Introduction
Esophageal carcinoma (EC) is one of the most common
malignant tumors worldwide, which is eighth in incidence and sixth
in leading cause of cancer-related deaths (1,2).
Esophageal squamous cell carcinoma (ESCC) accounts for nearly 90%
of EC cases in developing countries, especially in China (2). It is usually characterized by insidious
early symptoms, lack of specific markers for diagnosis and
evaluating prognosis, and poor prognosis (3). Despite its improvement in early
detection and treatment in recent years, the overall survival (OS)
of advanced ESCC patients remains poor, with a <30% five-year
survival rate in China (4). Hence, it
is necessary to find more efficient targets that can be used for
the diagnosis and treatment of ESCC patients.
According to the different locations of primary
cancers, ESCC can be divided into four types: Cervical ESCC, upper
thoracic ESCC, middle thoracic ESCC, and lower thoracic ESCC
(5). The incidence of cervical and
upper thoracic ESCC is much smaller than that of middle and lower
thoracic ESCC (6). Moreover, the
biological behavior of cervical and upper thoracic ESCC is closer
to head and neck squamous cell carcinoma, rather than ESCC
(5,7,8). Hence,
studies on middle and lower thoracic ESCC would be more meaningful
for EC research.
Tumor necrosis factor receptor (TNFR) is composed of
two members: tumor necrosis factor receptor 1 (TNFR1) and tumor
necrosis factor receptor 2 (TNFR2). TNFR1 is widely expressed in
different kinds of cells, and can mediate apoptosis induced by
tumor necrosis factor-α (TNF-α) (9).
Different to TNFR1 due to the lack of a cytoplasmic death domain
(DD), TNFR2 cannot activate the apoptotic machinery of cells, but
can play important roles in bone healing, anti-inflammation and
immune regulation through binding to TNF-α (10–12). In
recent years, with deeper and more extensive studies on cancer, the
high expression and promotion roles of TNFR2 have been reported in
several types of tumors, such as ovarian cancer (13,14) and
breast cancer (15). However, the
clinical significance of TNFR2 in EC remains unknown.
In the present study, TNFR2 expression was detected
in 431 tissue specimens obtained from ESSC patients by
immunohistochemistry (IHC) staining, and the positive correlation
of TNFR2 with the progression and poor prognosis of the total cases
was proven. Next, the clinical significance of TNFR2 in middle and
lower thoracic ESCC was studied. Finally, COX regression analysis
was performed to confirm the factors that can affect the prognosis
of ESCC patients.
Materials and methods
Collection of tissue samples
Approved by the Ethics Committee of the Affiliated
Hospital of Jining Medical University (Jining, China), we
retrospectively selected 431 primary ESCC specimens from EC
patients who had surgical removal from 2008 to 2014 in Affiliated
Hospital of Jining Medical University. The inclusion criteria and
exclusion criteria of this study were simple: i) patients with
middle or lower ESCC; and ii) patients did not receive
chemotherapy, radiotherapy or immunomodulatory therapy before
surgery. Follow-up of outpatients was performed by telephone and
the ending time was December, 2016.
IHC staining and scoring
ESCC wax samples were collected and cut into slides
of 4 µm thickness for hematoxylin and eosin (H&E) staining.
After H&E staining, tumor regions were marked under microscope
and tumor tissues were taken away using a trocar. Then many tumor
tissues from different samples were embeded into one paraffin
block. The paraffin block were cut into 4 µm thickness and
deparaffinized in xylene and rehydrated in graded ethanol, then
boiled in 10 mmol/l citrate buffer (pH 6.0) for 3 min at 100°C for
antigen unmasking. Then the sections were immersed in 3%
H2O2 for 10 min to block the endogenous
peroxidase and in goat serum blocking solution for 15 min to block
non-specific antigens. After incubated at room temperature for 2 h
in primary antibody of TNFR2 (1:400; Proteintech Group Inc.,
Chicago, IL, USA), sections were washed with PBS and incubated in
HRP goat anti-rabbit/mouse IgG polymer (Maixin Biotechnology
Development Co., Ltd., Fuzhou, China) at room temperature for 30
min. Finally, slides were stained with 3,3′-diaminobenzidine and
counterstained with hematoxylin. The scoring process was performed
by two independent pathologists who were blind to clinical
parameters and clinical outcomes of patients. The percentage of
stained cells was evaluated at ×400 magnification in at least 5
random fields. The proportion score represented the estimated
fraction of positive staining tumor cells (0≤25%; 26%≤1≤50%;
51%≤2≤75%; 3>75%). The intensity score represented the estimated
average staining intensity of positive tumor cells (0, negative; 1,
weak; 2, moderate; and 3, strong). The expression level of TNFR2
was evaluated using the product of proportion score and intensity
score at five fields and mean value was obtained (≤4 as low
expression, >4 as high expression).
