L1 (CD274, B7H1) expression in primary central nervous system
lymphomas (PCNSL). Clin Neuropathol. 33:42–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chapuy B, Roemer MG, Stewart C, Tan Y, Abo
RP, Zhang L, Dunford AJ, Meredith DM, Thorner AR, Jordanova ES, et
al: Targetable genetic features of primary testicular and primary
central nervous system lymphomas. Blood. 127:869–881. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pfreundschuh M, Kuhnt E, Trumper L,
Osterborg A, Trneny M, Shepherd L, Gill DS, Walewski J, Pettengell
R, Jaeger U, et al: CHOP-like chemotherapy with or without
rituximab in young patients with good-prognosis diffuse
large-B-cell lymphoma: 6-year results of an open-label randomised
study of the mabthera international trial (MInT) group. Lancet
Oncol. 12:1013–1022. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Shen W, Cao J, Wang J, Chen F, Wang
C, Zou S, Shen B, Zhao R, Li J and Shen Z: Treatment of
gastrointestinal diffuse large B cell lymphoma in China: A 10-year
retrospective study of 114 cases. Ann Hematol. 91:1721–1729. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim YR, Kim JS, Min YH, Hyunyoon D, Shin
HJ, Mun YC, Park Y, Do YR, Jeong SH, Park JS, et al: Prognostic
factors in primary diffuse large B-cell lymphoma of adrenal gland
treated with rituximab-CHOP chemotherapy from the consortium for
improving survival of lymphoma (CISL). J Hematol Oncol. 5:492012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aviles A, Neri N and Nambo MJ: The role of
genotype in 104 cases of diffuse large B-cell lymphoma primary of
breast. Am J Clin Oncol. 35:126–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hu S, Song Y, Li Y, Sun X, Su L, Zhang W,
Jia J, Bai O, Liang R, Li X, et al: Primary breast diffuse large B
cell lymphoma in the rituximab era: Outcomes of a multicenter
retrospective study by the lymphoma and leukemia committee of
chinese geriatric oncology society(LLC-CGOS). Blood.
128:42282016.
|
|
55
|
Sun Y, Joks M, Xu LM, Chen XL, Qian D, You
JQ and Yuan ZY: Diffuse large B-cell lymphoma of the breast:
Prognostic factors and treatment outcomes. Onco Ther. 9:2069–2080.
2016. View Article : Google Scholar
|
|
56
|
Nyman H, Adde M, Karjalainen-Lindsberg ML,
Taskinen M, Berglund M, Amini RM, Blomqvist C, Enblad G and Leppa
S: Prognostic impact of immunohistochemically defined germinal
center phenotype in diffuse large B-cell lymphoma patients treated
with immunochemotherapy. Blood. 109:4930–4935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choi WW, Weisenburger DD, Greiner TC,
Piris MA, Banham AH, Delabie J, Braziel RM, Geng H, Iqbal J, Lenz
G, et al: A new immunostain algorithm classifies diffuse large
B-cell lymphoma into molecular subtypes with high accuracy. Clin
Cancer Res. 15:5494–5502. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Horiguchi K, Hashimoto K, Hashizume M,
Masuo T, Suto M, Okajo J, Handa H, Kaneko Y, Yokoo H, Sasaki A, et
al: Primary bilateral adrenal diffuse large B-cell lymphoma
demonstrating adrenal failure. Intern Med. 49:2241–2246. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hosein PJ, Maragulia JC, Salzberg MP,
Press OW, Habermann TM, Vose JM, Bast M, Advani RH, Tibshirani R,
Evens AM, et al: A multicentre study of primary breast diffuse
large B-cell lymphoma in the rituximab era. Br J Haematol.
165:358–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Taniguchi K, Takata K, Chuang SS,
Miyata-Takata T, Sato Y, Satou A, Hashimoto Y, Tamura M, Nagakita
K, Ohnishi N, et al: Frequent MYD88 L265P and CD79B mutations in
primary breast diffuse large B-cell lymphoma. Am J Surg Pathol.
