Introduction
Lung cancer is one of the most common malignant
tumors in the world; it has a low 5-year survival rate of between
16–18% (1), and ranks first globally
for morbidity and mortality (2).
Approximately 80% of incidences of lung cancer are of non-small
cell lung cancer (NSCLC), the majority of which are middle or late
stage (IIIB/IV stage) when they are diagnosed, with corresponding
poor patient outcomes (3,4). The objective response rate of
platinum-based chemotherapy is only 30–40%, and median
progression-free survival is ~10 months (5). This treatment modality also induces
severe adverse reactions, which restricts clinical use (6). Molecular targeted drugs such as
erlotinib appear to demonstrate good clinical efficacy in the
treatment of advanced NSCLC (7).
Molecular targeted therapy has become a promising treatment
strategy for Asian patients with NSCLC. Unfortunately, following
8–12 months of effective treatment, patients treated with EGFR-TKIs
will inevitably develop secondary resistance to EGFR-TKIs,
resulting in tumor recurrence or metastasis (8). Therefore, resistance to erlotinib
results in the failure of therapy. For these aforementioned
reasons, the study of EGFR-TKI resistance in lung cancer is an
important direction of targeted therapy. β-elemene (β-ELE) is a
novel anticancer drug extracted from Curcuma zedoaria Roscoe
that has been widely used to treat malignant tumors (9). Recent studies have reported that β-ELE
can reverse the drug resistance of tumor cells (9,10). To the
best of our knowledge, at the time of writing there are no reports
concerning the reversal of erlotinib resistance by β-ELE in human
NSCLC cells. Therefore, the present study investigated the effects
and possible mechanism of action of β-ELE on the
erlotinib-resistant human NSCLC A549/erlotinib resistant (ER) cell
line in vitro. The results of the present study describe a
mechanism of β-ELE function, involving the decreased expression of
P-glycoprotein (P-gp), inhibition of P-gp-dependent drug efflux and
the increased intracellular concentration of anticancer drugs,
leading to the reversal of drug resistance in A549/ER cells.
Materials and methods
Reagents and equipment
The human lung adenocarcinoma cell line A549 was
purchased from Shanghai Fuxiang Biotechnological Co., Ltd,
(Shanghai, China) and its acquired erlotinib-resistant cell line
(A549/ER) was obtained from the Second Affiliated Hospital Tumor
Research Institute of Third Military Medical University (Chongqing,
China). The following reagents were used in the present study:
β-elemene (Dalian Huali Jingang Pharmaceutical Co., Ltd. Dalian,
China); erlotinib (Roche Applied Science, Penzburg, China); Roswell
Park Memorial Institute (RPMI)-1640 culture medium and fetal bovine
serum (FBS) (both Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA); penicillin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany); Streptomycin (Sigma-Aldrich; Merck KGaA);
radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime
Institute of Biotechnology Co., Ltd., Haimen, China); MTT cell
proliferation assay kits (Ameresco, Inc., Framingham, MA, USA);
dimethyl sulfoxide (DMSO; Shanghai Biological Engineering Co.,
Ltd., Shanghai, China); Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double dye kits (EMD Millipore,
Billerica, MA, USA); enhanced chemiluminescence (ECL) reagent kits,
PI and Rhodamine 123 (Rh123) (Sigma-Aldrich; Merck KGaA); mouse
anti-human monoclonal antibodies against P-gp (Abcam, Cambridge,
UK; cat. no., ab10333), β-actin (Abcam; cat. no., ab11003) and
horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin
(IgG) (Abcam; cat. no., ab6789). Inverted microscope (Olympus);
Western blot transfer system, Western blot electrophoresis
apparatus, Microplate Reader Model 680 and Gel imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA); and flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA).
Cell culture
Human NSCLC A549 and A549/ER cells were cultured in
RPMI-1640 medium supplemented with 10% FBS, 100 U/ml penicillin and
100 mg/l streptomycin in an atmosphere of 5% CO2 at
37°C. Cells in the exponential growth phase were used in the
following experiments.
MTT drug sensitivity assay
The sensitivity of A549 and A549/ER cells to
erlotinib was determined by MTT colorimetric assay. Exponentially
growing cells were plated in triplicate in 96-well plates at a
density of 5×103 cells per well and treated with
RPMI-1640 culture medium containing different concentrations of
erlotinib (0.01, 0.1, 1, 10 and 100 µmol/l) at 37°C for 24 or 48 h.
Following this, 20 µl MTT dye (5 mg/ml) was added for 4 h and then
100 µl DMSO per well was added with low speed oscillation for 10
min. Spectrometric absorbance at 570 nm wavelength was conducted
using a microplate reader to measure absorbance value of each well.
The experiment was repeated three times and selected averaged data
to generate a growth curve. The cell proliferation inhibitory rate
(%)=(1-value of the experimental group/value of the control group)
×100. The half-maximal inhibitory concentration (IC50)
was calculated by linear regression. The fold of drug
resistance=IC50 of resistant cells/IC50 of
sensitive cells (9).