Statistical analysis
SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. The association between the
expression of TNFR2 and clinical parameters was analyzed using the
Chi-square test. Survival curves were drawn using the Kaplan-Meier
method and compared using the log-rank test. Cox's proportional
hazards regression model was performed to identify factors which
can affect the OS of ESCC patients. P<0.05 was considered to
indicate a statistically significant difference.
Results
In the total cases, TNFR2 was positively correlated
with invasion depth, advanced clinical stage, low differentiation
degree and poor OS. A total of 431 specimens from different ESCC
patients were collected and stained using IHC to detect TNFR2
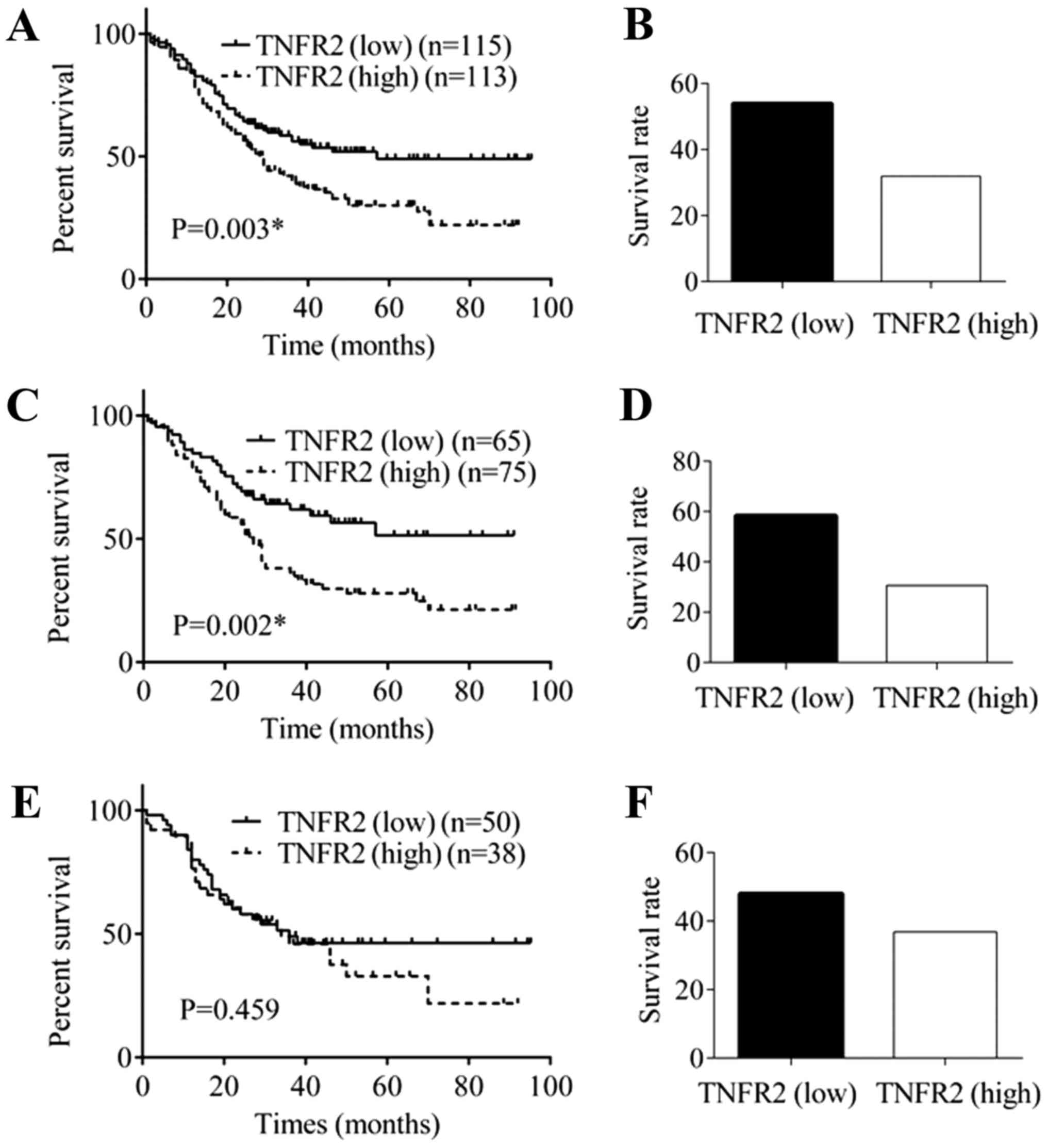
expression, higher than non-tumor esophageal tissues (Fig. 1). All ESCC cases were divided into two
groups including low expression of TNFR2 group and high expression
of TNFR2 group. As shown in Table I,
in the low expression of TNFR2 group, only 119 out of 192 cases had
an invasion depth greater than the muscularis, which was much less
than 187 out of 239 cases in the high expression of TNFR2 group,
and the difference was statistically significant (P<0.001).
