Introduction
The incidence of malignant melanoma is increasing
worldwide; approximately 160,000 cases were diagnosed in 2002
(1), and 232,000 in 2012 (2,3). Melanoma
is one of the most aggressive tumors, and the survival time of
melanoma patients with distant metastasis is only between 9
(4) and 18 (5) months. Melanomas are most commonly
localized in the skin but can arise anywhere in the body where
melanocytes exist (6). During early
embryogenesis, melanoblasts migrate from the neural crest to
various sites, including the mucosal surfaces of the body (e.g.,
lining the sinuses, nasal passages, oral cavity, vagina, and anus),
and these can transform into cancerous cells, resulting in mucosal
melanoma (7). Mucosal melanoma is a
rare form of this disease, making up only about 1.2% of white,
non-Hispanic cases in the United States (8) and 3% of Japanese cases (9,10).
Anorectal lesions are the most common type of
mucosal melanomas, followed by those of the eye, oral-nasopharynx,
and esophagus (11–13). Gastrointestinal melanoma (GM), a
malignant melanoma arising in the gastrointestinal tract, is a
major type of mucosal melanoma. However, there have been many
debates as to its true origin, e.g., the gut vs. a distant,
undetected primary lesion that has regressed, known as melanoma of
unknown primary.
Cancer stem cells (CSCs) are tumor cells with stem
cell properties. The concept of the melanoma stem cell was first
reported in 2005 (14). Melanoma stem
cells are thought to have self-renewal ability, multilineage
potential (15), and resistance to
chemotherapy (16,17). Several markers have been reported to
be melanoma stem cell markers, such as nestin (18), SOX2 (19), and ABCB5 (20).
The clinicopathological characteristics of melanoma
are drastically different with respect to primary site and tumor
stage. The prognosis for mucosal melanoma is extremely poor
compared to that for skin melanoma (SM), and the 5-year survival
rate associated with SM is 81% but that associated with mucosal
melanoma is only 25% (8) SM has been
clinicopathologically categorized into four types according to
Clark's classification (21): i)
lentigo maligna; ii) superficial spreading; iii) acral lentiginous;
and iv) nodular. Recently, Bastian et al (22) proposed a new classification for SM
that considers sun exposure and gene abnormalities (22,23). This
became a major classification with the advances in molecular
targeted therapy for melanomas with a BRAF mutation
(24). However, there are no
universal staging systems for GM (25). GM is difficult to diagnose at an early
stage because of the anatomical location of the disease. It is
usually challenging for pathologists to diagnose GM, especially
given the small amount of biopsy samples usually collected.
Previous studies have indicated GMs with BRAF and
NRAS mutations are significantly rare (23), whereas activating KIT mutations
have been reported in >30% of case (24,26) and
also NF1 mutations have been found in 20% of anorectal
melanoma cases (27). However, the
clinicopathological characteristics of GM have not been well
clarified. In this report, we attempt to determine the
clinicopathological differences between GM and SM.
Surgical resection is the first choice of treatment
for early-stage melanoma (Stages I and II), whereas treatment for
Stages III and IV melanomas with lymph-node and/or distant
metastasis has been evolving dramatically along with the
development of novel targeted therapies and immunotherapies.
Moreover, metastatic melanoma patients with the BRAF
mutations are commonly treated with dabrafenib or vemurafenib
(28). Therefore, accurate diagnosis
of GM is essential for implementing appropriate therapeutic
plans.
In the present study, we investigated the
clinicopathological characteristics of and presence of the
BRAFV600E mutation in GM patients, and compared
these findings to those of SM patients.
Materials and methods
Patients and tissues
Tissue samples [GM (n=10) and SM (n=31)] were
obtained from patients who had undergone surgical treatment at our
hospital from 1997 to 2015. This study was conducted in accordance
with the principles embodied in the Declaration of Helsinki (2008).
Approval for the study was obtained from the Human Research Ethics
Committees at the Tokyo Metropolitan Geriatric Hospital (no.
R17-33) and the Nippon Medical School Hospital (no. 29-07-805);
written informed consent was obtained from all patients.
Tissue processing and histological
assessment
Tissues were fixed in formalin and subjected to
standard processing and paraffin embedding. They were sliced into
3-µm thick sections for hematoxylin and eosin (H&E) staining
and immunohistochemical analysis. Diagnoses of pathological
specimens were made by more than two pathologists based on the
American Joint Committee on Cancer (AJCC, 2009) guidelines for SMs
and the Union for International Cancer Control (UICC, 7th edition)
guidelines for GMs.
