Introduction
Cervical cancer is the second most common female
malignant tumor following breast cancer. High risk human papilloma
virus (HPV) has been confirmed as a dominant factor responsible for
several anogenital diseases, particularly cervical intraepithelial
neoplasia (CIN) and cervical carcinoma (1). Of the more than two hundred HPV
genotypes, 16, 18, 31, 45, 52 and 58 are six particularly
important, as they are highly correlated with more than 90% of
cervical carcinoma cases (2–4). Persistent infection with high risk HPV
is the primary factor responsible for cervical cancer (3,4). Although
strains 16 and 18 are the two most common high-risk HPV genotypes
worldwide, genotype distribution studies in Chinese women have
shown that HPV58 is the third most common genotype found in Chinese
cervical carcinoma patients after HPV16 and −18 (5). Because HPV58 infection is not common in
western countries, current domestic and foreign research on HPV58
is lacking. However, the high rate of HPV58 infection in China
firmly indicates that studies investigating HPV58 therapeutic
vaccines are necessary.
HPV E7 is an early oncoproteins in HPV and
continuously present in cervical cancer cells. These proteins are
responsible for the carcinogenicity of HPV (1,6,7). Many studies have showed that sustained
expression of oncoproteins E7 is necessary for the tumorigenicity
and development of cervical cancer cells (8,9).
Accordingly, HPV E7 could be applied as target antigens for a
candidate HPV therapeutic vaccine due to their constitutive
expression and the maintenance of cellular transformation in
HPV-infected cells (3). Because of
continuous E7 expression, it is impossible that cervical carcinoma
cells can invoke immunologic escape through antigen loss (3). To date, E7 have been extensively
confirmed as ideal target antigens for the development of HPV
therapeutic vaccines against HPV-related CIN and cervical carcinoma
(10).
HPV therapeutic vaccines designed using specific
epitope peptides as target antigens can induce specific cellular
immune responses, avoid the immunosuppression induced by natural
proteins and enhance immune specificity and immune effects. At
present, this type of vaccine has become the focus of research in
the immunization field. Recently, researchers have found that the
cytotoxic T lymphocyte (CTL) epitope of the HPV16 E6 and E7
oncoproteins match human leukocyte antigen (HLA)-A2, which is
currently considered the most common human major histocompatibility
complex (MHC)-I molecule; the immunogenicity of these epitope has
been confirmed using transgenic mice (11). In vitro, lymphocytes from human
peripheral blood previously immunized with the HPV16 polypeptide
vaccine induced specific CTL immune responses and killed
HPV16-positive tumor cells (12,13).
However, the CTL epitope for HPV58 has not been found. In view of
the high HPV58 infection rate in China, we attempted to find
specific CTL epitope antigens in the HPV58 E7 proteins and to
prepare corresponding polypeptide vaccines to treat cervical
cancer.
To achieve our goal, we screened HLA-A2-restricted
HPV58 E7 CTL epitope peptides and evaluated their immune responses.
The resulting epitope peptides may serve as candidate epitope for
the preparation of an HPV58 therapeutic epitope peptide
vaccine.
Materials and methods
Peptide prediction and synthesis
The full-length amino acid sequences of the HPV58 E7
(accession no. AEJ33545.1) proteins were obtained from GenBank. The
HPV58 E7 protein is composed of 98 amino acids, and its sequence is
as follows:
MRGNNPTLREYILDLHPEPTDLFCYEQLCDSSDEDEIGLDRPDGQAQPATANYYIVTCCYTCGTTVRLCINSTTTDVRTLQQLLMGTCTIVCPSCAQQ.
The predicted peptides used for the identification
of CTL epitopes were selected on the basis of 3 different
prediction programs from internet websites that include an HLA
binding peptide prediction (http://www-bimas.cit.nih.gov/molbio/hla_bind/).
SYFPEITHI (http://www.syfpeithi.de/Scripts/MHCServer.dll/EpitopePrediction.htm),
and an MHC class I-binding prediction using an artificial neural
network (http://tools.immuneepitope.org/analyze/html_mhcibinding20090901/mhc_binding.html).
The predicted peptides each containing nine amino
acid were synthesized by solid phase peptide synthesis and purified
by reversed-phase high performance liquid chromatography by Abgent
(San Diego, CA, USA). The negative control peptide (irrelevant
peptide) from the SARS coronavirus (CoV) spike protein (LYLTQDLFL)
was synthesized and purified in the same way by GL Biochem Co.,
Ltd. (Shanghai, China). The mass of each peptide was 30 mg and the
purity of exceeded 93%.