40:324–334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Cao XX, Li J, Cai H, Zhang W, Duan MH and
Zhou DB: Patients with primary breast and primary female genital
tract diffuse large B cell lymphoma have a high frequency of MYD88
and CD79B mutations. Ann Hematol. 96:1867–1871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lin CH, Kuo KT, Chuang SS, Kuo SH, Chang
JH, Chang KC, Hsu HC, Tien HF and Cheng AL: Comparison of the
expression and prognostic significance of differentiation markers
between diffuse large B-cell lymphoma of central nervous system
origin and peripheral nodal origin. Clin Cancer Res. 12:1152–1156.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Braaten KM, Betensky RA, de Leval L, Okada
Y, Hochberg FH, Louis DN, Harris NL and Batchelor TT: BCL-6
expression predicts improved survival in patients with primary
central nervous system lymphoma. Clin Cancer Res. 9:1063–1069.
2003.PubMed/NCBI
|
|
64
|
Fukumura K, Kawazu M, Kojima S, Ueno T,
Sai E, Soda M, Ueda H, Yasuda T, Yamaguchi H, Lee J, et al: Genomic
characterization of primary central nervous system lymphoma. Acta
Neuropathol. 131:865–875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Derringer GA, Thompson LD, Frommelt RA,
Bijwaard KE, Heffess CS and Abbondanzo SL: Malignant lymphoma of
the thyroid gland: A clinicopathologic study of 108 cases. Am J
Surg Pathol. 24:623–639. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shimazu Y, Notohara K and Ueda Y: Diffuse
large B-cell lymphoma with central nervous system relapse:
Prognosis and risk factors according to retrospective analysis from
a single-center experience. Int J Hematol. 89:577–583. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yamamoto E, Ozaki N, Nakagawa M and Kimoto
M: Primary bilateral adrenal lymphoma associated with idiopathic
thrombocytopenic purpura. Leuk Lymphoma. 35:403–408. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sonneveld P, de Ridder M, van der Lelie H,
Nieuwenhuis K, Schouten H, Mulder A, van Reijswoud I, Hop W and
Lowenberg B: Comparison of doxorubicin and mitoxantrone in the
treatment of elderly patients with advanced diffuse non-Hodgkin's
lymphoma using CHOP versus CNOP chemotherapy. J Clin Oncol.
13:2530–2539. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bastion Y, Blay JY, Divine M, Brice P,
Bordessoule D, Sebban C, Blanc M, Tilly H, Lederlin P, Deconinck E,
et al: Elderly patients with aggressive non-Hodgkin's lymphoma:
Disease presentation, response to treatment, and survival-a Groupe
d'Etude des Lymphomes de l'Adulte study on 453 patients older than
69 years. J Clin Oncol. 15:2945–2953. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hayase E, Kurosawa M, Yonezumi M and
Suzuki S: Early relapse in the central nervous system-after
achieving complete response in primary vaginal lymphoma. Rinsho
Ketsueki. 53:229–234. 2012.(In Japanese). PubMed/NCBI
|
|
71
|
Yildirim Y: Primary ovarian large B-cell
lymphoma in patient with juvenile rheumatoid arthritis treated with
low dose Methotrexate. Gynecol Oncol. 97:249–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cohn DE, Resnick KE, Eaton LA, deHart J
and Zanagnolo V: Non-Hodgkin's lymphoma mimicking gynecological
malignancies of the vagina and cervix: A report of four cases. Int
J Gynecol Cancer. 17:274–279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hollender A, Kvaloy S, Nome O, Skovlund E,
Lote K and Holte H: Central nervous system involvement following
diagnosis of non-Hodgkin's lymphoma: A risk model. Ann Oncol.
13:1099–1107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
El-Galaly TC, Cheah CY, Hutchings M,
Mikhaeel NG, Savage KJ, Sehn LH, Barrington S, Hansen JW, Poulsen
MØ, Smith D, et al: Uterine, but not ovarian, female reproductive
organ involvement at presentation by diffuse large B-cell lymphoma
is associated with poor outcomes and a high frequency of secondary
CNS involvement. Br J Haematol. 175:876–883. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Primary adrenal lymphoma with secondary
central nervous system involvement: A case report and review of the
literature. Turk J Haematol. 30:405–408. 2013.PubMed/NCBI
|
|
76
|
Kraan W, Horlings HM, van Keimpema M,
Schilder-Tol EJ, Oud ME, Scheepstra C, Kluin PM, Kersten MJ,
Spaargaren M and Pals ST: High prevalence of oncogenic MYD88 and
CD79B mutations in diffuse large B-cell lymphomas presenting at
immune-privileged sites. Blood Cancer J. 3:e1392013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gonzalez-Aguilar A, Idbaih A, Boisselier
B, Habbita N, Rossetto M, Laurenge A, Bruno A, Jouvet A, Polivka M,
Adam C, et al: Recurrent mutations of MYD88 and TBL1XR1 in primary
central nervous system lymphomas. Clin Cancer Res. 18:5203–5211.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nagakita K, Takata K, Taniguchi K,
Miyata-Takata T, Sato Y, Tari A, Ohnishi N, Noujima-Harada M, Omote
S, Nakamura N, et al: Clinicopathological features of 49 primary
gastrointestinal diffuse large B-cell lymphoma cases; comparison
with location, cell-of-origin, and frequency of MYD88 L265P. Pathol
Int. 66:444–452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chua SL, Seymour JF, Streater J, Wolf MM,
Januszewicz EH and Prince HM: Intrathecal chemotherapy alone is
inadequate central nervous system prophylaxis in patients with
intermediate-grade non-Hodgkin's lymphoma. Leuk Lymphoma.
43:1783–1788. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Cheah CY, Herbert KE, O'Rourke K, Kennedy
GA, George A, Fedele PL, Gilbertson M, Tan SY, Ritchie DS, Opat SS,
et al: A multicentre retrospective comparison of central nervous
system prophylaxis strategies among patients with high-risk diffuse
large B-cell lymphoma. Br J Cancer. 111:1072–1079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Vassal G, Valteau D, Bonnay M, Patte C,
Aubier F and Lemerle J: Cerebrospinal fluid and plasma methotrexate
levels following high-dose regimen given as a 3-hour intravenous
infusion in children with nonHodgkin's lymphoma. Pediatr Hemat
Oncol. 7:71–77. 1990. View Article : Google Scholar
|
|
82
|
Arkenau HT, Chong G, Cunningham D, Watkins
D, Agarwal R, Sirohi B, Trumper M, Norman A, Wotherspoon A and
Horwich A: The role of intrathecal chemotherapy prophylaxis in
patients with diffuse large B-cell lymphoma. Ann Oncol. 18:541–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Boehme V, Schmitz N, Zeynalova S, Loeffler
M and Pfreundschuh M: CNS events in elderly patients with
aggressive lymphoma treated with modern chemotherapy (CHOP-14) with
or without rituximab: An analysis of patients treated in the
RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma
Study Group (DSHNHL). Blood. 113:3896–38902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cai QQ, Hu LY, Geng QR, Chen J, Lu ZH, Rao
HL, Liu Q, Jiang WQ, Huang HQ, Lin TY and Xia ZJ: New risk factors
and new tendency for central nervous system relapse in patients
with diffuse large B-cell lymphoma: A retrospective study. Chin J
Cancer. 35:872016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ichikawa S, Fukuhara N, Inoue A,
Katsushima H, Ohba R, Katsuoka Y, Onishi Y, Yamamoto J, Sasaki O,
Nomura J, et al: Clinicopathological analysis of primary adrenal
diffuse large B-cell lymphoma: Effectiveness of
rituximab-containing chemotherapy including central nervous system
prophylaxis. Exp Hematol Oncol. 2:192013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kridel R, Telio D, Villa D, Sehn LH,
Gerrie AS, Shenkier T, Klasa R, Slack GW, Tan K, Gascoyne RD, et
al: Diffuse large B-cell lymphoma with testicular involvement:
Outcome and risk of CNS relapse in the rituximab era. Br J
Haematol. 176:210–221. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Feugier P, Virion JM, Tilly H, Haioun C,
Marit G, Macro M, Bordessoule D, Recher C, Blanc M, Molina T, et
al: Incidence and risk factors for central nervous system
occurrence in elderly patients with diffuse large-B-cell lymphoma:
Influence of rituximab. Ann Oncol. 15:129–133. 2004. View Article : Google Scholar : PubMed/NCBI
|