Analyses of immunohistochemistry and
mitosis findings
Paraffin-embedded tissue sections were immunostained
using Histofine Simple Stain MAX PO (Nichirei, Tokyo, Japan) kits.
After deparaffinization, endogenous peroxidase activity was blocked
by incubating sections with 0.3% hydrogen peroxide in methanol for
30 min. Sections were incubated in either the absence (negative
control) or presence of a monoclonal anti-nestin antibody (diluted
1:100, incubated for 1 h at room temperature; MAB5326; EMD
Millipore, Billerica, MA, USA), anti-ABCB5 antibody (diluted 1:160,
incubated for 1 h at room temperature; SAB1300315; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), anti-SOX2 antibody (diluted 1:800,
incubated for 1 h at room temperature; GT15098; Neuromics, Edina,
MN, USA), anti-BRAF V600E antibody (diluted 1:50, incubated
overnight at 4°C; E19290; Spring Bioscience, Pleasanton, CA, USA),
and anti-MIB-1 antibody (diluted 1:100, incubated for 30 min at
room temperature; M7240; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). Bound antibodies were detected using
diaminobenzidine tetrahydrochloride as a chromogen.
Immunohistochemical review was performed separately by two of the
authors, who were blinded to clinical and outcome data. For the
evaluation of immunostaining, intensity and area of the positive
tumor cells were determined separately, based upon scores of 0–3+.
Intensity was scored as follows: 0, negative; 1+, low; 2+,
intermediate, and 3+, high. Area was scored as follows: 0, <25;
1+, 25–50; 2+, 50–75; 3+, >75%. Tissue magnification was −200.
Intensity and area scores were then multiplied for statistical
analysis.
We used representative H&E-stained slides to
count the number of normal mitoses and atypical mitoses at −400
magnification. Atypical mitosis was defined as anything other than
the typical form of normal mitosis, for example, asymmetrical,
ring, and multipolar mitoses. The total mitosis count included
atypical and normal mitoses. For statistical analysis, we averaged
the value of the findings from the two authors who provided
immunohistochemical reviews.
DNA Extraction, polymerase chain
reaction (PCR)
DNA was extracted from 10% formalin-fixed,
paraffin-embedded tissues using the TaKaRa DEXPAT™ kit (Takara Bio,
Inc., Otsu, Japan), according to the manufacturer's
instructions.
A BRAFV600E mutation was analyzed
in the total volume of the PCR mixture (20 µl), which contained the
following: 10 µl of TaqMan® Genotyping Master mix
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), 4 µl of
prepared gDNA sample, 4 µl of nuclease-free water, and 2 µl of
TaqMan® Mutation Detection Assay (Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions
(29). This mixture contains an
allele-specific probe to identify the presence of p.V600E
substitution in a BRAF gene. Amplification of the examined
region was performed in 96-well plates as follows: pre-denaturation
at 95°C for 10 min; 5 cycles each at 92°C (12 sec/cycle) and 58°C
(60 sec/cycle); 40 cycles at 92°C (15 sec/cycle) and at 60°C (60
sec/cycle). Each sample was sequenced by Quant Studio® 5
Real-Time PCR system (Thermo Fisher Scientific, Inc.).
Statistical analysis
Clinicopathological features were analyzed by using
Chi-square test and Student's t-test. The level of significance was
set at P<0.05 for all analyses. Statistical analyses were
performed using the StatViewJ version 5.0 software package (SAS
Institute, Inc., Cary, NC, USA).
Results
The clinicopathological characteristics of patients
with SM and GM are shown in Table I.
As no specific inclusion/exclusion criteria were set, we analyzed
all patients reviewed for this study. The SM patient group
comprised 17 men and 14 women with a mean range in age of 66.7
(24–87) years. The GM patient group comprised 5 men and 5 women
with a mean range in age of 75.7 (40–94) years. The mean age of the
GM patients was higher than that of the SM patients; however, the
difference was not statistically significant.
 | Table I.Clinicopathological characteristics
of SM and GM. |
Table I.