Isolation and culture of peripheral
blood mononuclear cells (PBMC)
A total of 100 ml peripheral blood from HLA-A2 (+)
healthy women volunteers was drawn with a vacuum bag from the
basilic vein. Our study using volunteers' samples was approved by
the ethical committee of Guangxi Medical University Affiliated
Tumor Hospital. Written informed consent was obtained from all
volunteers. Human PBMC were separated from the peripheral blood
using Ficoll density gradient separation method. The final
concentration of PBMC was 1×105, diluted with RPMI-1640
containing 10% FBS. The final volume added to the well was 100 ul.
These cells were inoculated at 24-well culture plate and stimulated
with 10 µg/ml of the six predicted peptides or negative control
peptide, respectively. At the same time, the cells were added 100
U/ml interleukin 2 every other day. Three times of stimulation by
the peptides and IL-2, the PBMC were collected for EILSPOT.
ELISPOT Assay on human PBMC
The human PBMC, which were HLA-A2 (+) and activated
by the 6 predicted peptides or negative control peptide, were
detected special immune spots by an ELISPOT kit (DKW22-1000-096S;
Dakewe Biotech Co., Ltd., Shenzhen, China). Briefly, a 96-well
nitrocellulose plate precoated with anti-human interferon (IFN)-γ
antibody was placed at 4°C overnight and then blocked the following
day with RPMI-1640 medium containing 10% FBS. PBMC were added to
the wells at a concentration of 1×105 cells/well in a
volume of 100 µl. These cells were stimulated with 10 µg/ml of the
six predicted peptides, negative control peptide,
phorbol-12-myristate-13-acetate (PHA) (5 ng/ml; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and PBS, respectively. PHA served
as the positive control, and the negative control peptide and PBS
served as the negative controls. The plate was incubated at 37°C in
5% carbon dioxide for 24 h. The next day, the plate was incubated
with a biotinylated anti-human IFN-γ antibody (primary antibody)
for 2 h at room temperature. Unbound primary antibody was removed
by washing with scrubbing solution, and a streptavidin-HRP antibody
(secondary antibody) was added. Following 1 h of incubation at room
temperature, the unbound secondary antibody was removed by washing
with scrubbing solution, and staining with AEC
(3-amino-9-ethylcarbazole) substrate solution was carried out for
20 min. The plate was washed and air dried for 24 h. The immune
spots, which represented individual IFN-γ-producing cells as
spot-forming cells (SFC) on the membrane, were enumerated by the
KS-ELISPOT automatic system (Dakewe Biotech Co., Ltd.). The
responses were considered positive when the IFN-γ associated spot
numbers produced by predicted peptides stimulation were above 50
SFC/well compared with the responses obtained with all the negative
control peptide, P-values ≤0.05 were considered significant.
Antigenicity verification of HPV58
E772-80 peptide
Before further immune evaluation to the peptide
HPV58 E772-80 (STTTDVRTL), a mice cervical cancer cell
line U14/LV-HPV58E6E7 and an adenovirus vector vaccine
AD-HPV16/18/58 mE6E7 containing HPV58 E7 antigen were constructed
by the authors. Our animal experiments were approved by the ethical
committee of GuangXi medical university affiliated tumor hospital.
Thirty female C57BL/6 mice, 6–8 weeks old, weighed approximately 20
to 25 g, were randomly divided into three groups as follows: PBS
group, AD-NC (empty adenovirus vector) group, and AD-HPV16/18/58
mE6E7 vaccine group. It is n=10 in each group. C57BL/6 mice were
subcutaneously injected on the back of the mice with
1×106/100 ul the mice cervical cancer U14/LV-HPV58E6E7
cells which can express HPV58 E6E7 fusion protein. The PBS, AD-NC
or AD-HPV16/18/58 mE6E7 vaccine was individually administered by
tattooing on day 7, 14, and 21 after the cancer cells challenge.
Two weeks after the last immunization, serum and splenocytes were
isolated individually from blood and spleen of the immunized mice.