MTT cytotoxicity assay
Exponentially growing cells were plated in
triplicate in 96-well plates at a density of 5×103 cells
per well. A final concentration of 5, 10, 20, 40 or 80 µg/ml β-ELE
was added to the experimental groups (RPMI-1640 culture medium was
used as vehicle control) was added to the control group at 37°C for
24 or 48 h. Subsequent MTT colorimetric assay was used to detect
β-ELE cytotoxicity as aforementioned. Using the linear regression
equation to calculate the IC50 and IC10 of
β-ELE incubation 24 or 48 h in A549/ER cells. In general,
non-cytotoxic doses are used as the reverse dose. It was identified
that there was no marked cytotoxic effect on the sensitive strain
and the resistant strain cells when the cell proliferation
inhibitory rate was <10% (IC10) (11). Therefore, the IC10 was set
as the non-toxic upper limit dose. Finally, a dose, which was lower
than IC10, was determined as the optimal reversal
concentration of β-ELE for subsequent experiments.
MTT drug resistance reversal
assay
Exponentially growing cells were plated in
triplicate in 96-well plates at a density of 5×103 cells
per well. A varying final concentration of 0.01, 0.1, 1, 10 and 100
µmol/l erlotinib and 15 µg/ml β-ELE which was determined by the
above method were added to the experimental group at 37°C for 24 h.
The same concentration gradient of erlotinib was added to the
positive control group, whereas PBS was added to the negative
control group. Subsequently, an MTT assay was performed. According
to IC50 to calculate reversal of drug resistance: Fold
reversal, FR=IC50 of resistant cells prior to
reversal/IC50 of the resistant cells following
reversal.
Apoptosis assay by annexin V-FITC/PI
staining
Exponentially growing A549/ER cells were plated in
triplicate in 96-well plates at a density of 5×103 cells
per well. The experiment groups were as follows: i) β-ELE
single-drug group, in which the final using concentration of β-ELE
was 15 µg/ml; ii) the erlotinib single-drug group, in which the
final concentration of erlotinib was 10 µmol/l; iii) the combined
β-ELE and erlotinib drug group, in which the concentrations used
was the same as single drug group; iv) the negative control group
containing only RPMI-1640 medium. After 24 h, the cells were
collected by centrifugation at 20–25°C and 1,000 × g for 5 min, and
100 µl Binding Buffer (included in the Annexin V-FITC/PI staining
apoptosis detection kit, EMD Millipore) suspension cell was added.
Next, 10 µl (20 µg/ml) Annexin V-FITC and 20 µl (50 µg/ml) PI were
added to cells for 5 to 15 min. Following this, 400 µl Binding
Buffer was added to the cells, which were assessed by flow
cytometry within 1 h. The excitation wavelength was set as 488 and
525 nm was set as the emission wavelength. The experiment was
repeated three times using CellQuest Pro software (version 5.1 BD
CellQuest Pro Software, BD Biosciences) to collect and analyze
data.
PI staining and flow cytometric
analysis of the effect of β-ELE on the cell cycle of A549/ER
cells
Exponentially growing erlotinib resistant A549/ER
cells were seeded in triplicate in 96-well plates at a density of
5×103 cells per well. The same aforementioned four
experimental group treatments were used. After 24 h, the cells were
collected by centrifugation at 20–25°C and 1,000 × g for 5 min.
Subsequently, 5 ml precooled 70% ethanol was added to fix the
samples overnight at 4°C. Next, the cells were incubated with 1 ml
PI for 15 min at 20–25°C, and the cells were assessed by flow
cytometry as aforementioned.
Rh123 retention assay
Exponentially growing A549/ER cells were plated in
triplicate in 96-well plates at a density of 5×103 cells
per well. The experiment groups were as follows: i) Non-toxic β-ELE
dose group using the final concentration of 15 µg/ml β-ELE, which
was determined as aforementioned; ii) the control group containing
RPMI-1640 only. After culturing for 24 h, the cells were
resuspended and 1×105/ml cell suspension was added into
a BD Falcon tube at 37°C for 30 min and 5 µg/ml Rh123 was added.
After incubation at 37°C for 30 min, cells were collected and
centrifuged at 1,500 × g for 2 min at 20–25°C. The supernatant was
aspirated and cell pellets were resuspended with the cold
RPMI-1640, and assessed by flow cytometry at an excitation
wavelength of 488 nm and emission wavelength of 530 nm. The mean
fluorescence intensity (MFI) was calculated to detect changes in
concentrations of Rh123 and thus evaluate the activity of
P-glycoprotein (P-gp).
Western blot analysis
Exponentially growing A549/ER cells were plated in
triplicate in 96-well plates at a density of 5×103 cells
per well. The experiment was divided into two groups as
aforementioned: Cells treated with non-toxic doses of β-ELE and the
vehicle control group. Cells were lysed using RIPA lysis buffer
following 24 h of treatment, and centrifuged at 13,000 × g and 4°C
for 10 min. The protein concentration quantification was detected
by the BCA method. A total of 50 µg protein per lane was separated
by 12% SDS-PAGE and transferred to polyvinylidene difluoride
membranes. Next, the membranes were blocked with 5% skim milk for 2
h, washed in tris-buffered saline plus 0.05% Tween-20 (TBST), and
incubated with mouse anti-human monoclonal antibodies against P-gp
(1:1,000 dilution) or β-actin (1:2,000 dilution) overnight at 4°C.
Afterwards, HRP-labeled secondary antibodies (1:2,000 dilution)
were added for 2 h at room temperature, followed visualization
using an ECL kit method. The expression of P-gp protein was
quantified using densitometry analysis [ChemiDoc™ XRS+
gel imaging system with Image Lab™ Software (version
2.0; Bio-Rad Laboratories, Inc.)].
Statistical analysis
Statistical analysis was performed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. Comparisons between multiple groups were
performed using one-way analysis of variance, followed by a
Dunnett's post-hoc test. Comparisons between the unpaired two
groups were performed using Student's t-test or χ2 test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Drug sensitivity of A549 and A549/ER
cells to erlotinib
To verify the differential drug sensitivity of A549
and A549/ER cells to erlotinib, each cell line was exposed to a
gradient of erlotinib concentrations for 24 or 48 h and an MTT
assay was performed. The results of this assay revealed that the
various experimental concentrations of erlotinib inhibited the
proliferation of A549 and A549/ER cells proliferation, regardless
of the time of incubation (Fig. 1).
The cell growth inhibition rate increased in a time- and
dose-dependent manner (Fig. 1). Using
linear regression, A549 cells incubated with erlotinib for 24 h
gave an IC50 value 113.21 µmol/ml; that of A549/ER cells
was 183.71 µmol/ml. The IC50 results were 50.25 and
83.81 µmol/ml for A549 and A549/ER cells incubated with erlotinib
for 48 h, respectively. According to the formula: The fold of drug
resistance=IC50 of resistant cells/IC50 of
sensitive cells, the drug resistance of A549/ER cells were 1.62 for
the 24 h erlotinib incubation and 1.67 for the 48 h erlotinib
incubation, indicating that A549/ER cells were stably resistant to
erlotinib.
Effects of β-ELE on A549/ER cell
toxicity
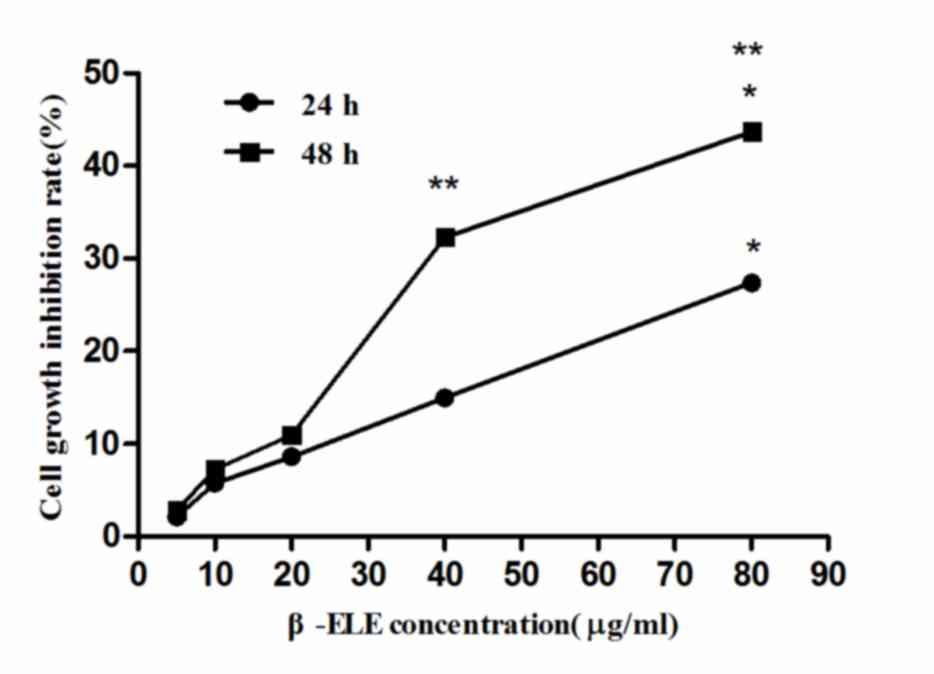
To verify the effects of β-ELE on A549/ER cells, MTT
assays were performed using different β-ELE doses. According to the
cell growth inhibition rate of variant β-ELE concentration, the
linear regression equation for 24 h incubation was calculated as
follows: x=3.064y-5.023, while the equation for 48 h incubation
was: x=1.667y-1.349, where × represents the β-ELE concentration and
y represents the cell proliferation inhibition rate. The results of
these assays revealed that β-ELE inhibited A549/ER cell growth in a
time- and dose-dependent manner (Table
I and Fig. 2). When the
concentration of β-ELE was <20 µg/ml, 24 or 48 h incubation of
A549/ER cell inhibition rates had no significant difference
(aP>0.05; Table I),
whereas doses of 40 and 80 µg/ml β-ELE had a significant effect on
the inhibition of A549/ER cell proliferation cP<0.01,
24 vs. 48 h; bP<0.05, 40 µg/ml group vs. 80 µg/ml
group). By using the above linear regression equation, β-ELE
inhibited the proliferation of A549/ER cells with an
IC50=148.18 µmol/ml at 24 h, an IC50=82.00
µmol/ml at 48 h, an IC10=25.62 µmol/ml at 24 h and an
IC10=15.32 µmol/ml at 48 h. Incubation of A549/ER cells
with 20 µg/ml β-ELE for 24 and 48 h gave growth inhibition rates of
8.59±2.82 and 10.93±2.65% respectively. On the basis of this
experimental data and according to the aforementioned principles
(the dose <IC10), 15 µg/ml β-ELE was selected as the
optimum concentration to reverse drug resistance for the following
assays.
 | Table I.Effect of β-ELE on A549/ER cell
growth (n=3). |
Table I.
Effect of β-ELE on A549/ER cell
growth (n=3).
|
| Cell proliferation
inhibition rate, % |
|---|
|
|
|
|---|
| β-ELE (µg/ml) | 24 h | 48 h |
|---|
| 5 | 2.10±0.99 |
2.80±1.49a |
| 10 | 5.75±1.49 |
7.28±1.69a |
| 20 | 8.59±2.82 |
10.93±2.65a |
| 40 |
14.96±2.27b |
32.32±2.66b,c |
| 80 |
27.38±2.98b |
43.69±2.17b,c |
Effect of non-toxic doses β-ELE on
drug-resistance of A549/ER cells
According to the cell growth inhibition rate of
different concentrations erlotinib, the linear regression equation
of the control group was: x=4.187y-25.64, whereas the equation for
experimental group is: x=1.81y-33.778 (where × represents the
erlotinib concentration and y represents the cell proliferation
inhibition rate). The results of the MTT assay revealed that the
rate of growth inhibition of β-ELE-treated A549/ER cells increased
as erlotinib concentration increased in the control group and the
experimental group (15 µg/ml β-ELE combined with erlotinib;
Fig. 3). Furthermore, the
IC50 value of the cells in the experimental group was
significantly lower than the IC50 value of the control
group, reduced from 183.71 in the control group to 56.72 µmol/l in
the experimental group, a degree of drug resistance reversal of
3.24 (Table II). These results
indicated that 15 µg/ml β-ELE combined with erlotinib increased the
inhibition of A549/ER cell proliferation, which is likely to be
associated with the β-ELE-dependent enhancement of the sensitivity
of A549/ER cells to erlotinib, thereby partially reversing drug
resistance in A549/ER cells.
 | Table II.Effect of non-toxic doses β-ELE on
drug-resistance of A549/ER cells (n=3). |
Table II.
Effect of non-toxic doses β-ELE on
drug-resistance of A549/ER cells (n=3).
|
| Cell proliferation
inhibition rate at 24 h, % |
|---|
|
|
|
|---|
| Erlotinib
(µmol/l) | Control | 15 µg/ml β-ELE |
|---|
| 0.01 | 1.91±0.78 |
11.32±1.52a |
| 0.1 | 5.29±1.02 |
15.35±1.76a |
| 1 | 9.42±1.78 |
23.24±1.70a |
| 10 | 14.30±1.97 |
39.62±3.25a |
| 100 | 26.23±2.75 |
65.14±3.41a |
Effect of non-toxic doses β-ELE on the
apoptosis of A549/ER cells
Apoptosis was analyzed using flow cytometry
following double-staining with annexin V-FITC and PI. As shown in
Table III, 15 µg/ml β-ELE alone led
to no significant induction of apoptosis in A549/ER cells
(P>0.05 in the β-ELE single drug group vs. control group;
Table III); a similar condition was
also observed in the group treated with 10 µmol/l erlotinib
(Fig. 4B). Although the difference of
early apoptotic cells (Q3) between the erlotinib-only drug group
vs. the control group was statistically significant (P<0.05),
the overall rate of apoptosis (Q2+Q3) in the erlotinib-only drug
group was only ~5%. β-ELE and erlotinib in combination evidently
induced A549/ER apoptosis; the early apoptotic rate was 16.84±1.61%
(combined drug group vs. the remaining three groups, P<0.01),
and the middle-late apoptotic rate was 6.20±0.41% (combined drug
group vs. the remaining three groups, P<0. 01; Fig. 4C). In conclusion, these results
indicated that β-ELE combined with erlotinib may enhance apoptosis
in A549/ER cells.
 | Table III.Effect of non-toxic doses of β-ELE on
inducing apoptosis of A549/ER cells (n=3). |
Table III.
Effect of non-toxic doses of β-ELE on
inducing apoptosis of A549/ER cells (n=3).
|
| Cells in a
quadrant, % |
|---|
|
|
|
|---|
| Group | Q2 | Q3 |
|---|
| β-ELE single drug
group |
3.34±0.34c |
2.11±0.49c |
| Erlotinib single
drug group | 4.04±0.37 |
4.22±0.28a |
| Combined drug
group |
6.20±0.41b |
16.84±1.61b |
| Control group | 3.44±0.30 | 1.63±0.27 |
Effect of non-toxic doses β-ELE on
A549/ER cell cycle
To determine whether β-ELE could influence the cell
cycle distribution of A549/ER cells, flow cytometry was performed
(Table IV). The number of A549/ER
cells in S phase increased following treatment with 15 µg/ml β-ELE
alone for 24 h. However, there was no statistically significant
difference compared with the control group, which contained only
RPMI-1640 medium (P>0.05). The combination of β-ELE and
erlotinib induced significantly higher rates
G0/G1 arrest than the other groups
(P<0.01).
 | Table IV.Effect of non-toxic doses β-ELE on
A549/ER cell cycle (n=3). |
Table IV.
Effect of non-toxic doses β-ELE on
A549/ER cell cycle (n=3).
|
| Cell cycle
distributions, % |
|---|
|
|
|
|---|
| Group |
G0/G1 | S |
G2/M |
|---|
| 15 µg/ml β-ELE | 53.16±2.89 |
30.47±2.85a | 16.37±2.86 |
| 10 µmol/l
erlotinib |
62.71±3.74b | 18.46±2.15 | 18.83±3.39 |
| 15 µg/ml β-ELE and
10 µmol/l erlotinib |
77.50±3.36c | 12.57±1.95 | 9.93±2.65 |
| Control group | 55.60±2.26 | 25.39±1.83 | 19.01±2.10 |
Effect of non-toxic doses β-ELE on
A549/ER cells P-gp function
Rh123 retention assays were used to assess P-gp
function in A549/ER cells (Fig. 5).
Following treatment with 15 µg/ml β-ELE for 24 h, the A549/ER MFI
increased 6.8 times compared with the control group, and MFI
increased 6.8 times relative to the control group (P<0.01),
indicating that non-toxic doses of β-ELE can effectively reduce
Rh123 efflux and increase the concentration of Rh123 in A549/ER
cells. The results of this assay indicated that non-toxic doses
β-ELE can reduce drug efflux in A549/ER cells, inhibiting the
transmembrane pumping function of P-gp.
Effect of non-toxic doses β-ELE on
A549/ER cells P-gp expression
The results of western blot analysis revealed that
levels of P-gp protein expression decreased significantly in
A549/ER cells incubated with 15 µg/ml β-ELE for 24 h, to
approximately one-third of the control group (0.25 vs. 0.74;
Fig. 6), indicating that 15 µg/ml
β-ELE serves a notable role in reversing drug resistance by
downregulating the expression of P-gp.
Discussion
Erlotinib is a commonly used molecular targeted drug
for the treatment of NSCLC. However, it is efficacious for a
limited time period as resistance will occur, resulting in the
failure of therapy (8). Therefore,
methods of overcoming erlotinib resistance and identification of
novel drugs that can delay or reverse the drug resistance have
become the subject of research in recent years (12,13).
Acquired drug resistance limits the long-term clinical success of
targeted therapies including EGFR inhibitors in patients with
EGFR mutant NSCLC. The most common mechanism of acquired
resistance, detected in 60% of patients, is a secondary mutation in
EGFR at position T790, which is located in exon 20 (14).
ELEs are novel anticancer drugs isolated from
Curcuma zedoaria Roscoe, commonly known as zedoary, and are
made up of the α, β, γ and δ forms (9). β-ELE has a chemical structure of
1-methyl-1-vinyl-2,4-diisopropenyl-cyclohexane, a molecular formula
C15H24, and a molecular weight of 204, β-ELE
confers the primary anti-tumor effect (9,15–17).
The current clinical use of elemene injection and
oral liquid is based on β-ELE as main ingredients, containing small
amounts of δ-ELE and γ-ELE. β-ELE injection has been widely used in
treatment of a variety of malignancies, including lung cancer
(18,19), liver cancer (20) and stomach cancer (21). β-ELE may elicit a good therapeutic
effect and has several advantages, including a broad antitumor
spectrum, mild side effects, low in liver and kidney toxicity and
no bone marrow suppression, meaning it is well tolerated by
patients (15). β-ELE can reverse
multidrug resistance (MDR) via the following mechanisms: Killing
the tumor cells directly, reducing tumor cell proliferation,
inhibiting tumor growth, inducing tumor cell apoptosis and cell
cycle arrest, inhibiting tumor angiogenesis and inhibiting tumor
cell migration (15). Using a β-ELE
and chemotherapy drugs in combination may prevent bone marrow
suppression caused by chemotherapy and improve the sensitivity of
tumor cells to chemotherapy drugs, potentially even reversing
resistance to chemotherapy drugs (15,18).
A prior study demonstrated that NP chemotherapy
combined with elemene can improve the efficacy of advanced NSCLC
(18). Gao et al (22) reported that β-ELE could reverse drug
resistance in the gefitinib-resistant human lung adenocarcinoma
PC9/ZD cell line. Yao et al (9,10)
investigated the potential mechanism by which β-ELE inhibited the
proliferation of the cisplatin-resistant human NSCLC A549/DDP cell
line; they revealed that the combination of β-ELE and cisplatin can
improve the sensitivity of A549/DDP cells to cisplatin and reverse
drug resistance. The mechanism may be associated with decreased
mitochondrial membrane potential and P-gp expression activated
intracellular redox system, regulation of apoptosis-signaling
proteins by increasing the protein expression of cytochrome c,
caspase-3, Bcl-2-associated death promoter and reducing protein
levels of B-cell lymphoma-2 and procaspase-3 in the A549/DDP cells
(9,10). Zhao et al (23) revealed that β-ELE can inhibit the
growth of NSCLC cells via extracellular signal-regulated kinase 1/2
and AMP-activated protein kinase-α-mediated inhibition of
transcription factor Sp1, followed by reduction in DNA
methyltransferase 1 expression.
β-ELE has been observed to reverse drug resistance
not only in lung cancer cells but also in other types of cancer
cells. Guo et al (24)
demonstrated that β-ELE inhibited the overexpression of P-gp and
the proliferation KB-C2 cells and enhanced the sensitivity of tumor
cells to colchicine, mitoxantrone, vincristine and taxane,
reversing drug resistance. Dong et al (25) identified that β-ELE may inhibit breast
cancer stem cell growth and reduce Breast cancer resistance protein
expression. In a previous study, Li et al (26) observed that β-ELE improve the
sensitivity of cisplatin-resistant human ovarian cancer MCAS and
A2780/CP70 cells to cisplatin. A recent study revealed that β-ELE
can reverse chemoresistance in breast cancer cells by altering the
expression of MDR-associated microRNAs, including Phosphatase and
tensin homolog and P-gp in adriacin- and docetaxel-resistant MCF-7
cells, consequently regulating the corresponding target genes
through the gene regulatory network to induce the development of
drug resistance (27).
The results of the present study revealed that β-ELE
induced inhibition of A549/ER cell proliferation in a time- and
dose-dependent manner in A549/ER cells. According to the
IC10, 15 µg/ml β-ELE was determined to be the non-toxic
dose and ultimately the test concentration for reversal of
resistance. A549/ER cell growth inhibition rates were significantly
increased (P<0.05) in each group following a 24-h incubation
with β-ELE and erlotinib, with IC50 values decreasing
significantly and the degree of drug resistance reversal was 3.24.
The aforementioned experimental results indicated that non-toxic
doses of β-ELE can enhance the sensitivity of A549/ER cells to
erlotinib, reversing drug resistance.
Following incubation with 15 µg/ml β-ELE for 24 h,
the rate of proliferation inhibition in A549/ER cells was
negligible, at ~6.5%. The results indicated that 15 µg/ml β-ELE
combined with erlotinib increased the inhibition of A549/ER cell
proliferation, which could be due to the enhancement that β-ELE
brings about in the sensitivity of A549/ER cells to erlotinib,
thereby partially reversing the drug resistance of A549/ER cells.
The cell apoptosis rate and cell cycle distribution were analyzed
by flow cytometry; the results indicated that treatment with 15
µg/ml β-ELE alone failed to induce A549/ER cell apoptosis, but in
combination with erlotinib an effect was observed (the overall
apoptosis rate increased from 5 to 23%). Similarly, treatment of
A549/ER cells with 15 µg/ml β-ELE alone for 24 h did not
significantly increase the degree of S phase arrest compared with
the control group (P>0.05). Treatment with 10 µmol/l erlotinib
alone resulted in a significantly higher degree of
G0/G1 phase arrest compared with the control
(P<0.05), with β-ELE in combination with erlotinib could cause a
more significant G0/G1 phase arrest
(P<0.01). The non-toxic dose of β-ELE may increase the drug
concentration of erlotinib within A549/ER cells, improving the
cytotoxic effect; thereby inducing tumor cells apoptosis and cell
cycle arrest in G0/G1 phase.
Drug resistance refers to the resistance of cancer
cells to a certain antitumor drug. MDR is a phenomenon wherein
cancer cells develop resistance to a number of antitumor drugs
(28). MDR is characterized by cancer
cells becoming resistant to a variety of drugs that have distinct
structures and mechanisms of action (29). The expression levels of P-gp serve an
important role in a number of resistance mechanisms. P-gp was the
first transmembrane protein to be identified to mediate drug
transport in the cell. There are two primary types of human P-gp,
P-gp I and P-gp II, which are encoded by the MDR1 and MDR2 genes,
respectively. The overexpression of P-gp in tumor cells is one of
the important contributing factors of MDR (30). P-gp pump the drugs to outside of the
cell via ATP hydrolysis (31),
exporting drugs away from their target, causing the tumor to be
insensitive to the anticancer drug. High expression of P-gp is
always associated with MDR. P-gp is an ATP-depended drug efflux
pump that is located on the cell membrane; it can reduce the
intracellular concentration of drugs by pumping drugs over the cell
membrane and lead to drug resistance (32). P-gp may efflux anticancer agents out
of cells, therefore decreasing their intracellular accumulation
(33). Yao et al (9) confirmed that β-ELE may significantly
downregulate the expression of P-gp in the A549/DDP cells membrane,
inhibit P-gp-mediated Rh-123 efflux and enhance the intracellular
anti-cancer drug accumulation in drug-resistant cancer cells,
ultimately leading to a reversal of drug resistance (9). The drug resistance of tumor cells can be
reversed by inhibiting the function of P-gp or downregulating the
expression of P-gp (34,35). Rh123 is a fluorescent substrate of
P-gp, and is widely used as an indicator of P-gp activity. Rh123 is
a cationic fluorescent dye that may penetrate the cell membrane,
and can be used to assess the expression and the activity levels of
P-gp on the surface of the cell via the detection of changes in
Rh123 fluorescence intensity in the tumor cells. The higher
fluorescence intensity of Rh123, the lower the activity of P-gp on
cell surface and the lower the rate of drug efflux. Using the
intracellular Rh123 aggregation test, it was found that
intracellular Rh123 fluorescence intensity was enhanced following
treatment of A549/ER cells with 15 µg/ml β-ELE for 24 h. The
results of western blot analysis also indicated that 15 µg/ml β-ELE
could significantly reduce P-gp protein levels at the A549/ER cell
membrane. These results indicated that 15 µg/ml β-ELE could
increase the intracellular accumulation of erlotinib in A549/ER
cells by downregulating the levels of P-gp at cell membrane,
reducing the efflux of erlotinib and improving the cytotoxic effect
of erlotinib on A549/ER cells, thereby inducing apoptosis in the
tumor cell and cell cycle arrest.
In conclusion, the results of the present study
indicated that treatment with β-ELE could reverse drug resistance
in erlotinib-resistant human NSCLC A549/ER cells in vitro.
Further analysis revealed that the mechanism of action of β-ELE may
involve the decreased expression of P-gp, the inhibition of P-gp
dependent drug efflux and the increase in concentration of
anticancer drugs in the cells. The present preliminary study
provides a potential mechanism of action for β-ELE that may explain
its ability to overcome erlotinib resistance. If the reversal of
drug resistance induced by β-ELE in erlotinib-resistant human NSCLC
cells can be supported by data from clinical trials, it could
improve the therapeutic effects in presently erlotinib-resistant
NSCLC patients.
Acknowledgements
The authors would like to thank the laboratory of
Fujian Medical University for their assistance in completing the
study.
Funding
The present study was supported by the Joint Funds
for the Innovation of Science and Technology, Fujian province,
China (grant no. 2017Y9031); Fujian Provincial Natural Science
Foundation, China (grant no. 2017J01295); Startup Fund for
scientific research, Fujian Medical University (grant no.
2016QH033) and the Key Specialty Discipline Construction Program of
Fujian and Nation.
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL designed and performed the experiments and was
the major contributor in writing the manuscript. LBL performed the
experiments and performed analysis and interpretation of data. XC
made substantial contributions to conception and design the study
and aided in drafting the manuscript. BZ made contributions to the
analysis and interpretation of data. TL made contributions to
conception and design the study, assisted with drafting and
revising important content of the manuscript, and gave final
approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tanoue LT, Tanner NT, Gould MK and
Silvestri GA: Lung cancer screening. Am J Respir Crit Care Med.
191:19–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
World Cancer Report 2014. Stewart B and
Wild CP: IARC Nonserial Publication;
|
|
3
|
Szasz A: Current status of oncothermia
therapy for lung cancer. Korean J Thorac Cardiovasc Surg. 47:77–93.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ichite N, Chougule M, Patel AR, Jackson T,
Safe S and Singh M: Inhalation delivery of a novel diindolylmethane
derivative for the treatment of lung cancer. Mol Cancer Ther.
9:3003–3014. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Ruysscher D, Van Meerbeeck J, Vande
casteele K, Oberije C, Pijls M, Dingemans AM, Reymen B, van
Baardwijk A, Wanders R, Lammering G, et al: Radiation-induced
oesophagitis in lung cancer patients. Is susceptibility for
neutropenia a risk factor? Strahlenther Onkol. 188:564–567. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with non-
small-cell lung cancer harbouring mutations of the epidermal growth
factor receptor (WJ-TOG3405): An open-label, randomised phase 3
trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu Y, Du Y, Liu H, Ma T, Shen Y and Pan
Y: Study of efficacy and safety of pulsatile administration of
high-dose gefitinib or erlotinib for advanced non-small cell lung
cancer patients with secondary drug resistance: A single center,
single arm, phase II clinical trial. Thorac Cancer. 7:663–669.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao C, Jiang J, Tu Y, Ye S, Du H and Zhang
Y: β-elemene reverses the drug resistance of A549/DDP lung cancer
cells by activating intracellular redox system, decreasing
mitochondrial membrane potential and P-glycoprotein expression, and
inducing apoptosis. Thorac Cancer. 5:304–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao CC, Tu YR, Jiang J, Ye SF, Du HX and
Zhang Y: β-elemene reverses the drug resistance of lung cancer
A549/DDP cells via the mitochondrial apoptosis pathway. Oncol Rep.
31:2131–2138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Ying W, Jia-li Y, Yan-Ting S and
Chun-ying L: Effects of Buzhong Yiqi decoction-medicated serum on
drug resistance of human lung adenocarcinoma cell line A549/DDP to
cisplatin. Chinese J Pathophysiol. 30:223–238. 2014.
|
|
12
|
Zhu CQ, da Cunha Santos G, Ding K,
Sakurada A, Cutz JC, Liu N, Zhang T, Marrano P, Whitehead M, Squire
JA, et al: Role of KRAS and EGFR as biomarkers of response to
erlotinib in National Cancer Institute of Canada Clinical Trials
Group Study BR.21. J Clin Oncol. 26:4268–4275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brevet M, Johnson ML, Azzoli CG and
Ladanyi M: Detection of EGFR mutations in plasma DNA from lung
cancer patients by mass spectrometry genotyping is predictive of
tumor EGFR status and response to EGFR inhibitors. Lung cancer.
73:96–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ercan D, Choi HG, Yun CH, Capelletti M,
Xie T, Eck MJ, Gray NS and Jänne PA: EGFR mutations and resistance
to Irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res.
21:3913–3923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X and HU X: Research progress on the
anti-tumor mechanism and reversal mechanism of drug resistance of
β-elemene. J Mod Oncol. 22:1711–1714. 2014.
|
|
16
|
Jianfeng JU, Weiping YU, Chunsheng FU and
Liming MA: Modern research and clinical application of β-elemene.
Qilu Pharmaceutical Affairs. 27:546–548. 2008.
|
|
17
|
Guangchao LI: Basic research and clinical
applications of β-elemene. J Changchun Univ Tradit Chin Med.
25:185–186. 2009.
|
|
18
|
Lihua W and Fang S: Elemene emulsion
combined with NP chemotherapy in the treatment of advanced NSCLC
patients. China Oncol. 20:547–550. 2010.
|
|
19
|
Li T, Yuean C, Chaosheng P, Wei W and
Nanzhan L: Elemene emulsion combined with chemotherapy in the
treatment of patients with advanced non-small cell lung cancer.
Chinese J Clin Med. 16:725–727. 2009.
|
|
20
|
Qirong P, Bi X, Long M, Tao C, Yongcai T,
Caixia H and Pengfei L: Short-term clinical observation of Elemene
injection used to treat metaphase and advanced primary liver
cancer. Chin J Integ Tradit West Med Liv Dis. 20:274–276. 2010.
|
|
21
|
Dan Y, Guangyu A and Hong D: Efficacy
observation of advanced gastric cancer treated with elemene and
Tegafur. World J Integ Tradit West Med. 8:264–266. 2013.
|
|
22
|
Gao FY, Zhang AQ and Sun Y: Reversing
Effect of β-elemene on human lung adenocarcinoma cell line PC9.
Chin Arch Tradit Chin Med. 32:131–133. 2014.
|
|
23
|
Zhao S, Wu J, Zheng F, Tang Q, Yang L, Li
L, Wu W and Hann SS: β-elemene inhibited expression of DNA
methyltransferase 1 through activation of ERK1/2 and AMPKa
signalling pathways in human lung cancer cells: The role of Sp1. J
Cell Mol Med. 19:630–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo HQ, Zhang GN, Wang YJ, Zhang YK,
Sodani K, Talele TT, Ashby CR Jr and Chen ZS: β-elemene, a compound
derived from Rhizoma zedoariae, reverses multidrug resistance
mediated by the ABCB1 transporter. Oncol Rep. 31:858–866. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong Y, Li L, Wang L, Zhou T, Liu JW and
Gao YJ: Preliminary study of the effects of β-elemene on MCF-7/ADM
breast cancer stem cells. Genet Mol Res. 14:2347–2355. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li QQ, Lee RX, Liang H, Wang G, Li JM,
Zhong Y and Reed E: β-elemene enhances susceptibility to cisplatin
in resistant ovarian carcinoma cells via downregulation of ERCC-1
and XIAP and inactivation of JNK. Int J Oncol. 43:721–728. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Zhang HD, Yao YF, Zhong SL, Zhao
JH and Tang JH: β-elemene reverses chemoresistance of breast cancer
cells by reducing resistance transmission via exosomes. Cell
Physiol Biochem. 36:2274–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gottesman MM: How cancer cells evade
chemotherapy: Sixteenth Richard and Hinda Rosenthal Foundation
Award Lecture. Cancer Res. 53:747–754. 1993.PubMed/NCBI
|
|
29
|
Gillet JP and Gottesman MM: Mechanisms of
multidrug resistance in cancer. Methods Mol Biol. 596:47–76. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin MS, Oldham ML, Zhang Q and Chen J:
Crystal structure of the multidrug transporter P- glycoprotein from
Caenorhabditis elegans. Nature. 490:566–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen F, Chu S, Bence AK, Bailey B, Xue X,
Erickson PA, Montrose MH, Beck WT and Erickson LC: Quantitation of
doxorubicin uptake, efflux, and modulation of multidrug resistance
(MDR) in MDR human cancer cells. J Pharmacol Exp Ther. 324:95–102.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aller SG, Yu J, Ward A, Weng Y,
Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL
and Chang G: Structure of P-glycoprotein reveals a molecular basis
for poly-specific drug binding. Science. 323:1718–1722. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakamura T, Oka M, Aizawa K, Soda H,
Fukuda M, Terashi K, Ikeda K, Mizuta Y, Noguchi Y, Kimura Y, et al:
Direct interaction between a quinoline derivative, MS-209, and
multidrug resistance protein (MRP) in human gastric cancer cells.
Biochem Biophys Res Commun. 255:618–624. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jovelet C, Bénard J, Forestier F,
Farinotti R, Bidart JM and Gil S: Inhibition of P-glycoprotein
functionality by vandetanib may reverse cancer cell resistance to
doxo-rubicin. Eur J Pharm Sci. 46:484–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Angelini A, Conti P, Ciofani G, Cuccurullo
F and Di Ilio C: Modulation of multidrug resistance p-glycoprotein
activity by antiemetic compounds in human doxorubicin-resistant
sarcoma cells (MES-SA/Dx-5): Implications on cancer therapy. J Biol
Regul Homeost Agents. 27:1029–1037. 2013.PubMed/NCBI
|