Clinicopathological characteristics
of SM and GM.
| Characteristic | SM | GM |
|---|
| Age (mean ±
SD) | 66.7±16.8 | 75.7±14.9 |
| Gender, n (%) |
|
|
|
Male | 17 (54.8) | 5 (50.0) |
|
Female | 14 (45.2) | 5 (50.0) |
| Location, n
(%) |
|
|
|
Acral | 13 (41.9) | – |
|
CSD | 4 (12.9) | – |
|
Mucosal | 2 (6.5) | – |
|
Non-CSD | 12 (38.7) | – |
|
Oesophagus | – | 1 (10.0) |
|
Rectum | – | 4 (40.0) |
| Anal
canal | – | 4 (40.0) |
| Small
intestine | – | 1 (10.0) |
| Cell morphology, n
(%) |
|
|
|
Spindle | 24
(77.4)a | 3 (30.0) |
|
Round | 7 (22.6) | 7 (70.0) |
| Melanin, n (%) |
|
|
|
Negative | 2
(6.5)a | 5 (50.0) |
|
Positive | 29 (93.5) | 5 (50.0) |
| T-classification, n
(%) |
|
|
| 1 | 8 (25.8) | 3 (30.0) |
| 2 | 11 (35.5) | 0 (0.0) |
| 3 | 11 (35.5) | 5 (50.0) |
| 4 | 1 (3.2) | 2 (20.0) |
| N-lymph node, n
(%) |
|
|
|
Negative | 21 (67.7) | 4 (40.0) |
|
Positive | 10
(32.3)a | 6 (60.0) |
| M-metastasis, n
(%) |
|
|
|
Negative | 30 (96.8) | 8 (80.0) |
|
Positive | 1
(3.2)a | 2 (20.0) |
| UICC stage, n
(%) |
|
|
| I | 8 (25.8) | 3 (30.0) |
| II | 11 (35.5) | 0 (0.0) |
|
III | 11 (35.5) | 5 (50.0) |
| IV | 1 (3.2) | 2 (20.0) |
T4 SM of the back shows an elevated nodule on the
epithelium (Fig. 1A). Malignant
melanoma of the rectum shows a thickened glandular epithelium with
tumor cell invasion (Fig. 1B).
Findings of melanoma in situ (Fig.
1C, SM) or of junctional components (Fig. 1D, GM) adjacent to the invasive tumor
was considered evidence for identifying a lesion as primary rather
than metastatic. Melanin deposition in melanoma cells (Fig. 1E) and around tumor cells (Fig. 1F) are shown.
The morphological characteristics of SMs (Fig. 2A) and GMs (Fig. 2B) show that GMs were more likely to
display round cells (Fig. 2B) than
were SMs (Fig. 2A) (70 vs. 23%,
P=0.02). Furthermore, GM lesions were more likely to be amelanotic
(Fig. 2B) than were SMs (Fig. 2C) (50 vs. 7%, P=0.001). The incidence
of lymph-node metastasis was significantly higher in GMs than in
SMs (60 vs. 32%, P<0.04), and distant metastases were more
likely to be detected in GMs (10 vs. 6.5%, P=0.02) at the point of
resection. The percentage of UICC stage IV patients in GMs was
higher than that of AJCC stage IV patients in SMs (30 vs. 3.2%,
P=0.03).
Mitosis rates and MIB-1 labeling indices are shown
in Table II. The MIB-1 indices
tended to be higher in GMs than in SMs; however, the difference was
not statistically significant. The rate of mitoses was
significantly higher in GMs (Fig. 2D)
than in SMs (35.8 vs. 14.5, P=0.02), whereas the difference in
number of atypical mitoses was not statistically significant.
 | Table II.MIB-1 indices and mitoses. |
Table II.
MIB-1 indices and mitoses.
| Indices | SM | GM |
|---|
| MIB-1 index | 25.6±19.1 | 35.0±18.1 |
| Stage
I–II | 22.2±17.2 | 21.7±10.4 |
| Stage
III–IV | 30.8±21.5 | 40.7±18.1 |
| Normal mitosis | 10.6±15.8 |
27.1±24.7a |
| Stage
I–II | 6.5±9.6 | 2.7±2.3 |
| Stage
III–IV | 17.1±21.4 |
37.5±22.1b |
| Atypical
mitosis | 3.9±7.5 | 8.7±7.8 |
| Stage
I–II | 2.1±3.3 | 1.2±0.9 |
| Stage
III–IV | 6.6±11.0 |
11.9±2.6b |
| Total mitosis | 14.5±22.7 |
35.8±30.7a |
| Stage
I–II | 8.6±12.8 | 3.8±3.5 |
| Stage
III–IV | 23.8±31.3 |
49.4±26.2b |
Next, we compared advanced melanoma (Stage III and
IV) to early melanoma (Stage I and II) (Table II). Results for Stage III and IV GMs
were significantly higher than those for Stage I and II GMs with
respect to normal mitosis (2.7 vs. 37.5, P=0.03), atypical mitosis
(1.2 vs. 11.9, P=0.03) and total mitosis (3.8 vs. 49.4, P=0.02).