The peptide HPV58 E772-80 was used as antigen to
activate the immunized mice serum and splenocytes before ELISA and
ELISPOT assay. HPV58 related serum specific antibody was detected
by enzyme-linked immunosorbent assay (ELISA) using an ELISA kit
following the manufacturer's instructions (DKW-F1; Dakewe Biotech
Co., Ltd.). IFN-γ-spots of the splenocytes were detected using an
ELISPOT kit (DKW22-2000-096S; Dakewe Biotech Co., Ltd.). Briefly, a
96-well nitrocellulose plate precoated with anti-mice IFN-γ
antibody was placed at 4°C overnight and then blocked the following
day with RPMI-1640 medium containing 10% FBS. Immunized mice
splenocytes were added to the wells at a concentration of
1×105 cells/well in a volume of 100 µl. These cells were
stimulated with 10 µg/ml of the peptide HPV58 E772-80.
The plate was incubated at 37°C in 5% carbon dioxide for 24 h. The
next day, the plate was incubated with a biotinylated anti-mice
IFN-γ antibody (primary antibody) for 2 h at room temperature.
Unbound primary antibody was removed by washing with scrubbing
solution, and a streptavidin-HRP antibody (secondary antibody) was
added. Following 1 h of incubation at room temperature, the unbound
secondary antibody was removed by washing with scrubbing solution,
and staining with AEC (3-amino-9-ethylcarbazole) substrate solution
was carried out for 20 min. The plate was washed and air dried for
24 h. The immune spots, which represented individual
IFN-γ-producing cells as SFC on the membrane, were enumerated by
the KS-ELISPOT automatic system (Dakewe Biotech Co., Ltd.). The
production of IFN-γ associated spots indicated that the peptide
HPV58 E772-80 can activate the mice splenocytes which
were immumed by the AD-HPV16/18/58 mE6E7 vaccine containing HPV58E7
gene. The responses were considered positive when the spot numbers
produced in AD-HPV16/18/58 mE6E7 vaccine group were above 50
SFC/well compared with the responses obtained with PBS group and
AD-NC group, P-values ≤0.05 were considered significant.
Statistical analysis
The SPSS 22.0 software (IBM Corp., Armonk, NY, USA)
was used for all statistical analyses. IFN-ELISPOT assay and ELISA
were evaluated using an analysis of variance (ANOVA). Results are
expressed as means ± standard deviation (SD). To identify
significant differences between groups, Student's t-test was used.
For all comparisons, P<0.05 was considered to indicate a
statistically significant difference. All findings were confirmed
in at least one additional independent experiment.
Results
Peptide prediction and analysis
results
Several peptide prediction programs were used to
assist in the identification of candidate CTL epitope. According to
the prediction results of the three programs, the top-ranked six
peptides in HPV58 E7 were selected are shown in Table I.
 | Table I.Prediction results for HPV58 E7
epitope peptides. |
Table I.
Prediction results for HPV58 E7
epitope peptides.
| Peptide number | The initial position
of the amino acid | The terminal position
of the amino acid | Amino acid
sequence |
|---|
| P1 | 7 | 15 | TLREYILDL |
| P2 | 14 | 22 | DLHPEPTDL |
| P3 | 69 | 77 | CINSTTTDV |
| P4 | 72 | 80 | STTTDVRTL |
| P5 | 79 | 87 | TLQQLLMGT |
| P6 | 83 | 91 | LLMGTCTIV |
Evaluation of HPV58 E7
peptide-specific T cell immunity in HLA-A2 (+) human PBMC
IFN-γ ELISPOT assay was performed on the HLA-A2 (+)
human PBMC using a final concentration of 10 µg/ml of the 6
candidate peptides of HPV58 E7. IFN-γ-positive spots were detected
in the PHA (the positive control), P2 and P4 groups, whereas other
experimental groups and the two negative control groups (negative
control peptide group and PBS group) exhibited no or few spots
formation (Fig. 1). The average
numbers of IFN-γ-positive spots in the PHA, the negative control
peptide, PBS, P1, P2, P3, P4, P5 and P6 groups were 737±17.54
SFC/1×105, 0, 0,0, 50.61±5.37 SFC/1×105,
5.62±4.78 SFC/1×105, 266±34.42 SFC/1×105,
13.33±1.53 SFC/1×105, 21.61±5.37 SFC/1×105,
respectively. The average numbers of IFN-γ-positive spots in the
PHA, P2 and P4 groups were significantly higher than other
experiment groups and the two negative control groups (P<0.05;
Fig. 1).
 | Figure 1.HPV58 E7-specific T-cell response to
the candidate peptides in human leukocyte antigen-A2 (+) human PBMC
as measured by IFN-γ enzyme-linked immunospot assay. IFN-γ-positive
spots were detected in the PHA, P2 and P4 groups, whereas other
experimental groups and the two negative control groups exhibited
no or few spots formation. The average numbers of IFN-γ-positive
spots in the PHA, P2 and P4 groups were 737±17.54
SFC/1×105, 50.61±5.37 SFC/1×105, 266±34.42
SFC/1×105, respectively. The spot numbers in these three
groups were significantly higher than other experiment groups and
the two negative control groups. *P<0.05 vs. the PBS group.
#P<0.05 vs. the irrelevant peptide group. IFN,
interferon; PBS, phosphate-buffered saline; SFC, spot-forming
cells; HPV58 E7, human papillomavirus; PBMC, peripheral blood
mononuclear cells; PHA, positive control. |
Antigenicity verification of the peptide
HPV58 E772-80
The humoral immunogenicity of the
peptide HPV58 E772-80
On day 14 after the last immunization, blood was
collected from the orbital veins of the mice, and sera were
isolated. The peptide HPV58 E772-80 was added to 96-well
plate as stimulation antigen. Then the serum of mice immunized with
PBS, AD-NC, and AD-HPV16/18/58 mE6E7 vaccine was added to 96-well
plate. Specific humoral immune response associated with HPV58E7 was
detected by ELISA. After ELISA was completed, the absorbance of
each hole of 96 holes was detected. Absorbance value more than 0.1
can be judged as positive. The maximum dilution of the positive
results was regarded as the antibody titer of the predicted
peptides. As shown in Fig. 2, on the
14th day of the last immunization, with the prediction peptide
HPV58 E772-80 as antigen, the AD-HPV16/18/58 mE6E7
vaccine containing HPV58 E7 protein can produce specific antibody
in the immunized mice serum. The antibody titers of vaccine group
were 1:25,600, but the antibody titers of PBS group and AD-NC group
were 1:400. Compared with the two control groups, the antibody
titers of AD-HPV16/18/58 mE6E7 vaccine were significantly increased
(P<0.05). HPV58 E772-80 peptide can activate humoral
immunogenicity of the mice immunized by the vaccine containing
HPV58E7 antigen.
Cellular immunogenicity of the peptide
HPV58 E772-80
To test cellular immunogenicity of the peptide HPV58
E772-80, PBMC from the immunized mice were isolated and
added to a 24-well plate at a concentration of 2×105
cells/well and in a volume of 200 µl. Then the cells were
stimulated with 10 µg/ml of the peptide HPV58 E772-80.
IL-2 100 u/ml was added to the cells every other day. The
splenocytes were co-cultured with the peptide HPV58
E772-80 for a week. Seven days later, the splenocytes
from the immunized mice were tested directly ex vivo in IFN-γ
Elispot assays against the peptide HPV58 E772-80.
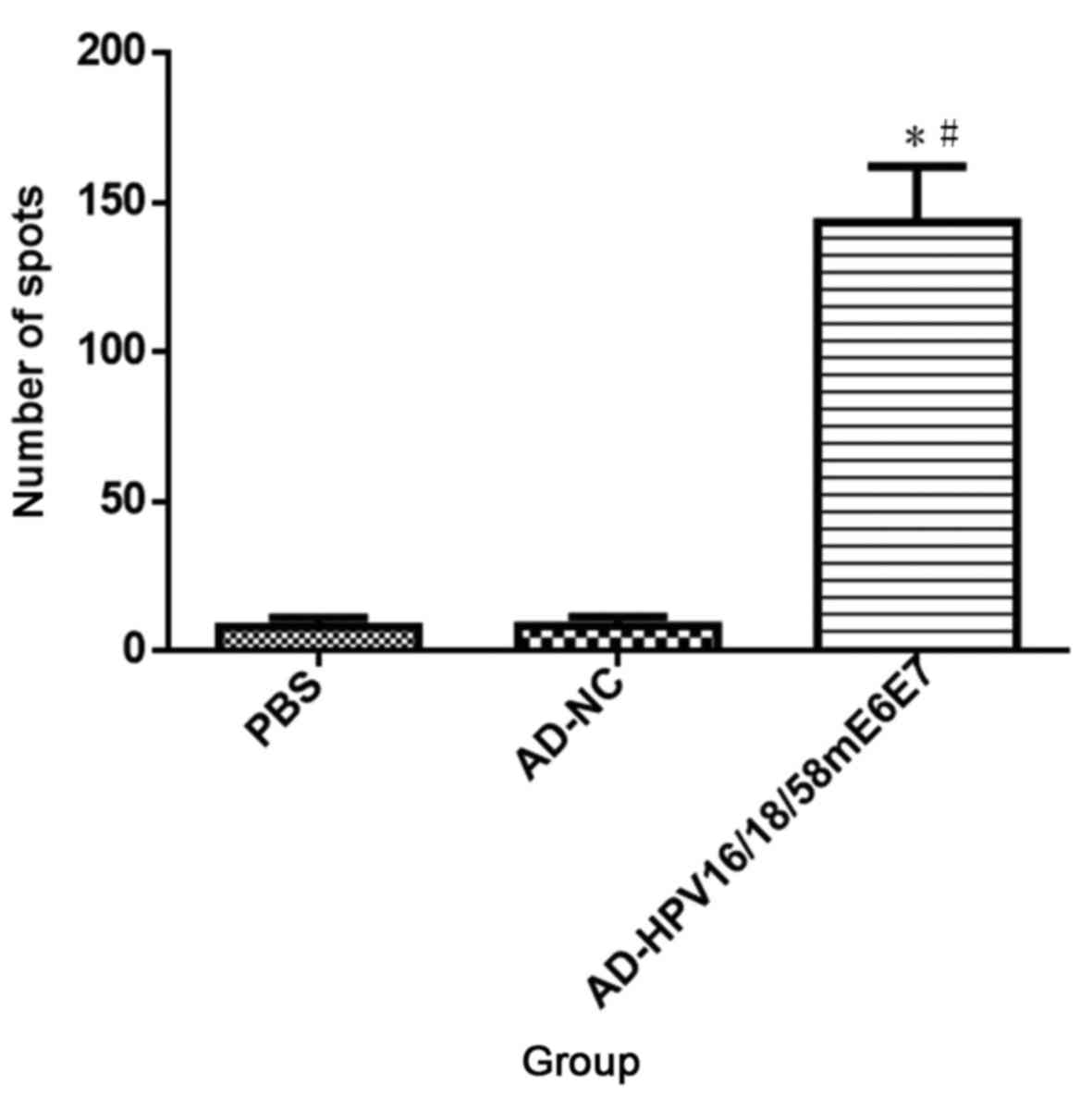
AD-HPV16/18/58 mE6E7 vaccine can induce specific cellular immune
responses against the HPV58 E772-80 peptide in the
C57BL/6 mice after three immunizations, the average number of
IFN-γ-positive spots was 143±32.13 SFC/1×105. The
positive spots were significantly increased compared with the
negative groups as PBS (8±5.29 SFC/1×105, P<0.01) and
AD-NC empty vector (8±5.13 SFC/1×105, P<0.01)
(Fig. 3). HPV58 E772-80
peptide can also activate cellular immunogenicity of the mice
immunized by the vaccine containing HPV58 E7 antigen.
Discussion
Peptide vaccines have attracted increasing attention
because they possess the ability to induce a single specific immune
response. At present, studies evaluating HPV therapeutic
polypeptide vaccines have focused on the HPV E6 and E7 proteins and
their CTL epitope (14). The HPV E6
and E7 proteins contain multiple CTL epitope that have high
affinity for HLA class I molecules. Therefore, we can identify
epitope peptides with both strong immunogenicity and high
specificity (15). This process is
key for the preparation of HPV therapeutic polypeptide vaccines.
Despite the high HPV58 infection rate in south China, where HPV58
is third after HPV16 and 18, the HPV58 infection rate is very low
in western countries; moreover, HPV58 infection distribution
differs by region. Research and development of HPV58 peptide
vaccines has not received much attention because no accepted CTL
epitope has been identified to date. Thus, the study and
development of related therapeutic vaccines are difficult.
Specific cellular immunity mediated by CTL plays a
key role in cancer immunity. Effector T cells mainly recognize CTL
epitopes that are bound to MHC-I molecules to induce cytotoxic
cytotoxicity (16). HLA-I molecules
are key to antigen presentation and activation of T cells. It is
very important for the antigen presentation of virus antigen, tumor
antigen, and so on. HLA-A2 is the most specific HLA-I molecule. Its
distribution has significant ethnic, ethnic and geographical
differences. Studies have shown that the frequency of allele
distribution varies from 10 to 40% in different ethnic groups.
HLA-A2 (+) accounts for approximately 53% of Chinese population
(17). Therefore, it is important to
study the HPV58 E6 and E7 HLA-A2-restricted CTL epitope peptide in
Chinese population. At present, some HLA-A2 (+) CTL epitopes in
HPV16 E7 protein, such as E711-19 and
E749-57, have been identified and accepted (18–20). At
present, many studies have confirmed the effectiveness of these
epitopes as antigen targets (21–24).
However, there are few studies about HPV58. Chan identified three
HLA-A11-restricted HPV58 E7 peptides (amino acid positions 78 to
86, 74 to 82, and 88 to 96) showed a positive response, but without
HLA-A2-restricted E7 peptides (25).
Sabah confirmed the peptide region for the E7 protein ranged from27
to 33amino acids and a 9-m epitopes, SSDEDEIGL, in HPV58 E7 were
the most potential T cell epitope. And the peptide sequences could
interact with as many as seven MHC-1 alleles and showed population
coverage up to 90.31% (26).
The CTL epitope for the HPV E6 and E7 antigens were
screened by acid elution or the use of synthetic overlapping
peptides. However, these two methods are costly and time consuming;
additionally, the methods can result in a high miss rate because
four to eight amino acid residues are separated by two overlapping
peptide segments. Time and money can be saved by using
internationally recognized epitope prediction sites. Therefore, in
our study, HPV58 E7 HLA-A2-restricted CTL epitope peptides were
predicte d using several related websites: the HLA binding peptide
prediction website HLA Peptide Binding Predictions, http://www-bimas.cit.nih.gov/molbio/hla_bind/;
SYFPEITH, http://www.syfpeithi.de/; and epitope
prediction and analysis tools, http://tools.immune epitope.org/main/ (27). After a comprehensive analysis, we
preliminarily identified 6 CTL antigen peptides in HPV58 E7 that
were subsequently named P1 (E77-15: TLREYILDL), P2
(E714-22: DLHPEPTDL), P3 (E769-77:
CINSTTTDV), and P4 (E772-80: STTTDVRTL), P5
(E779-87: TLQQLLMGT), P6 (E783-91:
LLMGTCTIV).
In the next study, HLA-A2 (+) PBMC were collected
and co-cultured with the six predicted peptides, respectively. In
the evaluation of HPV58 E7 peptide-specific T cell immunity in
HLA-A2 (+) human PBMC, IFN-γ-production was evident by ELISPOT
assay in the P2 and P4 groups. The average numbers of
IFN-γ-positive spots in groups P2 and P4 were significantly
increased compared to other four experiment groups and the two
negative control groups. And P4 showed the strongest response.
Therefore, we chose P4 (E772-80: STTTDVRTL) to continue
our study. To be viewed as good peptide epitope, a particular
sequence must have some key properties. Firstly, the epitope has to
be fairly well conserved among HPV's proteome. Secondly, the
epitope has capacity that ensures processivity. In addition, the
processed peptide has to be able to interact with MHC alleles, and
finally the interacting MHC allele should have good population
coverage. E772-80 was chose from HPV58 E7 conserved
sequence and fulfilled all the parameters mentioned here.
In the further antigenicity verification of the
peptide HPV58 E772-80, this peptide can activate not
only humoral but also cellular immune reaction of AD-HPV16/18/58
mE6E7 vaccine containing HPV58 E7 antigen, but not the negative
control groups as PBS and AD-NC. the antibody titers of the vaccine
group were significantly increased. In cellular immunoassays, the
average number of IFN-γ-positive spots in the AD-HPV16/18/58 mE6E7
vaccine group wa significantly enhanced compared with PBS group and
AD-NC group. This result preliminarily confirmed that the peptide
HPV58 E772-80 can be used an HLA-A2-restricted CTL
epitope peptide. It can be used as a candidate peptide for peptide
vaccine development.
Overall, peptide vaccines are considered to be safe,
easy to produce and stable (28).
However, their low immunogenicity must first be overcome. Another
limitation of peptide-based vaccines is the need to match the
patient's HLA that makes it difficult to develop a peptide-based
vaccine applicable to the whole population. HPV58
E772-80 is a HLA-A2-restricted CTL epitope peptide and
has good antigenicity. HLA-A2 (+) accounts for more than 50% of
Chinese population. Therefore, this result provided a potential
target epitope for HPV58 E7 protein and lay the foundation for the
construction and identification of HPV58 peptide vaccines.
Acknowledgements
The authors would like to thank Dr Zhijun Yang and
Dr Qi Wang for their expert advice in molecular biology, cell
culture and animal experimentation.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no. 81260386), the Natural
Science Foundation of Guangxi (grant no. 2013GXNSFAA019192), the
Patent project of Guangxi colleges and Universities (grant no.
KY2015ZL021), and the Research and Development Project of Guangxi
Medical and Health Appropriate Technology (grant no. S201511).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HW performed the experiments and wrote the
manuscript. LC predicted and selected the epitopes. WM performed
the animals experiment about immunity. YZ divided the lymphocyte
from peripheral blood. LQ carried out the immunity test. MC
analyzed the experiments data. LL designed and conducted the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bosch FX, Manos MM, Muñoz N, Sherman M,
Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R and Shah KV:
Prevalence of human papillomavirus in cervical cancer: a worldwide
perspective. International biological study on cervical cancer
(IBSCC) study group. J Natl Cancer Inst. 87:796–802. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maleki Z: Human papilloma virus
vaccination: Review article and an update. World J Obstet Gynaecol.
5:16–27. 2016. View Article : Google Scholar
|
|
4
|
Varughese J and Richman S: Cancer care
inequity for women in resource-poor countries. Rev Obstet Gynecol.
3:122–132. 2010.PubMed/NCBI
|
|
5
|
Lo KW, Wong YF, Chan MK, Li JC, Poon JS,
Wang VW, Zhu SN, Zhang TM, He ZG, Wu QL, et al: Prevalence of human
papillomavirus in cervical cancer: A multicenter study in China.
Int J Cancer. 100:327–331. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Callejas-Valera JL, Iglesias-Bartolome R,
Amornphimoltham P, Palacios-Garcia J, Martin D, Califano JA,
Molinolo AA and Gutkind JS: mTOR inhibition prevents rapid-onset of
carcinogen-induced malignancies in a novel inducible HPV-16 E6/E7
mouse model. Carcinogenesis. 37:1014–1025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McLachlin CM: Human papillomavirus in
cervical neoplasia. Role, risk factors and implications. Clin Lab
Med. 20:257–270, v. 2000.
|
|
8
|
Ullman CG and Emery VC: Transforming
proteins of human papillomaviruses. Rev Med Virol. 6:39–55. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Münger K, Baldwin A, Edwards KM, Hayakawa
H, Nguyen CL, Owens M, Grace M and Huh K: Mechanisms of human
papillomavirus-induced oncogenesis. J Virol. 78:11451–11460. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wain G: The human papillomavirus (HPV)
vaccine, HPV related diseases and cervical cancer in the
post-reproductive years. Maturitas. 65:205–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bounds CE, Hu J, Cladel NM, Balogh K and
Christensen ND: Vaccine generated immunity targets an HPV16 E7
HLA-A2.1-restricted CD8(+) T cell epitope relocated to an early
gene or a late gene of the cottontail rabbit papillomavirus (CRPV)
genome in HLA-A2.1 transgenic rabbits. Vaccine. 29:1194–1200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Mizuno MT, Singhal MC, Hu SL,
Galloway DA, Hellström I and Hellström KE: Induction of cytotoxic T
lymphocytes specific for a syngeneic tumor expressing the E6
oncoprotein of human papillomavirus type 16. J Immunol.
148:2617–2621. 1992.PubMed/NCBI
|
|
13
|
Nurkkala M, Wassén L, Nordström I,
Gustavsson I, Slavica L, Josefsson A and Eriksson K: Conjugation of
HPV16 E7 to cholera toxin enhances the HPV-specific T-cell recall
responses to pulsed dendritic cells in vitro in women with cervical
dysplasia. Vaccine. 28:5828–5836. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng Y, Zhang Y, Ma Y, Wan J, Shi C and
Huang L: Enhancement of immunotherapeutic effects of HPV16E7 on
cervical cancer by fusion with CTLA4 extracellular region. J
Microbiol. 46:728–736. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gan L, Jia R, Zhou L, Guo J and Fan M:
Fusion of CTLA-4 with HPV16 E7 and E6 enhanced the potency of
therapeutic HPV DNA vaccine. PLoS One. 9:e1088922014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren F, Xu Y, Mao L, Ou R, Ding Z, Zhang X,
Tang J, Li B, Jia Z, Tian Z, et al: Heat shock protin 110 improves
the antitumor effects of the cytotoxic T lymphocyte epitope
E7(49–57) in mice. Cancer Biol Ther. 9:134–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rötzschke O, Falk K, Stevanović S, Jung G
and Rammensee HG: Peptides motif of closely related HLA class I
molecules encompass substantial differences. Eur J Immunol.
22:2453–2456. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Speidel K, Osen W, Faath S, Hilgert I,
Obst R, Braspenning J, Momburg F, Hämmerling GJ and Rammensee HG:
Priming of cytotoxic T lymphocytes by five heat-aggregated antigens
in vivo: Conditions, efficiency, and relation to antibody
responses. Eur J Immunol. 27:2391–2399. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schäfer K, Müller M, Faath S, Henn A, Osen
W, Zentgraf H, Benner A, Gissmann L and Jochmus I: Immune response
to human papilomavirns 16 L1E7 chimeric virus-1ike particles:
Induction of cytotoxic T cells and specific tumor protection. Int J
Cancer. 81:881–888. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu YS, Hao F, Song ZQ, Zhong B, Hao J and
Ye Q: Screening and Identification of Predicted Epitopes of
HLA-A2-restricted Cytotoxic T Lymphocytes Derived from the HPV16 E7
Antigen. Chin J Dermatol. 37:283–284. 2004.(In Chinese).
|
|
21
|
Riemer AB, Keskin DB, Zhang G, Handley M,
Anderson KS, Brusic V, Reinhold B and Reinherz EL: A conserved
E7-derived cytotoxic T lymphocyte epitope expressed on human
papillomavirus 16-transformed HLA-A2+ epithelial cancers. J Biol
Chem. 285:29608–29622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harro CD, Pang YY, Roden RB, Hildesheim A,
Wang Z, Reynolds MJ, Mast TC, Robinson R, Murphy BR, Karron RA, et
al: Safety and immunogenicity trial in adult volunteers of a human
papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer
Inst. 93:284–292. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Ou R, Tang J, Deng X, Wu Y, van
Velkinburgh JC, Ni B and Xu Y: Enhanced anti-tumor effects of
HPV16E7(49–57)-based vaccine by combined immunization with
poly(I:C) and oxygen-regulated protein 150. Cancer Epidemiol.
37:172–178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao Y, Huang W, Yang X, Sun W, Liu X, Cun
W and Ma Y: HPV-16 E6 and E7 protein T cell epitopes prediction
analysis based on distributions of HLA-A loci across populations:
An in silico approach. Vaccine. 31:2289–2294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan PK, Liu SJ, Cheung TH, Yeo W, Ngai
SM, Cheung JL, Chong P and Man S: T-cell response to human
papillomavirus type 58 L1, E6, And E7 peptides in women with
cleared infection, cervical intraepithelial neoplasia, or invasive
cancer. Clin Vaccine Immunol. 17:1315–1321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sabah SN, Gazi MA, Sthity RA, Husain AB,
Quyyum SA, Rahman M and Islam MR: Designing of epitope-focused
vaccine by targeting E6 and E7 conserved protein sequences: An
immuno-informatics approach in human papillomavirus 58 isolates.
Interdiscip Sci. 17 Sep. 2016, (Epub ahead of print). PubMed/NCBI
|
|
27
|
Tu SH, Huang HI, Lin SI, Liu HY, Sher YP,
Chiang SK, Chong P, Roffler S, Tseng GC, Chen HW and Liu SJ: A
novel HLA-A2-restricted CTL epitope of tumor-associated antigen L6
can inhibit tumor growth in vivo. J Immunother. 35:235–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rudolf MP, Man S, Melief CJ, Sette A and
Kast WM: Human T-cell responses to HLA-A-restricted high binding
affinity peptides of human papillomavirus type 18 proteins E6 and
E7. Clin Cancer Res. 7(3 Suppl): 788s–795s. 2001.PubMed/NCBI
|