Furthermore, 129 out of 192 cases were at stage III, which was much
less than 184 out of 239 cases in the high expression of TNFR2
group, and the difference was statistically significant (P=0.03).
Moreover, 88 out of 192 cases has a low differentiation degree,
which was much less than 143 out of 239 cases in the high
expression of TNFR2 group, and the difference was statistically
significant (P=0.005). Otherwise, there was no significant
difference in age, gender, tumor size, tumor location and lymph
node involvement between these two groups. In addition, in the low
expression of TNFR2 group, follow-up was performed for 115 cases. A
total of 53 cases died during the follow-up period, and the
survival rate was 53.91%. In the high expression of TNFR2 group,
follow-up was performed in 113 cases. A total of 77 cases died
during the follow-up period, and the survival rate was 31.86%
(Fig. 2A). The Kaplan-Meier analysis
revealed that OS in the high expression of TNFR2 group was worse,
compared with that in the low expression of TNFR2 group, and the
difference was statistically significant (P=0.003; Fig. 2B).
 | Table I.Correlation of TNFR2 with clinical
parameters of the patients with total ESCC. |
Table I.
Correlation of TNFR2 with clinical
parameters of the patients with total ESCC.
|
|
| TNFR2 |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Cases | Low (192) | High (239) | P-value |
|---|
| Age |
|
|
|
|
| ≤60 | 195 | 92 | 103 | 0.331 |
|
>60 | 236 | 100 | 136 |
|
| Sex |
|
|
|
|
| Male | 245 | 106 | 139 | 0.558 |
|
Female | 186 | 86 | 100 |
|
| Invasion depth |
|
|
|
|
|
≤Muscularis | 125 | 73 | 52 |
<0.001a |
|
>Muscularis | 306 | 119 | 187 |
|
| Tumor size |
|
|
|
|
| ≤4
cm | 271 | 115 | 156 | 0.271 |
| >4
cm | 160 | 77 | 83 |
|
| Tumor location |
|
|
|
|
|
Lower | 152 | 72 | 80 | 0.418 |
|
Middle | 279 | 120 | 159 |
|
| Clinical stage |
|
|
|
|
|
I/II | 118 | 63 | 55 | 0.030a |
|
III | 313 | 129 | 184 |
|
|
Differentiation |
|
|
|
|
|
Low | 231 | 88 | 143 | 0.005a |
|
Moderate/high | 200 | 104 | 96 |
|
| Lymph node
involvement |
|
|
|
|
| No | 222 | 99 | 123 | 0.984 |
|
Yes | 209 | 93 | 116 |
|
In middle thoracic ESCC patients,
TNFR2 was positively correlated with invasion depth, advanced
clinical stage and poor OS
The middle thoracic esophagus is the most common
location of ESCCs. The 279 middle thoracic ESCC cases were divided
into two groups: Low expression of TNFR2 group (n=120) and
high expression of TNFR2 group (n=159). As shown in Table II, in the low expression of TNFR2
group, only 73 out of 120 cases had an invasion depth greater than
the muscularis, which was much less than 127 out of 159 cases in
the high expression of TNFR2 group, and the difference was
statistically significant (P=0.001). Furthermore, 77 out of 120
cases were at stage III, which was much less than 125 out of 159
cases in the high expression of TNFR2 group, and the difference was
statistically significant (P=0.01). In low expression of TNFR2
group, follow-up was performed in 65 cases. A total of 27 cases
died during the follow-up period, and the survival rate was 58.46%.