The rate of mitoses in Stage III and IV SMs tended to be higher
than that in Sage I, II; however, the difference was not
statistically significant.
Expression levels of stem cell markers nestin,
ABCB5, and SOX2 were similar between GMs and SMs (Table III). Stage III and IV SMs and GMs
were associated with higher expression of these markers than were
Stage I and II SMs and GMs. Only nestin expression was
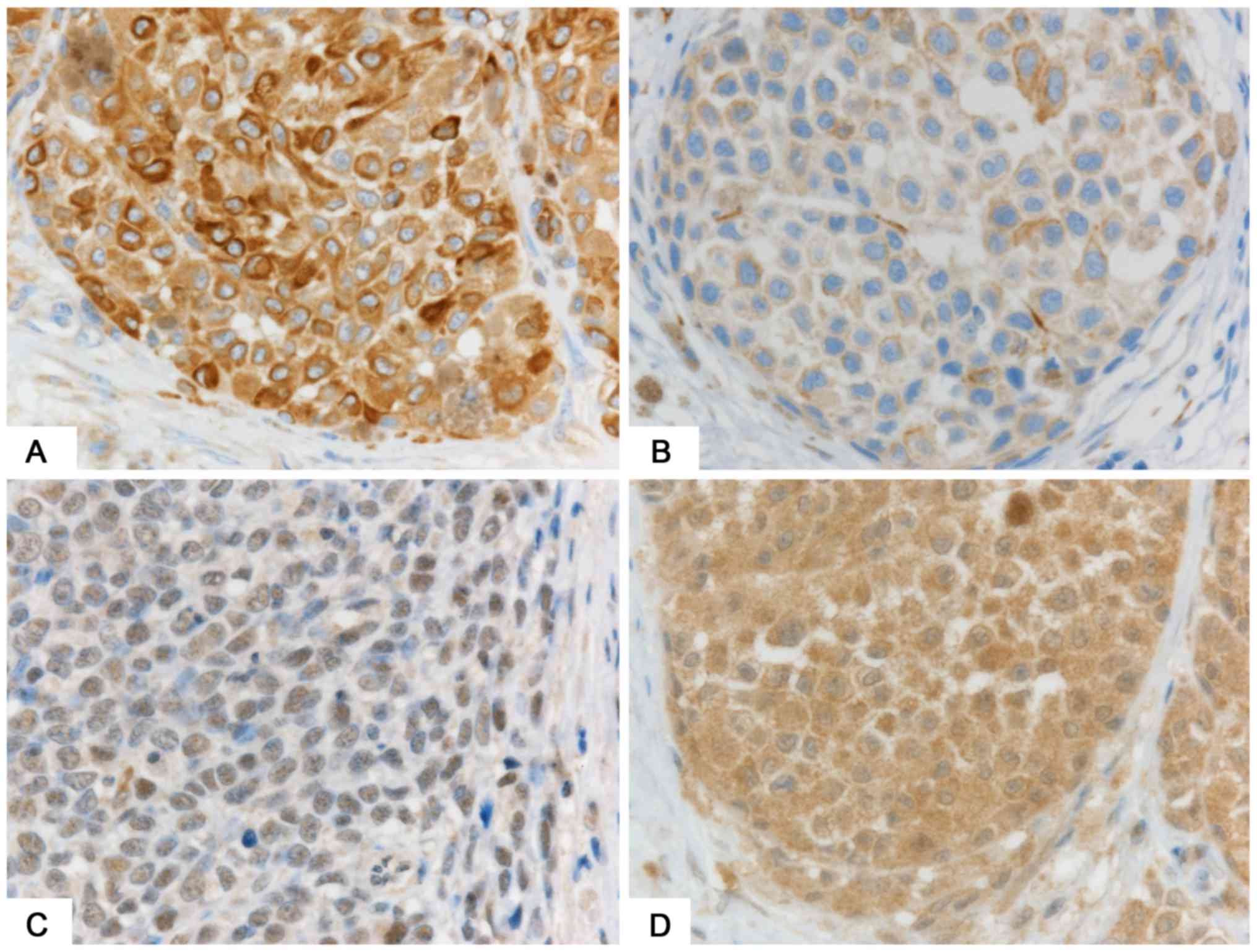
statistically significantly higher in Stage III, IV SMs (Fig. 3A) than in Stage I and II SMs (9.6 vs.
6.3, P=0.02; Fig. 3B).
 | Table III.Score of positive cells of
immunohistochemical markers. |
Table III.
Score of positive cells of
immunohistochemical markers.
| Marker | SM | GM |
|---|
| Nestin | 7.6±3.9 | 6.7±2.8 |
| Stage
I–II | 6.3±3.9 | 5.7±2.5 |
| Stage
III–IV |
9.6±2.9b | 7.1±3.0 |
| ABCB5 | 1.3±0.6 | 1.2±0.6 |
| Stage
I–II | 1.2±0.6 | 1.0±0.0 |
| Stage
III–IV | 1.4±0.7 | 1.3±0.8 |
| SOX2 | 0.9±1.3 | 0.8±1.4 |
| Stage
I–II | 3.0±3.9 | 1.5±0.5 |
| Stage
III–IV | 2.3±2.0 | 5.5±5.0 |
| BRAF | 3.3±3.3 |
1.0±1.5a |
| Stage
I–II | 2.9±3.2 | 1.0±1.0 |
| Stage
III–IV | 4.1±3.3 | 1.0±1.2 |
We used PCR to examine BRAF V600E
mutations. Mutations were positive in 6 cases (all SMs) and
negative in 15 cases (12 SMs and 3 GMs). The remaining 20 cases (13
SM and 7 GM) were not evaluated because the samples were old and
small in quantity. Immunohistochemically, the expression of
BRAFV600E was lower in GMs (Fig. 3C) than in SMs (Fig. 3D and Table
III) (1.0 vs. 3.3, P=0.01). Cases with the
BRAFV600E mutation showed higher expression of
the BRAFV600E protein than did cases without the
mutation (6.7 vs. 1.9, P=0.002) (Table
IV).
 | Table IV.Results of BRAF mutation by
polymerase chain reaction and immunohistochemistry. |
Table IV.
Results of BRAF mutation by
polymerase chain reaction and immunohistochemistry.
| Type |
BRAFV600E mutation by
PCR | Immunohistochemical
score of BRAFV600E expression |
|---|
| Wild type | 15 | 1.9±2.8 |
| Mutant type | 6 |
6.7±1.6a |
| N.D. | 20 | – |
Discussion
Our investigation revealed that, compared with SM
patients, patients with GMs were generally older and more likely to
display round cells and to be amelanotic. Moreover, they had a
higher incidence of lymph-node and distant metastases, were more
often in an advanced stage of the disease at the point of
resection, and had a lower frequency of BRAFV600E
mutations than did SM patients. In our analysis, we found the peak
age at diagnosis to be in the 60s for SMs and 70s for GMs, which is
consist with a previous report (8).
Melanoma tumor cells vary greatly in size, shape,
and morphological characteristics. Epithelioid and spindle-shaped
cells are two major SM-cell types. Generally, lentigo maligna and
acral lentiginous types tend to show a predominance of
spindle-shaped cells among their invasive dermal components,
whereas superficial-spreading and nodular lesions tend to be
composed largely of epithelioid cells (30). In previous reports, approximately 70%
of anorectal melanomas showed an epithelioid phenotype (27,31), which
is consistent with our study results. Amelanotic melanoma was found
in 50% of GMs in our study, as opposed to 21% in a previous report
concerning anorectal melanoma (31),
suggesting a higher incidence of amelanotic features in GMs than in
SMs.
Cell proliferation is one of the major
characteristics of malignant tumors. Numerous studies have shown
that mitotic rate is associated with the prognosis for SM (32,33). In
the present study, the number of normal and total mitoses were
found to be higher in GM, than in SM, suggesting that GM might be
more aggressive than SM, thereby resulting in GM's poorer
prognosis. However, the MIB-1 index did not show a significant
difference between GM and SM. We believe that this lack of a MIB-1
index difference has a few possible explanations. First, the
mitosis number was counted by the hot spot area, whereas the MIB-1
index was evaluated by the entire region on the slide. Furthermore,
the mitosis number included abnormal mitoses, which may count
alterations related to chromosomal abnormalities. Finally, the
MIB-1 index considers all cells except those in the G0 phase of the
cell cycle. These disparities between mitosis and the MIB-1 index
may have influenced our results.
Previous studies have suggested that melanoma stem
cells play important roles in cell proliferation as well as in
tumor initiation, development, recurrence, and resistance to
chemotherapy (15,17). SRY (sex-determining region Y)-box
(SOX2) is a transcription factor expressed by neural progenitor
cells that also regulates the nestin core enhancer (19). Recent reports have shown that SOX-2
contributes to cell invasion (34),
while regulating the self-renewal ability and tumorigenicity of
human melanoma-initiating cells (35). ABCB5 is a members of the ATP-binding
cassette (ABC) superfamily of transporters and is targeted in
chemotherapeutic drug-efflux pumps (20). Immunostaining of ABCB5 has been shown
to have higher expression in invasive than in in situ
melanoma, suggesting that it is a key player in the development of
melanoma (36). Various types of stem
cell markers have been identified for SM, while little is known
about stem cell markers for GM. We found expression levels of
nestin, ABCB5, and SOX2 to be similar in GM and SM, suggesting that
these stem cell marker-related proteins might play similar roles in
both diseases. However, nestin expression was higher in advanced
stages of SM than in early stages. Nestin is an intermediate
filament protein that regulates cell proliferation via
cyclin-dependent kinases and AKT (37). Similarly, it has been reported that
nestin plays important roles in cell proliferation and metastasis
of melanoma (18,38–40). Taken
together, these findings suggest that nestin plays important roles
in melanoma stem cells, and has potential as a novel targeted
therapy.
We confirmed a low frequency of
BRAFV600E mutation in GMs as compared with that
in SMs. A recent meta-analysis showed that immunohistochemistry is
highly sensitive and specific for the detection of the
BRAFV600E mutation and highly comparable to
genetic methodology in melanoma cases (41). Findings of a few analyses have
indicated that BRAF and NRAS mutations in GM are
exceedingly rare (23), whereas
activating KIT mutations are found in >30% of cases
(24,26). The present study also found a high
concordance between immunostaining and PCR in detecting BRAF
V600E. Therefore, immunohistochemical staining might
be more useful than PCR, especially with a small number of biopsy
samples.
Our study has several imitations. Primarily, the
number of cases was small and most of the patients were elderly,
especially for the GM group. However, our study might provide
useful information for making differential diagnoses of GMs.
Further study is needed to elucidate the mechanisms of GM's poor
prognosis and to achieve early detection, thereby improving patient
outcomes. In conclusion, we have identified differences in
clinicopathological characteristics between GM and SM. In addition,
we suggest a possible focus for a new molecular targeted
treatment.
Acknowledgements
The authors would like to thank Dr. Seiichi Shinji
(Surgery for Organ Function and Biological Regulation, Nippon
Medical School, Tokyo, Japan) for his support in case presentations
and Ms. Yasuko Hasegawa for her immunohistochemical work.
Funding
This work was supported by a Grant-in-Aid for Young
Scientists (grant no. 15K19705).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MA, YM and TA conceived and designed the
experiments. TA and HS contributed to the acquisition of data. MA
selected the data and performed the experiments. MA and YM analyzed
the data and wrote the paper. TA and HS critically revised the
manuscript. All authors were involved in data interpretation and
writing the manuscript, and have all read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Human Research Ethics
Committees at the Tokyo Metropolitan Geriatric Hospital (approval
no. R17-33) and the Nippon Medical School Hospital (approval no.
29–07-805); written informed consent was obtained from all
patients.
Consent for publication
Written informed consent was obtained from all
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erdmann F, Lortet-Tieulent J, Schüz J,
Zeeb H, Greinert R, Breitbart EW and Bray F: International trends
in the incidence of malignant melanoma 1953-2008-are recent
generations at higher or lower risk? Int J Cancer. 132:385–400.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E586. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee ML, Tomsu K and Von Eschen KB:
Duration of survival for disseminated malignant melanoma: Results
of a meta-analysis. Melanoma Res. 10:81–92. 2000.PubMed/NCBI
|
|
5
|
Balch CM, Gershenwald JE, Soong SJ,
Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG,
Ding S, et al: Final version of 2009 AJCC melanoma staging and
classification. J Clin Oncol. 27:6199–6206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baderca F, Vincze D, Balica N and Solovan
C: Mucosal melanomas in the elderly: Challenging cases and review
of the literature. Clin Interv Aging. 9:929–937. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nordlund JJ: The lives of pigment cells.
Clin Geriatr Med. 5:91–108. 1989.PubMed/NCBI
|
|
8
|
Chang AE, Karnell LH and Menck HR: The
National Cancer Data Base report on cutaneous and noncutaneous
melanoma: A summary of 84,836 cases from the past decade. The
American College of Surgeons Commission on Cancer and the American
Cancer Society. Cancer. 83:1664–1678. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Umeda M, Mishima Y, Teranobu O, Nakanishi
K and Shimada K: Heterogeneity of primary malignant melanomas in
oral mucosa: An analysis of 43 cases in Japan. Pathology.
20:234–241. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujisawa Y, Takahashi T, Yamamoto A,
Yamazaki N, Saida T and Ishihara K: Ohtsuka: Statistics for
malignant melanoma in Japan: A nationwide survey conductied during
the calender years 2006 2007. Skin Cancer. 23:267–279. 2008.
View Article : Google Scholar
|
|
11
|
Chen H, Cai Y, Liu Y, He J, Hu Y, Xiao Q,
Hu W and Ding K: Incidence, surgical treatment, and prognosis of
anorectal melanoma from 1973 to 2011: A population-based SEER
analysis. Medicine (Baltimore). 95:e27702016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arai T, Yanagisawa A, Kondo F, Aida J and
Takubo K: Clinicopathologic characteristics of esophageal primary
malignant melanoma. Esophagus. 13:17–24. 2016. View Article : Google Scholar
|
|
13
|
Cheung MC, Perez EA, Molina MA, Jin X,
Gutierrez JC, Franceschi D, Livingstone AS and Koniaris LG:
Defining the role of surgery for primary gastrointestinal tract
melanoma. J Gastrointest Surg. 12:731–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang D, Nguyen TK, Leishear K, Finko R,
Kulp AN, Hotz S, van Belle PA, Xu X, Elder DE and Herlyn M: A
tumorigenic subpopulation with stem cell properties in melanomas.
Cancer Res. 65:9328–9337. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schatton T, Murphy GF, Frank NY, Yamaura
K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM,
Weishaupt C, et al: Identification of cells initiating human
melanomas. Nature. 451:345–349. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brinckerhoff CE: Cancer stem cells (CSCs)
in melanoma: There's smoke, but is there fire? J Cell Physiol.
232:2674–2678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Klein WM, Wu BP, Zhao S, Wu H,
Klein-Szanto AJ and Tahan SR: Increased expression of stem cell
markers in malignant melanoma. Mod Pathol. 20:102–107. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka S, Kamachi Y, Tanouchi A, Hamada H,
Jing N and Kondoh H: Interplay of SOX and POU factors in regulation
of the Nestin gene in neural primordial cells. Mol Cell Biol.
24:8834–8846. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frank NY, Margaryan A, Huang Y, Schatton
T, Waaga-Gasser AM, Gasser M, Sayegh MH, Sadee W and Frank MH:
ABCB5-mediated doxorubicin transport and chemoresistance in human
malignant melanoma. Cancer Res. 65:4320–4333. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clark WH Jr, From L, Bernardino EA and
Mihm MC: The histogenesis and biologic behavior of primary human
malignant melanomas of the skin. Cancer Res. 29:705–727.
1969.PubMed/NCBI
|
|
22
|
Bastian BC, Olshen AB, LeBoit PE and
Pinkel D: Classifying melanocytic tumors based on DNA copy number
changes. Am J Pathol. 163:1765–1770. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Curtin JA, Busam K, Pinkel D and Bastian
BC: Somatic activation of KIT in distinct subtypes of melanoma. J
Clin Oncol. 24:4340–4346. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Curtin JA, Fridlyand J, Kageshita T, Patel
HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Bröcker EB, LeBoit PE, et
al: Distinct sets of genetic alterations in melanoma. N Engl J Med.
353:2135–2147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santi R, Simi L, Fucci R, Paglierani M,
Pepi M, Pinzani P, Merelli B, Santucci M, Botti G, Urso C and Massi
D: KIT genetic alterations in anorectal melanomas. J Clin Pathol.
68:130–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang HM, Hsiao SJ, Schaeffer DF, Lai C,
Remotti HE, Horst D, Mansukhani MM and Horst BA: Identification of
recurrent mutational events in anorectal melanoma. Mod Pathol.
30:286–296. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chapman PB, Hauschild A, Robert C, Haanen
JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, et
al: Improved survival with vemurafenib in melanoma with BRAF V600E
mutation. N Engl J Med. 364:2507–2516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Richter A, Grieu F, Carrello A, Amanuel B,
Namdarian K, Rynska A, Lucas A, Michael V, Bell A, Fox SB, et al: A
multisite blinded study for the detection of BRAF mutations in
formalin-fixed, paraffin-embedded malignant melanoma. Sci Rep.
3:16592013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clark WH Jr, Elder DE, Guerry D IV,
Braitman LE, Trock BJ, Schultz D, Synnestvedt M and Halpern AC:
Model predicting survival in stage I melanoma based on tumor
progression. J Natl Cancer Inst. 81:1893–1904. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tariq MU, Ud Din N, Ud Din NF, Fatima S
and Ahmad Z: Malignant melanoma of anorectal region: A
clinicopathologic study of 61 cases. Ann Diagn Pathol. 18:275–281.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chu VH, Tetzlaff MT, Torres-Cabala CA,
Prieto VG, Bassett R Jr, Gershenwald JE, McLemore MS, Ivan D, Wang
WL, Ross MI and Curry JL: Impact of the 2009 (7th edition) AJCC
melanoma staging system in the classification of thin cutaneous
melanomas. Biomed Res Int. 2013:8987192013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mandalà M, Galli F, Cattaneo L, Merelli B,
Rulli E, Ribero S, Quaglino P, de Giorgi V, Pigozzo J, Sileni VC,
et al: Mitotic rate correlates with sentinel lymph node status and
outcome in cutaneous melanoma greater than 1 millimeter in
thickness: A multi-institutional study of 1524 cases. J Am Acad
Dermatol. 76:264–73.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Girouard SD, Laga AC, Mihm MC, Scolyer RA,
Thompson JF, Zhan Q, Widlund HR, Lee CW and Murphy GF: SOX2
contributes to melanoma cell invasion. Lab Invest. 92:362–370.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Santini R, Pietrobono S, Pandolfi S,
Montagnani V, D'Amico M, Penachioni JY, Vinci MC, Borgognoni L and
Stecca B: SOX2 regulates self-renewal and tumorigenicity of human
melanoma-initiating cells. Oncogene. 33:4697–4708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Setia N, Abbas O, Sousa Y, Garb JL and
Mahalingam M: Profiling of ABC transporters ABCB5, ABCF2 and
nestin-positive stem cells in nevi, in situ and invasive melanoma.
Mod Pathol. 25:1169–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsuda Y, Ishiwata T, Yoshimura H,
Yamahatsu K, Minamoto T and Arai T: Nestin phosphorylation at
threonines 315 and 1299 correlates with proliferation and
metastasis of human pancreatic cancer. Cancer Sci. 108:354–361.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akiyama M, Matsuda Y, Ishiwata T, Naito Z
and Kawana S: Nestin is highly expressed in advanced-stage
melanomas and neurotized nevi. Oncol Rep. 29:1595–1599. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ladstein RG, Bachmann IM, Straume O and
Akslen LA: Nestin expression is associated with aggressive
cutaneous melanoma of the nodular type. Mod Pathol. 27:396–401.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Akiyama M, Matsuda Y, Ishiwata T, Naito Z
and Kawana S: Inhibition of the stem cell marker nestin reduces
tumor growth and invasion of malignant melanoma. J Invest Dermatol.
133:1384–1387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anwar MA, Murad F, Dawson E, Abd Elmageed
ZY, Tsumagari K and Kandil E: Immunohistochemistry as a reliable
method for detection of BRAF-V600E mutation in melanoma: A
systematic review and meta-analysis of current published
literature. J Surg Res. 203:407–415. 2016. View Article : Google Scholar : PubMed/NCBI
|