Introduction
Epstein-Barr virus (EBV), a human γ herpes virus,
may exist in humans for a long time without producing any symptoms
(1). A variety of human malignancies,
including Burkitt's lymphoma (2),
nasopharyngeal carcinoma (3) and
gastric cancer have been reported to be associated with EBV
(4). EBV-associated gastric carcinoma
(EBVaGC) has been reported to account for ≤10% of total gastric
carcinoma (5). It is difficult to
remove and eliminate EBVaGC cells thoroughly by surgical, radio and
chemotherapeutic methods. Therefore, it is necessary to identify
novel therapeutic approaches to treat EBVaGC by inhibiting tumor
cell growth or survival.
At present, traditional Chinese herbs are widely
being studied to treat numerous diseases (6,7). One herb,
artemisinin, the active constituent of Artemisia annua L.,
along with its derivatives, has been used as an effective
anti-malarial agent (8).
Dihydroartemisinin (DHA), one of the main active metabolites of
arteminisin, has been reported to exhibit antitumor activity in
various cancer cells in vitro and in vivo in mice
(9,10). DHA inhibits cell proliferation by
inducing G1 arrest and apoptosis in human nasopharyngeal carcinoma
cells. DHA, as a putative signal transducer and activator of
transcription 3 (STAT3) inhibitor, suppresses the growth of head
and neck squamous cell carcinoma by targeting Janus kinase 2/STAT3
signaling. DHA prevents breast cancer-induced osteolysis by
inhibiting breast cancer cells and osteoclasts. DHA combined with
holotransferrin, which increases the concentration of ferrous iron
in cancer cells, was reported to effectively kill a type of
radiation-resistant human breast cancer cell in vitro
(11). However, there are few studies
about the effect of DHA on EBVaGC.
EBVaGC expresses a well-defined set of latent viral
genes, including latent membrane protein 2A (LMP2A). It was
reported that LMP2A is expressed in ~50% of EBVaGCs
(12). As a transmembrane protein, it
functions in multiple signal transduction pathways and is involved
in the tumorigenic processes in EBVaGC (12). LMP2A is associated with DNA
methyltransferases and induces expression of phosphorylated-STAT3,
which causes upregulation of DNA methyltransferase (DNMT)1
(13) and DNMT3B (14) in EBVaGC cells. Downregulating
LMP2A may also inhibit apoptosis through upregulation of the
cellular survivin gene via the nuclear factor-κB pathway (15). Therefore, LMP2A may be a
potential target for treatment of EBVaGC.
In the present study, the effect of DHA on the
growth of EBVaGC cells was explored and the LMP2A-associated
mechanisms were investigated, with the aim of finding a potential
candidate for EBVaGC therapy.
Materials and methods
Cell line and culture
The EBVaGC GT-38 cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). Cells were
maintained at 37°C in RPMI-1640 medium supplemented with 10% fetal
bovine serum (MP Biomedicals, LLC, Santa Ana, CA, USA), 100 U/ml
penicillin and 100 mg/l streptomycin in a humidified environment
with 5% CO2.
MTT assay
GT-38 cells were seeded onto 96-well plates at
6×104/ml. No DHA was added to the negative control
group; equal amounts of PBS were added instead, and 5, 10, 20 and
40 µg/ml DHA were added to the experimental groups of GT-38 cells
for 48 h. Each group was repeated six times. A total of 20 µl MTT
solution (5 mg/ml) was added to each well following 0, 12, 24, 36,
48 and 60 h of incubation at 37°C, respectively, and the plates
were incubated for 4 h at 37°C. Dimethyl sulfoxide (DMSO; 100 µl)
was then added to each well, and the plates were rotated for 15
min. The absorbance was measured at 450 nm with a microplate reader
(SPECTRA MAX 190; Molecular Devices, LLC, Sunnyvale, CA, USA). The
inhibition of cancer cell viability was calculated as follows:
Inhibition rate (%)=(1-optical density
(OD)treatment/ODcontrol) ×100.