In the high expression of TNFR2 group, follow-up was performed in
75 cases. A total of 52 cases died during the follow-up period, and
the survival rate was 30.67% (Fig.
2C). The Kaplan-Meier analysis revealed that OS in the high
expression of TNFR2 group was worse than that in the low expression
of TNFR2 group, and the difference was statistically significant
(P=0.002; Fig. 2D).
 | Table II.Correlation of TNFR2 with clinical
parameters of patients with middle thoracic ESCC. |
Table II.
Correlation of TNFR2 with clinical
parameters of patients with middle thoracic ESCC.
|
|
| TNFR2 |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Cases | Low (120) | High (159) | P-value |
|---|
| Age |
|
|
|
|
|
≤60 | 126 | 56 | 70 | 0.716 |
|
>60 | 153 | 64 | 89 |
|
| Sex |
|
|
|
|
|
Male | 212 | 91 | 121 | 0.959 |
|
Female | 67 | 29 | 38 |
|
| Invasion depth |
|
|
|
|
|
≤Muscularis | 79 | 47 | 32 | 0.001a |
|
>Muscularis | 200 | 73 | 127 |
|
| Tumor size |
|
|
|
|
| ≤4
cm | 175 | 71 | 104 | 0.318 |
| >4
cm | 104 | 49 | 55 |
|
| Clinical stage |
|
|
|
|
|
I/II | 77 | 43 | 34 | 0.01a |
|
III | 202 | 77 | 125 |
|
|
Differentiation |
|
|
|
|
|
Low | 146 | 55 | 91 | 0.07 |
|
Moderate/high | 133 | 65 | 68 |
|
| Lymph node
involvement |
|
|
|
|
| No | 154 | 69 | 85 | 0.544 |
|
Yes | 125 | 51 | 74 |
|
In lower thoracic ESCC patients, TNFR2
was positively correlated with low differentiation degree
Lower thoracic esophagus is the second common
location of ESCCs. The 152 lower thoracic ESCC cases were divided
into two groups: low expression of TNFR2 group (n=72) and
high expression of TNFR2 group (n=80). As shown in Table III, in the low expression of TNFR2
group, only 33 out of 72 cases had a low differentiation degree,
which was much less than 52 out of 80 cases in the high expression
of TNFR2 group, and the difference was statistically significant
(P=0.022). In low expression of TNFR2 group, follow-up was
performed in 50 cases. A total of 26 cases died during the
follow-up period, and the survival rate was 48.00%. In the high
expression of TNFR2 group, follow-up was performed in 38 cases. A
total of 24 cases died during the follow-up period, and the
survival rate was 36.84% (Fig. 2E).
The Kaplan-Meier analysis revealed that OS in the high expression
of TNFR2 group was worse than that in the low expression of TNFR2
group, but the difference was not statistically significant
(P=0.459; Fig. 2F).
 | Table III.Correlation of TNFR2 with clinical
parameters of patients with lower thoracic ESCC. |
Table III.
Correlation of TNFR2 with clinical
parameters of patients with lower thoracic ESCC.
|
|
| TNFR2 |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Cases | Low (72) | High (80) | P-value |
|---|
| Age |
|
|
|
|
|
≤60 | 69 | 36 | 33 | 0.329 |
|
>60 | 83 | 36 | 47 |
|
| Sex |
|
|
|
|
|
Male | 33 | 15 | 18 | 0.846 |
|
Female | 119 | 57 | 62 |
|
| Invasion depth |
|
|
|
|
|
≤Muscularis | 46 | 26 | 20 | 0.159 |
|
>Muscularis | 106 | 46 | 60 |
|
| Tumor size |
|
|
|
|
| ≤4
cm | 96 | 44 | 52 | 0.736 |
| >4
cm | 56 | 28 | 28 |
|
| Clinical stage |
|
|
|
|
|
I/II | 41 | 20 | 21 | 0.857 |
|
III | 111 | 52 | 59 |
|
|
Differentiation |
|
|
|
|
|
Low | 85 | 33 | 52 | 0.022a |
|
Moderate/high | 67 | 39 | 28 |
|
| Lymph node
involvement |
|
|
|
|
| No | 68 | 30 | 38 | 0.516 |
|
Yes | 84 | 42 | 42 |
|
COX regression analysis of factors
which can affect OS of ESCC patients
In order to further investigate the potential
factors that can influence the prognosis of patients, COX
regression analysis was performed. In the total cases, the
univariate COX regression analysis revealed that clinical stage,
lymph node involvement, and invasion depth could affect the OS of
ESCC patients, and the difference was statistically significant
(P=0.01, <0.001 and 0.001). The multivariate COX regression
analysis revealed that lymph node involvement and invasion depth
could affect the OS of ESCC patients, and the difference was
statistically significant (P<0.001 and 0.048; Table IV). In middle thoracic ESCC patients,
the univariate COX regression analysis revealed that clinical
stage, lymph node involvement, and invasion depth could affect the
OS of ESCC patients, and the difference was statistically
significant (P=0.026, 0.012, and 0.002). The multivariate COX
regression analysis revealed that clinical stage, lymph node
involvement, and invasion depth could affect the OS of ESCC
patients, and the difference was statistically significant
(P=0.013, 0.031, and 0.001; Table V).
In lower thoracic ESCC patients, the univariate COX regression
analysis revealed that only lymph node involvement could affect the
OS of ESCC patients, and the difference was statistically
significant (P<0.001; Table
VI).
 | Table IV.Univariate and multivariate analysis
identify factors influencing the overall survival of patients with
total ESCC. |
Table IV.
Univariate and multivariate analysis
identify factors influencing the overall survival of patients with
total ESCC.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | 0.845 | 0.566–1.284 | 0.430 |
|
|
|
| Age | 1.107 | 0.784–1.562 | 0.564 |
|
|
|
| Clinical stage | 0.585 | 0.389–0.881 | 0.010a | 0.586 | 0.205–1.679 | 0.320 |
| Differentiation
degree | 1.133 | 0.803–1.598 | 0.477 |
|
|
|
| Location | 0.999 | 0.702–1.423 | 0.996 |
|
|
|
| Size | 1.069 | 0.747–1.528 | 0.717 |
|
|
|
| Lymph node
involvement | 0.459 | 0.32–0.659 |
<0.001a | 2.058 | 1.43–2.961 | <0.001a |
| Invasion depth | 0.511 | 0.339–0.77 | 0.001a | 2.882 | 1.007–8.245 | 0.048a |
 | Table V.Univariate and multivariate analysis
identify factors influencing the overall survival of patients with
middle thoracic ESCC. |
Table V.
Univariate and multivariate analysis
identify factors influencing the overall survival of patients with
middle thoracic ESCC.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex | 0.817 | 0.488–1.369 | 0.443 |
|
|
|
| Age | 0.889 | 0.573–1.380 | 0.600 |
|
|
|
| Clinical stage | 0.55 | 0.325–0.93 | 0.026a | 0.202 | 0.057–0.713 | 0.013a |
| Differentiation
degree | 1.186 | 0.764–1.84 | 0.447 |
|
|
|
| Size | 1.188 | 0.747–1.888 | 0.467 |
|
|
|
| Lymph node
involvement | 0.559 | 0.356–0.878 | 0.012a | 1.656 | 1.048–2.617 | 0.031a |
| Invasion depth | 0.415 | 0.239–0.721 | 0.002a | 9.932 | 2.673–36.911 | 0.001a |
 | Table VI.Univariate analysis identify factors
influencing the overall survival of patients with lower thoracic
ESCC. |
Table VI.
Univariate analysis identify factors
influencing the overall survival of patients with lower thoracic
ESCC.
|
| Univariate
analysis |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value |
|---|
| Sex | 0.893 | 0.433–1.839 | 0.758 |
| Age | 1.607 | 0.92–2.808 | 0.096 |
| Clinical stage | 0.647 | 0.337–1.242 | 0.191 |
| Differentiation
degree | 1.064 | 0.608–1.861 | 0.828 |
| Size | 0.899 | 0.51–1.584 | 0.712 |
| Lymph node
involvement | 0.333 | 1.181–0.612 |
<0.001a |
| Invasion depth | 0.687 | 0.369–1.277 | 0.235 |
Discussion
ESCC is a type of cancer that originates from
esophageal squamous epithelial cells. Its pathogenesis is not only
hereditary, but can be induced by unhealthy lifestyles, such as
eating food that contains nitrite, drinking, smoking and so on
(16,17). Hence, its incidence in males is
significantly higher than that in females (18). In the present study, the number of
enrolled males (n=245) was greater than females
(n=186), which is consistent with the incidence of ESCC
between different genders. According to different location of
primary cancers, ESCC can be divided into four types. