Introduction
Ovarian cancer is categorized according to histology
as serous, mucinous, endometrioid, clear cell, undifferentiated or
unclassified carcinoma, or as a malignant Brenner tumor (1). Due to the lack of specific symptoms and
effective detection methods, the majority of cases of ovarian
cancer are detected at advanced stages (2). Surgery and chemotherapy using paclitaxel
and platinum are representative treatments of ovarian cancer. In
the early stages of disease, the aforementioned treatment
rehabilitates 80–90% of patients (2,3), and
10–15% of patients recover completely. However, >70% of patients
relapse and succumb within 5-years (2). In the later stages of disease, ovarian
cancer spreads and attaches to the abdominal cavity, which is a
common metastasis site for progressive ovarian cancer (4,5). At the
site of metastasis, ovarian cancer metastasizes to ascites as
single cells or multicellular aggregates, which form spheroids
(6,7).
The spheroids also exhibit chemoresistance to paclitaxel and
platinum, which is implicated as a major contributory factor in
relapse (8,9). Thus, molecular studies using ovarian
spheroids provide a robust model to elucidate the underlying
mechanisms of chemoresistance and relapse following treatment in
ovarian cancer. In previous studies, these spheroids have been
mimicked by organotypic culture, 3-dimensional (3D) sphere culture
systems, for understanding the mechanisms of spheroid development
(10–12).
MicroRNAs (miRNAs/miRs) are small non-coding RNAs of
19–25 nucleotides in length that modulate gene silencing at the
post-transcriptional level by interfering with translation and
accelerating the degradation of specific target transcripts
(13). These miRNAs are synthesized
as pri-miRNA, which is sequentially cleaved to mature miRNA by
RNase III enzymes, such as Drosha and Dicer. The mature miRNAs
assemble into the RNA-induced silencing complex (RISC) (14). The RISC-mature miRNA complex is bound
with the 3′-untranslated region (UTR) of specific target
transcripts at the seed regions, which are located in the
5′-teminal of miRNA and complementarily matched with 3′UTR of
target genes (15). Thus, the in
silico miRNA target is predicted by the sequence of the seed
site (16,17). The miRNA-mediated translational
interference is implicated in various physiological phenomena
(18,19). Notably, deregulation of miRNA
expression is a major factor in the initiation, progression,
metastasis and chemoresistance of a wide spectrum of different
types of cancer (20,21). For example, the let-7 family, commonly
referred to as tumor suppressors, is downregulated in head, neck,
lung, breast, ovarian and prostate cancer (22). Notably, in chemoresistant cancer, the
miRNA expression pattern is definitively altered (20,22). Thus,
miRNAs are implicated as signature genetic biomarkers in
chemoresistance of various types of cancer. In the present study,
the alteration of miRNA expression in 3D sphere-cultured SK-OV3ip1
cells was examined, in addition to the investigating the
association between the miRNA expression profile and characteristic
features of 3D sphere-cultured SK-OV3ip1 cells.
Materials and methods
Cell culture
SKOV3ip1 cells were obtained from Professor A.K.
Sood, University of Texas MD Anderson Cancer Center, (Texas, USA).
Cells were cultured in RPMI 1640 medium (Corning Incorporated,
Corning, NY, USA) supplemented with 10% fetal bovine serum (FBS,
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
IU/ml penicillin and 100 µg/ml streptomycin (23). Cultures were incubated at 37°C in a
humidified atmosphere of 5% CO2. In addition, 3D
sphere-cultured SKOV3ip1 cells were cultured in ultra-low
attachment 6-well plates (Corning Incorporated), in the same
culture conditions.
Cell viability assay
Cell viability was determined by crystal violet
assay. To determine cell viability in conventional 2-dimensional
(2D) monolayer cultures and a 3D sphere culture model, SKOV3ip1
cells were seeded at a density of 5×104 cells/well in
conventional 24-well plates and incubated with the indicated
concentration (0, 6.25, 12.5, 25, 50 and 100 nM) of paclitaxel at
37°C. At 72 h post-paclitaxel treatment, 3D sphere
cultured-SKOV3ip1 cells were transferred to conventional 24-well
plates and incubated for 12 h at 37°C. Attached viable SKOV3ip1
cells were stained with 0.2% crystal violet solution for 5 min at
37°C. For colorimetric analysis, crystal violet dye was extracted
using 1% SDS/PBS and the absorbance was determined at 570 nm using
an EMax PLUS microplate reader (Molecular Devices, LLC, Sunnyvale,
CA, USA).
miRNA microarray
Total RNA was extracted from each sample (2D
monolayer cultures and a 3D sphere cultured SKOV3ip1) using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Total RNAs were labeled with Cy3 using
pCp (Agilent Technologies, Inc., Santa Clara, CA, USA) and an
Agilent miRNA labeling kit (Agilent Technologies, Inc.). Salt and
debris were removed using Micro Bio-Spin P-6 columns (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Purified labeled total RNAs
were loaded into SurePrint G3 Human V16 miRNA 8×60 K array (Agilent
Technologies, Inc.), with 1,205 annotated miRNA sequences, and
hybridized at 65°C for 20 h. After hybridization, the microarray
was washed with washing solution (Gene Expression Wash Buffer pack;
Agilent Technologies, Inc.) and dried. The microarray was scanned
using Agilent Microarray Scanner (Agilent Technologies, Inc.), and
digitalized by Agilent Feature Extraction Software (Agilent
Technologies, Inc.). Gene expression fold-change was analyzed by
comparing between digitalized miRNA profile data using GeneSpring
GX version 11.5 (Agilent Technologies, Inc.).
In silico analysis of putative target
genes
Target genes of 3D sphere culture-specific miRNAs
were identified by three online bioinformatics database, namely,
PITA (http://genie.weizmann.ac.il),
microRNAorg (http://www.microrna.org) and
TargetScan (http://www.targetscan.org).
Kyoto Encyclopedia of Genes and
Genomes (KEGG) analysis of putative targets
KEGG analysis of putative target genes was
determined using the Database for Annotation, Visualization and
Integrated Discovery Bioinformatics Resource 6.7 (DAVID, http://david.abcc.ncifcrf.gov). A list of putative
target genes was uploaded and analyzed in DAVID for identifying
associated KEGG pathways. Associated KEGG pathways were selected
according to the threshold of the Expression Analysis Systematic
Explorer Score (<0.1), a modified Fisher's Exact P-Value.
Statistical analysis
The experimental data were statistically analyzed
using the Student's t-test for two groups using Excel 2016 version
1,707 Microsoft Corporation, Redmond, WA, USA). P<0.05 was
considered to indicate a statistically significant difference. All
experimental data are expressed as the mean ± standard deviation
from three independent experiments.
Results
Increased chemoresistance in spheroid
culture model of SKOV3ip1 cells
To compare miRNA expression profiles between
conventional 2D monolayer-cultured SKOV3ip1 and 3D sphere-cultured
SKOV3ip1, SKOV3ip1 cells were cultured in ultra-low attachment
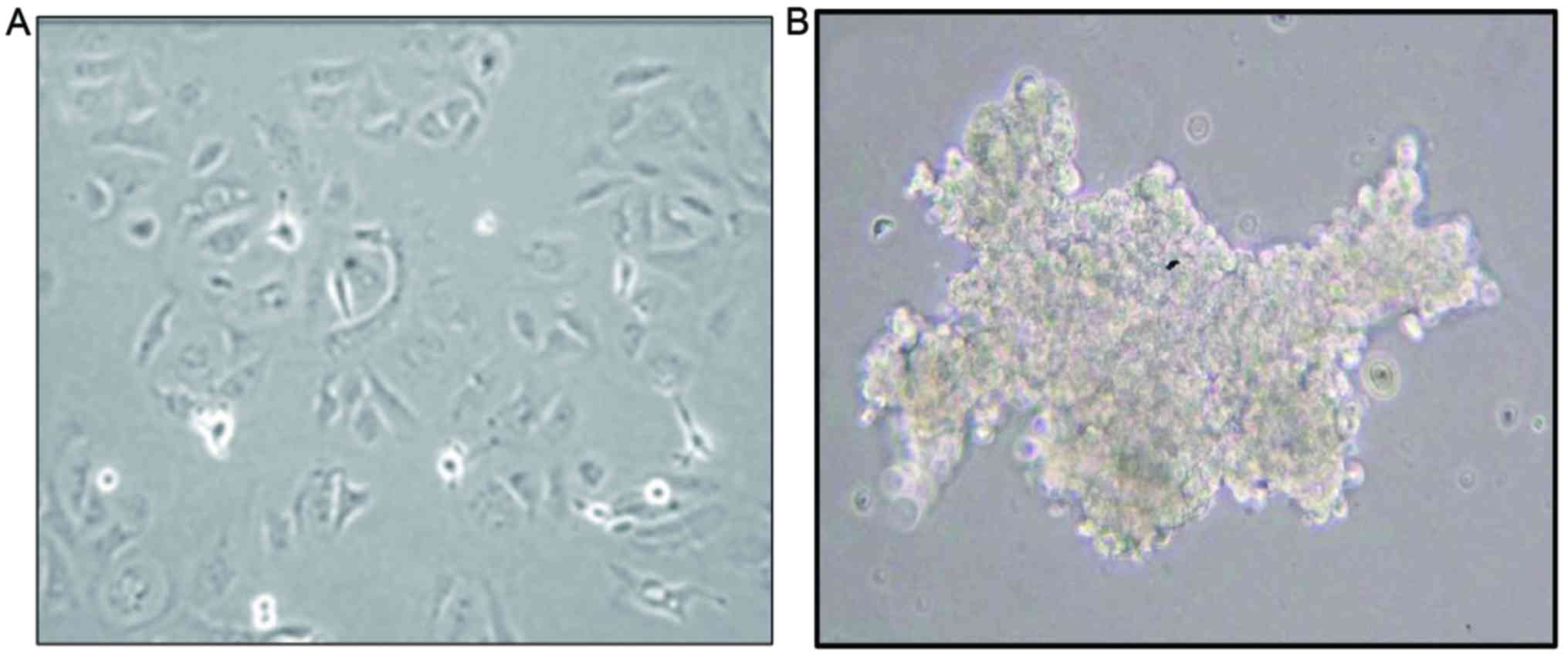
culture plates. As presented in Fig.
1, in the conventional 2D sphere culture system (Fig. 1A), SKOV3ip1 formed loose sheet-like
aggregates and did not accumulate as compact spheroids, unlike in
3D monolayer-cultured SKOV3ip1 (Fig.
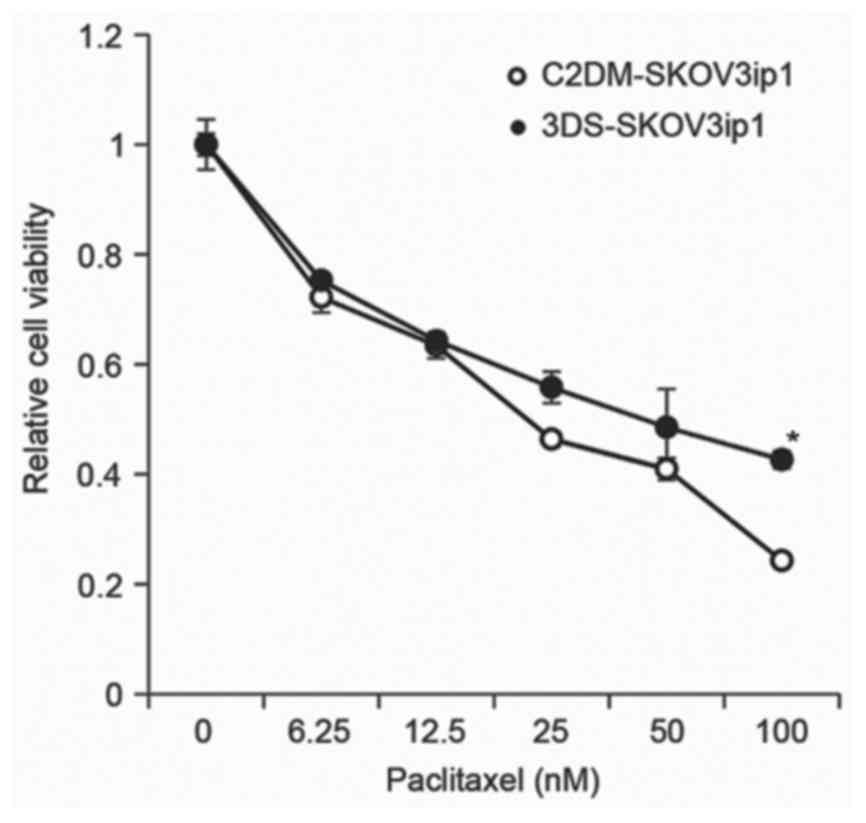
1B). To determine whether paclitaxel differentially affected
the cell viability in 2D monolayer and 3D sphere culture,
paclitaxel-induced cell cytotoxicity was evaluated in the two
systems. The cell viability of conventional 2D monolayer-cultured
and 3D sphere-cultured SKOV3ip1 cells was decreased by paclitaxel
in a dose-dependent manner. However, colorimetric cell viability
analysis demonstrated that 3D sphere-cultured SKOV3ip1 cells
exhibited greater resistance to paclitaxel than monolayer-cultured
SKOV3ip1 (Fig. 2).
Differences in miRNA expression
profile between normal culture and spheroid culture
The miRNA expression profile was examined to compare
differentially expressed miRNAs, between the conventional 2D
monolayer-cultured SKOV3ip1 and 3D sphere-cultured SKOV3ip1 systems
by SurePrint G3 Human V16 miRNA 8×60 K array, probed with 1,205
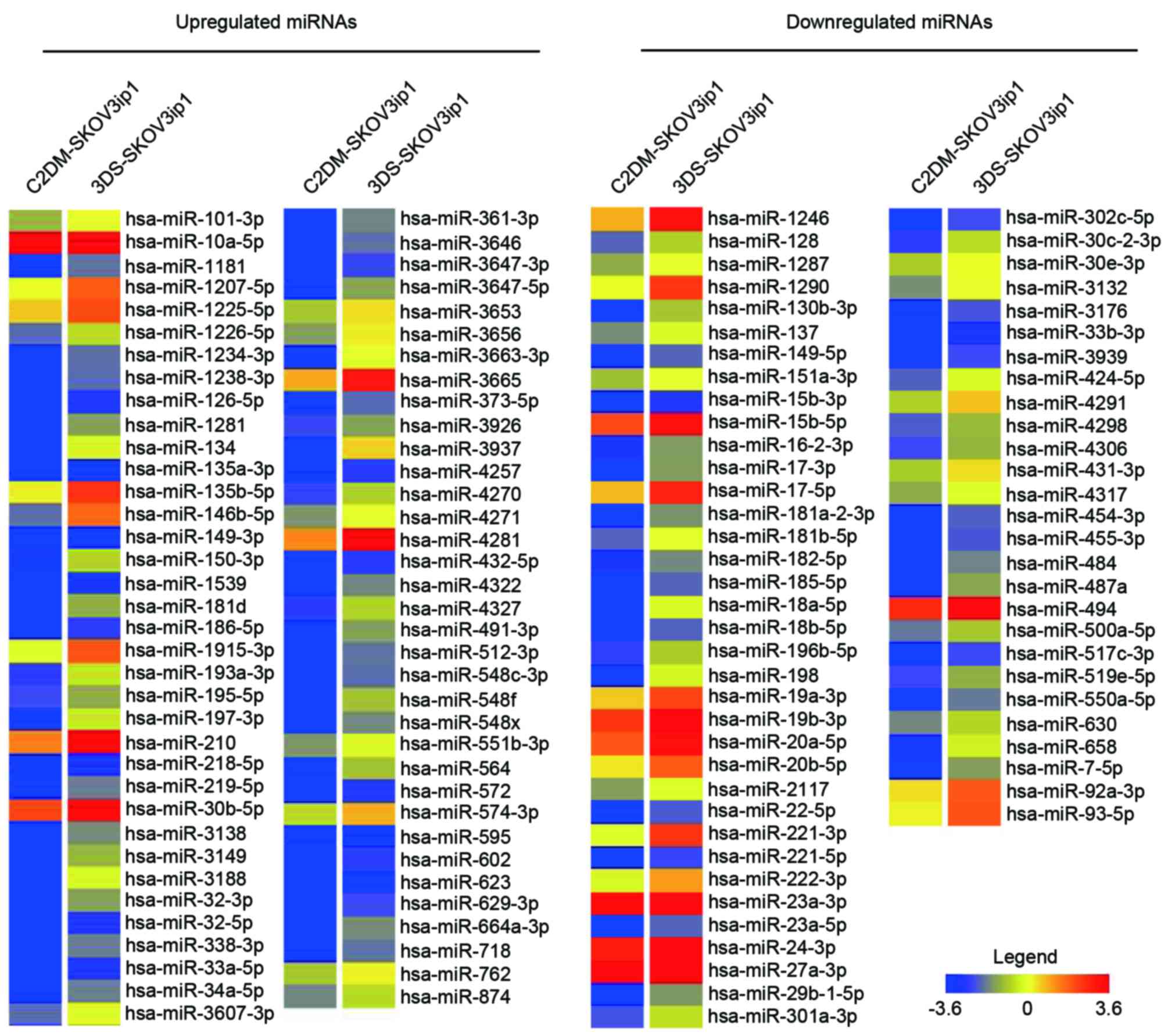
annotated miRNAs. As demonstrated in Fig.
3, 71 and 63 miRNAs were upregulated and downregulated,
respectively, in the 3D spheroid culture model compared with the
conventional 2D monolayer culture system in the SKOV3ip1 cells
(Fig. 1). From 134 spheroid culture
differentially expressed miRNAs, miR-3937 was the most upregulated
by 965.61 fold, and miR-18a-5p was the most downregulated by 849.08
fold.
In addition, identified target genes of spheroid
culture-specific miRNAs were identified using miRNA target gene
prediction databases, namely, PITA, microRNAorg and TargetScan
(Table I). A total of 96 putative
target genes of spheroid culture-specific upregulated miRNAs were
identified by all databases. Additionally, 662 putative target
genes of spheroid culture-specific downregulated miRNAs were
identified using all 3 target prediction systems. In addition, the
analysis of the association between target genes of spheroid
culture specific miRNAs and the biological functions of these
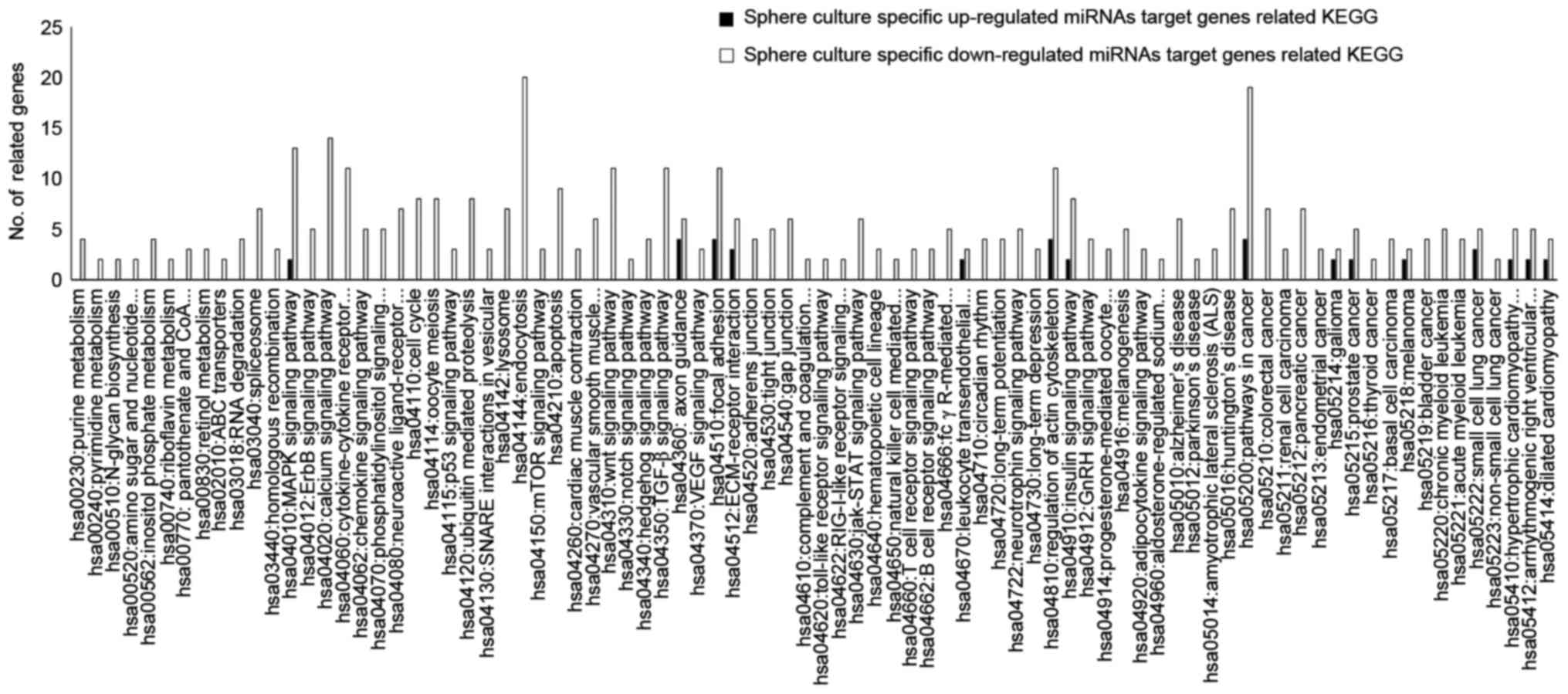
target genes was conducted using KEGG pathways analysis. The data
presented in Fig. 4 and Table II demonstrates that the target genes
of specific upegulated and downregulated miRNAs in spheroid culture
were linked with various KEGG pathways.
 | Table I.Number of 3-dimensional sphere
culture-specific miRNA targets using three miRNA target prediction
databases. |
Table I.
Number of 3-dimensional sphere
culture-specific miRNA targets using three miRNA target prediction
databases.
| Database | Target miRNAs, n | Overlapping miRNAs in
all three databases, n |
|---|
| Upregulated
miRNAs |
| 96 |
|
microRNAorg | 709 |
|
|
TargetScan | 808 |
|
| PITA | 156 |
|
| Downregulated
miRNAs |
| 662 |
|
microRNAorg | 2,695 |
|
|
TargetScan | 1,465 |
|
| PITA | 969 |
|
 | Table II.List of KEGG pathways associated with
target genes of specific upregulated and downregulated miRNAs in
spheroid culture. |
Table II.
List of KEGG pathways associated with
target genes of specific upregulated and downregulated miRNAs in
spheroid culture.
| KEGG
pathway-associated target genes of specific upregulated miRNAs in
spheroid culture | Axon guidance,
ECM-receptor interaction, small cell lung cancer, focal adhesion,
regulation of actin cytoskeleton, pathways in cancer, glioma,
melanoma, prostate cancer, ARVC, HCM, dilated cardiomyopathy,
leukocyte transendothelial migration, the insulin signaling pathway
and the MAPK signaling pathway |
| KEGG
pathway-associated target genes of specific downregulated miRNAs in
spheroid culture | Endocytosis, the
TGF-β signaling pathway, the calcium signaling pathway, circadian
rhythm, apoptosis, pathways in cancer, the Wnt signaling pathway,
pancreatic cancer, colorectal cancer, oocyte meiosis, pantothenate,
CoA biosynthesis, the cell cycle, focal adhesion, ECM-receptor
interaction, bladder cancer, the insulin signaling pathway, gap
junctions, ubiquitin mediated proteolysis, the regulation of the
actin cytoskeleton, the MAPK signaling pathway, lysosomes,
homologous recombination, the phosphatidylinositol signaling
system, chronic myeloid leukemia, ARVC, spliceosome, inositol
phosphate metabolism, basal cell carcinoma, the hedgehog signaling
pathway, RNA degradation, acute myeloid leukemia, small cell lung
cancer, hypertrophic cardiomyopathy, vascular smooth muscle
contraction, the ErbB signaling pathway, prostate cancer, SNARE
interactions in vesicular transport, Fc γ R-mediated phagocytosis,
long-term potentiation, riboflavin metabolism, cytokine-cytokine
receptor interaction, melanogenesis, axon guidance, adherens
junctions, the mTOR signaling pathway, endometrial cancer,
amyotrophic lateral sclerosis, retinol metabolism, Huntington's
disease, dilated cardiomyopathy, the neurotrophin signaling
pathway, the Jak-STAT signaling pathway, thyroid cancer, glioma,
the GnRH signaling pathway, Alzheimer's disease, the adipocytokine
signaling pathway, the tumor protein p53 (p53) signaling pathway,
tight junctions, long-term depression, renal cell carcinoma,
melanoma, the VEGF signaling pathway, the B cell receptor signaling
pathway, cardiac muscle contraction, aldosterone-regulated sodium
reabsorption, ABC transporters, amino sugar and nucleotide sugar
metabolism, N-glycan biosynthesis, hematopoietic cell lineage,
progesterone-mediated oocyte maturation, the notch signaling
pathway, non-small cell lung cancer, neuroactive ligand-receptor
interaction, the T-cell receptor signaling pathway, the chemokine
signaling pathway, purine metabolism, complement and coagulation
cascades, the RIG-I-like receptor signaling pathway, leukocyte
transendothelial migration, pyrimidine metabolism, the Toll-like
receptor signaling pathway, Parkinson's disease and natural killer
cell-mediated cytotoxicity |
Discussion
In gynecology, ovarian cancer is the most lethal
malignancy (1,2). Unlike for other types of cancer, the
development of ovarian cancer is closely associated with a specific
metastatic tumor microenvironment (6,7). In an
ovarian cancer-specific microenvironment, metastatic cell spheres
gain a resistance to anticancer agents, including paclitaxel
(8,9).
In particular, the emergence of chemoresistance in recurrent
ovarian cancer is observed, which is a major hurdle in overcoming
ovarian cancer (8,9). Therefore, there is an urgent requirement
to better understand the mechanisms underlying the formation of
multicellular aggregation, and to overcome mechanisms of
chemoresistance to anticancer drugs. In Figs. 1 and 2,
an in vitro multicellular aggregation was generated using
the 3D sphere culture system in SKOV3ip1 cells. Additionally, it
was demonstrated that 3D sphere-cultured SKOV3ip1 cells resisted
paclitaxel-mediated cell death compared to 2D monolayer cultured
SKOV3ip1 cells. In order to identify miRNAs that regulated the
formation of multicellular aggregated 3D spheres and
chemoresistance, the miRNA profile in conventional 2D
monolayer-cultured SKOV3ip1 and 3D sphere-cultured SKOV3ip1 cells
was displayed. As demonstrated in Fig.
3, of the 134 differentially expressed miRNAs identified, 71
were upregulated and 63 were downregulated in the 3D sphere culture
system, compared with the conventional 2D monolayer culture system.
To determine the association between miRNA-mediated regulation of
target gene expression and 3D sphere cultivation of ovarian cancer,
putative target genes of 3D sphere culture-specific miRNAs were
identified (Fig. 3). Additionally,
putative target genes of the 3D sphere culture-specific
miRNA-associated KEGG pathway were analyzed (Fig. 4). Notably, the MAPK signaling pathway,
regulation of the actin cytoskeleton, focal adhesion and pathways
in cancer were highly associated with target genes of upregulated
and downregulated miRNAs. The MAPK signaling pathway, regulation of
the actin cytoskeleton and focal adhesion are implicated in
anticancer drug chemoresistance (24–30).
Paclitaxel induces p38 and ERK/MAPK-mediated apoptosis and cell
death (25–27). Furthermore, p38, MAPK and epidermal
growth factor receptor were constitutively activated in paclitaxel
chemoresistance cells, which induces P38 MAPK-mediated E3
ubiquitin-protein ligase Mdm2 degradation and p53-mediated drug
resistance (26). MAPK
signaling-mediated anticancer drug chemoresistance is apparent in
various types of cancer, including breast, ovarian and lung cancer
(25,26,28).
Additionally, paclitaxel promotes microtubule assembly and
stabilization, which is a key mechanism for inducing anticancer
activity (29). Dysregulation of the
interactions between microtubules and cell adhesion induced
paclitaxel chemoresistance in ovarian cancer (30). Additionally, regulation of focal
adhesion induced integrin-mediated spherical formation (31,32). Thus,
miRNA-mediated regulation of the MAPK signaling pathway, regulation
of the actin cytoskeleton and focal adhesion induced paclitaxel
resistance and sphere formation in 3D sphere-cultured SKOV3ip1
cells.
In conclusion, the results of the present study
demonstrated that sphere-formed SKOV3ip1 cells acquired
chemoresistance to paclitaxel and cell-cell interaction, which is
associated with alteration of miRNA profiles in SKOV3ip1 ovarian
cancer cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
2015R1D1A1A01060688).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HJC, SKA, TJK and JHL conceived and designed the
experiments. HJC performed the experiments. HJC, SKA, TJK and JHL
performed the validation and formal analysis and curated and
analyzed the data. HJC, SKA, TJK and JHL wrote the manuscript. HJC,
SKA, TJK and JHL reviewed and revised the manuscript. JHL acquired
funding. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cannistra SA: Cancer of the ovary. N Engl
J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kindelberger DW, Lee Y, Miron A, Hirsch
MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW,
Birch C, et al: Intraepithelial carcinoma of the fimbria and pelvic
serous carcinoma: Evidence for a causal relationship. Am J Surg
Pathol. 31:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshida S, Furukawa N, Haruta S, Tanase Y,
Kanayama S, Noguchi T, Sakata M, Yamada Y, Oi H and Kobayashi H:
Expression profiles of genes involved in poor prognosis of
epithelial ovarian carcinoma: A review. Int J Gynecol Cancer.
19:992–997. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saad AF, Hu W and Sood AK:
Microenvironment and pathogenesis of epithelial ovarian cancer.
Horm Cancer. 1:277–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cormio G, Rossi C, Cazzolla A, Resta L,
Loverro G, Greco P and Selvaggi L: Distant metastases in ovarian
carcinoma. Int J Gynecol Cancer. 13:125–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naora H and Montell DJ: Ovarian cancer
metastasis: Integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan XD, Li M, Yuan Y, Mao N and Pan LY:
Biological comparison of ovarian cancer resistant cell lines to
cisplatin and Taxol by two different administrations. Oncol Rep.
17:1163–1169. 2007.PubMed/NCBI
|
|
10
|
Soriţău O, Tomuleasa CI, Páll E, Virág P,
Fischer-Fodor E, Foris V, Barbos O, Tatomir C, Kacsó G and Irimie
A: Enhanced chemoresistance and tumor sphere formation as a
laboratory model for peritoneal micrometastasis in epithelial
ovarian cancer. Rom J Morphol Embryol. 51:259–264. 2010.PubMed/NCBI
|
|
11
|
Xu F, Celli J, Rizvi I, Moon S, Hasan T
and Demirci U: A three-dimensional in vitro ovarian cancer
coculture model using a high-throughput cell patterning platform.
Biotechnol J. 6:204–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu G, Yin F, Wu H, Hu X, Zheng L and Zhao
J: In vitro ovarian cancer model based on three-dimensional agarose
hydrogel. J Tissue Eng. 5:2041731413520438. 2014.doi:
10.1177/2041731413520438. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hannon GJ: RNA interference. Nature.
418:244–251. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lehrbach NJ and Miska EA: Regulation of
pre-miRNA Processing. Adv Exp Med Biol. 700:67–75. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lenkala D, Gamazon ER, LaCroix B, Im HK
and Huang RS: MicroRNA biogenesis and cellular proliferation.
Transl Res. 166:145–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Didiano D and Hobert O: Molecular
architecture of a miRNA-regulated 3′ UTR. RNA. 14:1297–1317. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peterson SM, Thompson JA, Ufkin ML,
Sathyanarayana P, Liaw L and Congdon CB: Common features of
microRNA target prediction tools. Front Genet. 5:232014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kutanzi KR, Yurchenko OV, Beland FA,
Checkhun VF and Pogribny IP: MicroRNA-mediated drug resistance in
breast cancer. Clin Epigenetics. 2:171–185. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li SD, Zhang JR, Wang YQ and Wan XP: The
role of microRNAs in ovarian cancer initiation and progression. J
Cell Mol Med. 14:2240–2249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyerinas B, Park SM, Hau A, Murmann AE
and Peter ME: The role of let-7 in cell differentiation and cancer.
Endocr Relat Cancer. 17:F19–F36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu D, Wolf JK, Scanlon M, Price JE and
Hung MC: Enhanced c-erbB-2/neu expression in human ovarian cancer
cells correlates with more severe malignancy that can be suppressed
by E1A. Cancer Res. 53:891–898. 1993.PubMed/NCBI
|
|
24
|
Zhao Y, Shen S, Guo J, Chen H, Greenblatt
DY, Kleeff J, Liao Q, Chen G, Friess H and Leung PS:
Mitogen-activated protein kinases and chemoresistance in pancreatic
cancer cells. J Surg Res. 136:325–335. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bacus SS, Gudkov AV, Lowe M, Lyass L, Yung
Y, Komarov AP, Keyomarsi K, Yarden Y and Seger R: Taxol-induced
apoptosis depends on MAP kinase pathways (ERK and p38) and is
independent of p53. Oncogene. 20:147–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SH, Seong MA and Lee HY: p38
MAPK-induced MDM2 degradation confers paclitaxel resistance through
p53-mediated regulation of EGFR in human lung cancer cells.
Oncotarget. 7:8184–8899. 2016.PubMed/NCBI
|
|
27
|
Okano J and Rustgi AK: Paclitaxel induces
prolonged activation of the Ras/MEK/ERK pathway independently of
activating the programmed cell death machinery. J Biol Chem.
276:19555–19564. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ribeiro JR, Schorl C, Yano N, Romano N,
Kim KK, Singh RK and Moore RG: HE4 promotes collateral resistance
to cisplatin and paclitaxel in ovarian cancer cells. J Ovarian Res.
9:282016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McGrail DJ, Khambhati NN, Qi MX, Patel KS,
Ravikumar N, Brandenburg CP and Dawson MR: Alterations in ovarian
cancer cell adhesion drive taxol resistance by increasing
microtubule dynamics in a FAK-dependent manner. Sci Rep.
5:95292015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang N, Zhang H, Yao Q, Wang Y, Dai S and
Yang X: TGFBI promoter hypermethylation correlating with paclitaxel
chemoresistance in ovarian cancer. J Exp Clin Cancer Res. 31:62012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gallant ND, Michael KE and García AJ: Cell
adhesion strengthening: Contributions of adhesive area, integrin
binding, and focal adhesion assembly. Mol Biol Cell. 16:4329–4340.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jin H and Varner J: Integrins: Roles in
cancer development and as treatment targets. Br J Cancer.
90:561–565. 2004. View Article : Google Scholar : PubMed/NCBI
|