Apoptosis assay
According to protocol of the Annexin V-FITC
Apoptosis Detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), following treatment with 0 and 20 µg/ml DHA for 48 h,
GT-38 cells were harvested by centrifugationat 500 × g for 5 min at
room temperature, resuspended in binding buffer and successively
incubated with 5 µl Annexin V-fluorescein isothiocyanate and 5 µl
propidium iodide (PI) for 15 min at room temperature. A flow
cytometer (FACS Calibur; BD Biosciences, Franklin Lakes, NJ, USA)
was then used to analyze the apoptosis results.
Analysis of cell cycle by flow
cytometry
Following pre-incubation with Dulbecco's modified
Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 24 h, 0 and 20 µg/ml DHA was added to GT-38 cells for 48
h. The cells were centrifuged at 500 × g for 5 min at room
temperature, fixed in 70% cooled ethanol, and then dyed with PI for
30 min in the dark. The cells were measured by a flow cytometer
(FACS Calibur; BD Biosciences), and the data on the percentage of
cells at each stage of the cell cycle was harvested.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from groups of GT-38 cells,
which were treated with 0 and 20 µg/ml DHA for 48 h, using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
then treated with DNaseI (Roche Diagnostics, Basel, Switzerland) to
remove contaminating genomic DNA prior to the preparation of cDNA.
Reverse transcription was performed using the cDNA Synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) The following reagents
were added into a sterile, nuclease free tube on ice in the
following order: 1 µl total RNA, 1 µl Oligo (dT) 18 primer, 10 µl
nuclease-free water, 4 µl 5X Reaction Buffer, 1 µl RiboLock RNase
Inhibitor (20 U/µl), 2 µl 10 mM dNTP Mix and 1 µl RevertAid M-MuLV
RT (200 U/µl). The total volume was 20 µl. The mix was incubated
for 5 min at 25°C followed by 60 min at 42°C. The reaction was
terminated by heating at 70°C for 5 min. cDNA (5 µl) from each
sample was used to perform PCR. The sequences of primers for
LAMP2A were as follows (13),
forward: 5′-ATGACTCATCTCAACACATA-3′ (nt.166874-166893), reverse:
5-CATGTTAGGCAAATTGCAA-3 (nt.166380-166361). The product size of
LMP2A was 280 bp. Glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was used as a control, and the primers of GAPDH were as
follows, forward: 5′-ACGGATTTGGTCGTATTGGG-3′ and reverse:
5′-TGATTTTGGAGGGATCTCGC-3′. The qPCR was performed with 1 µl cDNA
using SYBR Green Taq Mix (Takara Bio, Inc., Otsu, Japan) on ABI
PRISM 7500 Real-time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The expression of target gene was evaluated
using a relative quantification method (2−∆∆Cq)
(16), with GAPDH as the internal
reference. The thermocycling conditions were as follow: 95°C for 5
min, followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec and
72°C for 1 min, with a final extension step of 72°C for 5 min.
Western blot analysis
Protein was extracted from GT-38 cells treated with
0 or 20 µg/ml DHA for 48 h using radioimmunoprecipitation assay
buffer (150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% nonidet
P-40 and 50 mM Tris pH 8.0) with the addition of 2 mM
phenylmethylsulfonyl fluoride. Protein concentrations were
determined with a The QuantiProbicinchoninic acid (BCA) Assay kit
(Sigma-Aldrich; Merck KGaA), and 20 µg protein was loaded on 15%
SDS-PAGE gels and then transferred to polyvinylidene fluoride
membranes for western blotting. Membranes were blocked at 4°C
overnight with 5% skim milk, and incubated with a 1:200 dilution of
rat anti-EBV LMP2A antibody at 4°C overnight (cat. no.
ab59028; Abcam, Cambridge, UK). The blots were washed with
phosphate buffered saline (PBS) with 0.05% Tween-20 3 times and
then incubated with a donkey anti-rat secondary antibody conjugated
with horseradish peroxidase, diluted by 1:2,000 (cat. no. A18739;
Thermo Fisher Scientific, Inc.).
In vivo tumor growth xenograft
models
A total of 12 BALB/c-nude mice (4–6-week-old; male;
SPF degree, and 20–24 g of body weight) were purchased from the
Sino-British Sippr/BK Lab Animal Co., Ltd., (Shanghai, China).
Animals were housed in a specific pathogen-free room within the
animal facilities, and were maintained at the average temperature
of 23°C and the relative humidity of 40–70% in a 12-h light/dark
cycle. The access to the food was restricted. All animals were
allowed to acclimate to their new environment for 1 week prior to
the initiation of the model. Mice were randomly divided into 2
groups (6 mice/group). The animal use protocol was approved by the
Institutional Animal Care and Use Committee of Xi'an Jiaotong
University Second Affiliated Hospital (Xi'an, China). The GT-38
xenograft tumor model was developed by injecting a 5×106
GT-38 cell suspension into the right flank of the BALB/c-nude mice.
Tumor nodules were allowed to grow to a volume of ~150
mm3 prior to initiating treatment. Tumor-bearing
BALB/c-nude mice were randomly assigned to two groups and treated
with 25 mg/kg DHA or DMSO. The tumor volume and total body weight
were measured every 2 days. The tumor volume was calculated using
the following equation: Tumor volume (V)=length × width × width ×
0.52. These experiments were replicated 3 times.
Statistical analysis
The continuous variables are presented as the mean ±
standard deviation. The statistical analysis was performed using
Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
The differences between the control group and experimental groups
were determined by one-way analysis of variance (ANOVA) followed by
a Student Newman-Keuls post-hoc test. As treatment and time course
were investigated, two-way ANOVA followed by a Student Newman-Keuls
post-hoc test was also used. P<0.05 was considered to indicate a
statistically significant difference.
Results
DHA inhibits GT-38 cell viability
The MTT assay revealed that DHA inhibited the
viability of GT-38 cells in a dose-dependent manner (Fig. 1A). With increasing concentrations of
DHA, the absorbance at 450 nm decreased and the rate of inhibition
increased (P<0.05; n=6; Fig. 1A).
It was also observed that DHA exerted a time-dependent inhibition
of viability in GT-38 cells (Fig.
1B). Following treatment with DHA for 0, 12, 24, 36, 48 and 60
h, the absorbance at 450 nm decreased and the rate of inhibition
increased (P<0.05; n=6; Fig. 1B).
Since there was no significant difference in cell growth inhibition
between 48 and 60, 48 h was selected as the DHA treatment time for
the subsequent experiments.
DHA treatment induces apoptosis and
alters the cell cycle profile in GT-38 cells
Flow cytometric analysis revealed that the apoptotic
rate was 6.25±0.38% and17.33±0.79% following treatment with 0 and
20 µg/ml DHA for 48 h, respectively (Fig.
2). The proportion of apoptosis was significantly increased in
the 20 µg/ml DHA group compared with the control group (P<0.05;
n=6; Fig. 2).
It was revealed that the proportion of
G0/G1 phase cells was 19.15±1.28% following
treatment with 20 µg/ml DHA for 48 h, while the proportion of
G0/G1 phase cells was 6.75±0.37% in the
control group. The present results also demonstrated that the
percentage of G2/M phase cells was 18.33±0.49%
subsequent to being treated with 20 µg/ml DHA for 48 h, while the
percentage of G2/M phase cells was 31.15±1.78% in the
control group (Fig. 2A and B). The
frequency of GT-38 cells in the G0/G1 phase
was significantly increased while the frequency in the
G2/M was decreased following treatment with 20 µg/ml
DHA, as compared with the control group (P<0.05; n=6; Fig. 2C and D). No significant difference was
observed in the S-phase phase between the control group and the DHA
treated group.
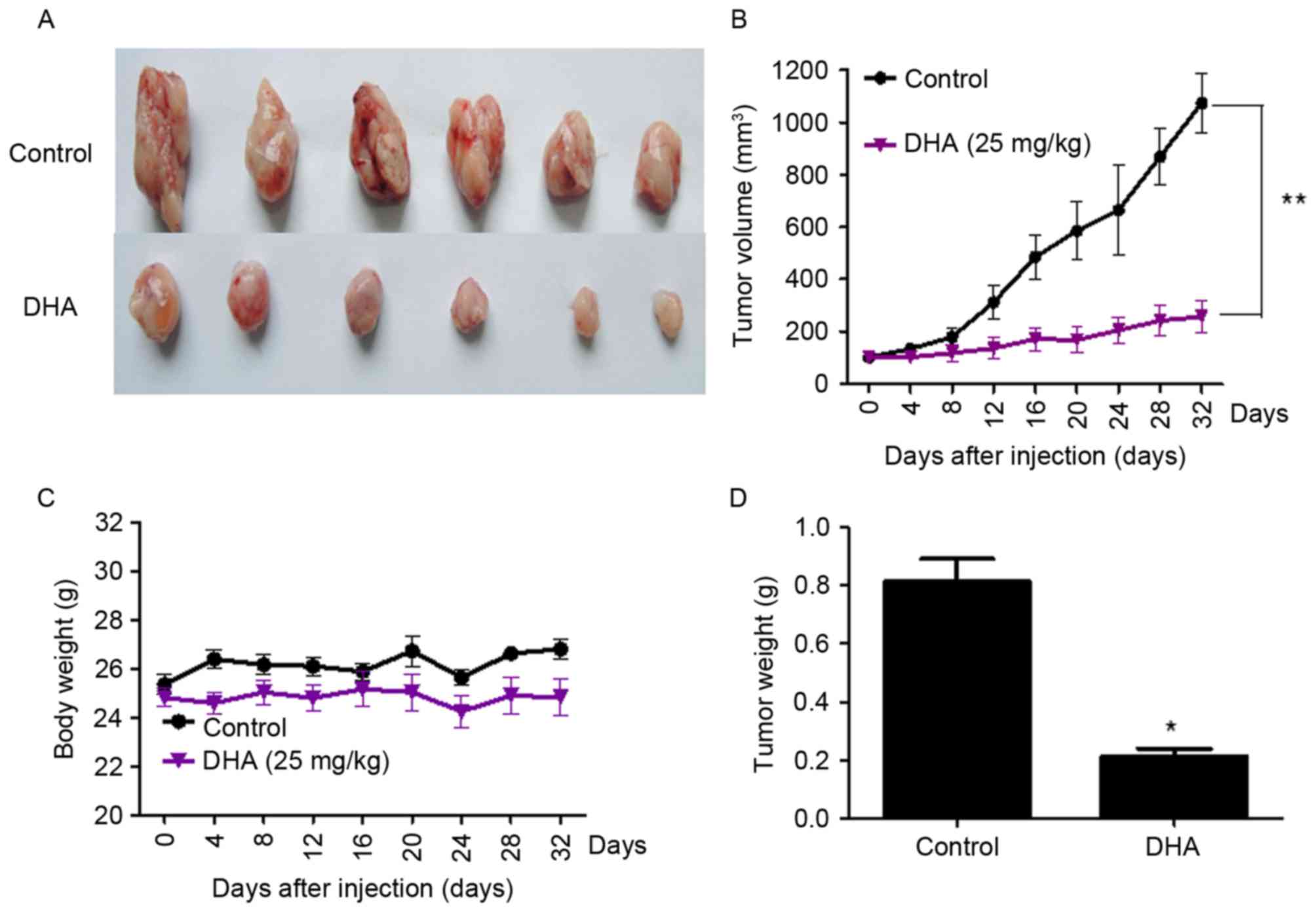
DHA treatment inhibits the growth of
GT-38 cell-transplanted tumors in vivo
Subcutaneous tumors were observed in all nude mice
(n=12) inoculated with GT-38 cells (tumorigenicity rate of 100%).
Tumor-bearing mice treated with 25 mg/kg DHA exhibited a
significant reduction in tumor volume compared with untreated mice.
Tumor volumes were 1195.40±21.3 mm3 and 220.98±12.5
mm3 in untreated and DHA-treated mice bearing GT-38
tumors, respectively (P<0.01; n=6; Fig. 3). No significant changes of mice body
weight between the control and DHA treated group were observed,
which indicated that DHA treatment in vivo had no
significant toxicity (Fig. 3).
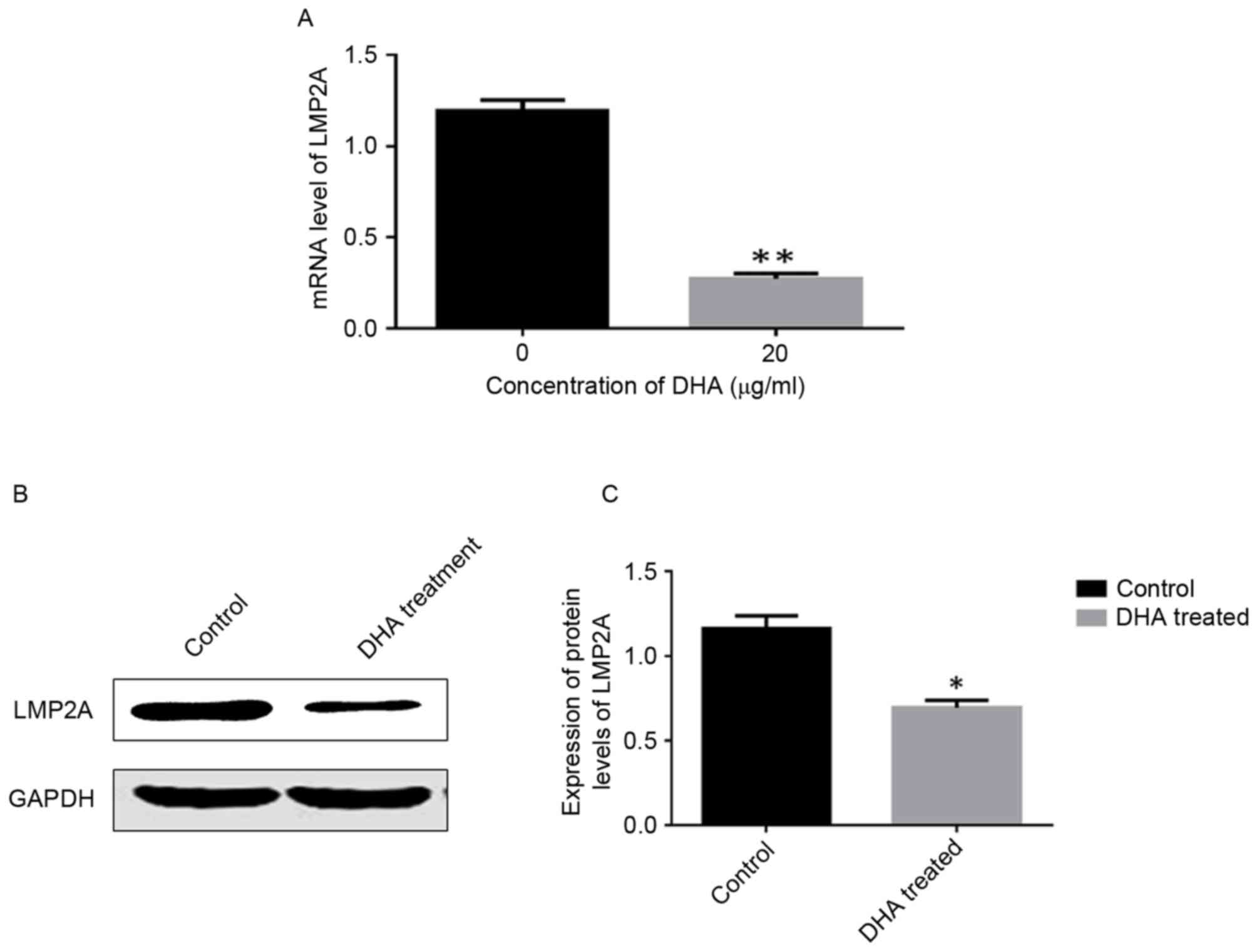
DHA treatment downregulates LMP2A
expression in GT-38 cells
The RT-PCR results revealed significantly lower
LMP2A mRNA expression levels in DHA-treated cells compared
with untreated cells (P<0.05; Fig.
4A). Western blotting results demonstrated that the protein
expression levels of LMP2A were significantly lower in
DHA-treated cells compared with control cells. (P<0.05; Fig. 4B and C).
Discussion
The morbidity and mortality of gastric cancer has
decreased in developed countries due to improved prognosis for
early stage cancer (17). However, it
remains the second leading cause of cancer-associated mortality
globally (17). EBVaGC comprised 10%
of gastric carcinoma cases in 2014, which is estimated to exceed
75,000 cases/year across the world (18). Treatment of recurrent and metastatic
disease remains a challenge, particularly in developing
countries.
DHA, a semi-synthetic derivative of artemisinin
isolated from the traditional Chinese herb A. annua, is used
as a first-line anti-malarial drug with low toxicity (19). In previous years, artemisinin and its
derivatives have been identified as a promising drug to induce
cancer cell apoptosis and inhibit viral infection (20). It was reported that DHA may inhibit
pancreatic cancer cells in vitro through downregulating the
expression of proliferating cell nuclear antigen and cyclin D1,
upregulating cyclin dependent kinase inhibitor 1 expression and
inducing apoptosis by reducing the ratio of B-cell lymphoma-2
(Bcl-2)/Bcl-2 associated X protein and increasing the activation of
caspase-9 in a dose-dependent manner (9). It was also reported that DHA inhibited
pancreatic cancer cells in subcutaneous BxPC-3 xenograft tumors in
mice (9). Furthermore, DHA may also
inhibit cervical cancer cells by upregulation of kinase inhibitor
protein, a suppressor of metastasis, and downregulation of Bcl-2
(10). In addition, Sun et al
(21) reported that the proliferation
rate and colony-forming abilities of gastric cancer cells were
significantly suppressed by DHA, together with significant
suppression of the expression of proliferation markers
(proliferating cell nuclear antigen, cyclin E and cyclin D1) and
the upregulation of p21 and p27. However, it remains unknown
whether artemisinin and its derivatives work as growth inhibitors
in EBVaGC cells.
The present study investigated the inhibitory effect
of DHA on EBVaGC cells. The present data demonstrated that DHA
significantly inhibited the viability of EBVaGC cells in a
dose-dependent and time-dependent manner. This was in accordance
with previous studies, which also demonstrated a dose-dependent and
time-dependent inhibition of different types of cancer cell
viability following DHA treatment (9–11). The
present results also demonstrated that DHA significantly increased
the apoptotic rate of GT-38 cells. The same cancer cell inhibitory
effect of DHA was also reported by Lee (22), and they reported that DHA was
effective in inhibiting ovarian cancer cell proliferation at doses
of micromolar levels and in a time-dependent manner. The present
results also demonstrated that DHA treatment altered the cell cycle
profile of GT38 cells, resulting in a significant increase in the
percentage of G0/G1 phase cells. As more GT38
cells were arrested in the G0/G1 phase, the
potency of cancer cells to divide and proliferate was decreased.
Another study also reported that DHA may suppress the growth of
rhabdomyosarcoma cells through arresting the cell cycle at the
G0/G1 phase (23).
LMP2A is an EBV-encoded transmembrane
protein, which functions in numerous signal transduction pathways
and is involved in EBVaGC tumorigenesis (12,24). To
understand the mechanism of DHA on the apoptosis of EBVaGC cells,
the effect of DHA on LMP2A expression was studied. The
present data revealed that the mRNA and protein levels of
LMP2A were significantly downregulated in the DHA-treated
group compared with the control group. LMP2A may be detected
in the majority of EBVaGC and functions in maintaining latent
pattern, promoting viral gene expression, inhibiting apoptosis and
serving an important function in the pathogenesis of EBVaGC
(13–15,25–27). It
was observed that DHA downregulated them RNA and protein levels of
LMP2A, and the decreasing LMP2A expression induced
the observed cell viability inhibition and cell apoptosis of EBVaGC
cells. DHA may therefore inhibit cell viability and induce cell
apoptosis of EBVaGC cells via downregulating the expression of
LMP2A. It was reported that LMP2A upregulates the
expression of survivin, which confers resistance to serum
deprivation-induced apoptosis in EBV-infected gastric carcinoma
cells (15). However, additional
studies are required to investigate the mechanism of how
LMP2A mediates the suppressing effect of DHA on GT-38 cell
viability.
In summary, the present study demonstrated that DHA
may significantly suppress cell growth and induce apoptosis in
EBVaGC cells by downregulating LMP2A expression. This
provides an attractive therapeutic candidate for treating human
EBVaGC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XLW and QL conceived and designed the study. WG, LZ,
HY, WKP, YW and QY performed the experiments. WG wrote the paper.
WKP, YW, XLW and QL reviewed and edited the manuscript. All authors
read and approved the manuscript.
Ethics approval and consent to
participate
The animal use protocol was approved by the
Institutional Animal Care and Use Committee of Xi'an Jiaotong
University Second Affiliated Hospital (Xi'an, China).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Kieff E: Epstein-Barr virus and its
replicationFundamental Virology. Fields BN, Knipe DM and Howley PM:
Lippincott-Raven; Philadelphia, PA: pp. 1109–1162. 1996
|
|
2
|
Epstein MA, Achong BG and Barr YM: Virus
particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet.
1:702–703. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunvén P, Klein G, Henle G, Henle W and
Clifford P: Epstein-Barr virus in Burkitt's lymphoma and
nasopharyngeal carcinoma. Antibodies to EBV associated membrane and
viral capsid antigens in Burkitt lymphoma patients. Nature.
228:1053–1056. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shibata D and Weiss LM: Epstein-Barr
virus-associated gastric adenocarcinoma. Am J Pathol. 140:769–774.
1992.PubMed/NCBI
|
|
5
|
Wu MS, Shun CT, Wu CC, Hsu TY, Lin MT,
Chang MC, Wang HP and Lin JT: Epstein-Barr virus-associated gastric
carcinomas: Relation to H. pylori infection and genetic
alterations. Gastroenterology. 118:1031–1038. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang Y, Gao H and Turdu G: Traditional
Chinese medicinal herbs as potential AChE inhibitors for
anti-Alzheimer's disease: A review. Bioorg Chem. 75:50–61. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen CY, Jiang JG, Yang L, Wang DW and Zhu
W: Anti-ageing active ingredients from herbs and nutraceuticals
used in traditional Chinese medicine: pharmacological mechanisms
and implications for drug discovery. Br J Pharmacol. 174:1395–1425.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chaturvedi D, Goswami A, Saikia PP, Barua
NC and Rao PG: Artemisinin and its derivatives: A novel class of
anti-malarial and anti-cancer agents. Chem Soc Rev. 39:435–454.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen H, Sun B, Pan S, Jiang H and Sun X:
Dihydroartemisinin inhibits growth of pancreatic cancer cells in
vitro and in vivo. Anticancer Drugs. 20:131–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu CJ, Zhou L and Cai Y:
Dihydroartemisinin induces apoptosis of cervical cancer cells via
upregulation of RKIP and downregulation of bcl-2. Cancer Biol Ther.
15:279–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh NP and Lai H: Selective toxicity of
dihydroartemisinin and holotransferrin toward human breast cancer
cells. Life Sci. 70:49–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo B, Wang Y, Wang XF, Gao Y, Huang BH
and Zhao P: Expression of Epstein-Barr virus genes in
EBV-associated gastric carcinomas. World J Gastroenterol.
11:629–633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hino R, Uozaki H, Murakami N, Ushiku T,
Shinozaki A, Ishikawa S, Morikawa T, Nakaya T, Sakatani T, Takada K
and Fukayama M: Activation of DNA methyltransferase 1 by EBV latent
membrane protein 2A leads to promoter hypermethylation of PTEN gene
in gastric carcinoma. Cancer Res. 69:2766–2774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Liang Q, Cheung KF, Kang W, Lung
RW, Tong JH, To KF, Sung JJ and Yu J: Genome-wide identification of
Epstein-Barr virus-driven promoter methylation profiles of human
genes in gastric cancer cells. Cancer. 119:304–312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hino R, Uozaki H, Inoue Y, Shintani Y,
Ushiku T, Sakatani T, Takada K and Fukayama M: Survival advantage
of EBV-associated gastric carcinoma: Survivin up-regulation by
viral latent membrane protein 2A. Cancer Res. 68:1427–1435. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Gao Y, Luo B and Zhao Y:
Construction and antiapoptosis activities of recombinant adenoviral
expression vector carrying EBV latent membrane protein 2A.
Gastroenterol Res Pract. 2011:1828322011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crew KD and Neugut AI: Epidemiology of
gastric cancer. World J Gastroenterol. 12:354–362. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yau TO, Tang CM and Yu J: Epigenetic
dysregulation in Epstein-Barr virus-associated gastric carcinoma:
Disease and treatments. World J Gastroenterol. 20:6448–6456. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dhingra V, Rao Vishweshwar K and Narasu
Lakshmi M: Current status of artemisinin and its derivatives as
antimalarial drugs. Life Sci. 66:279–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun H, Meng X, Han J, Zhang Z, Wang B, Bai
X and Zhang X: Anti-cancer activity of DHA on gastric cancer-an in
vitro and in vivo study. Tumour Biol. 34:3791–3800. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee S: Artemisinin, promising lead natural
product for various drug developments. Mini Rev Med Chem.
7:411–422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiao Y, Ge CM, Meng QH, Cao JP, Tong J and
Fan SJ: Dihydroartemisinin is an inhibitor of ovarian cancer cell
growth. Acta Pharmacol Sin. 28:1045–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Odaka Y, Xu B, Luo Y, Shen T, Shang C, Wu
Y, Zhou H and Huang S: Dihydroartemisinin inhibits the mammalian
target of rapamycin-mediated signaling pathways in tumor cells.
Carcinogenesis. 35:192–200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Longnecker R: Epstein-Barr virus latency:
LMP2, a regulator or means for Epstein-Barr virus persistence? Adv
Cancer Res. 79:175–200. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
zur Hausen A, Brink AA, Craanen ME,
Middeldorp JM, Meijer CJ and van den Brule AJ: Unique transcription
pattern of Epstein-Barr virus (EBV) in EBV-carrying gastric
adeno-carcinomas: Expression of the transforming BARF1 gene. Cancer
Res. 60:2745–2748. 2000.PubMed/NCBI
|
|
27
|
Miller CL, Lee JH, Kieff E and Longnecker
R: An integral membrane protein (LMP2) blocks reactivation of
Epstein-Barr virus from latency following surface immunoglobulin
crosslinking. Proc Natl Acad Sci USA. 91:772–776. 1994. View Article : Google Scholar : PubMed/NCBI
|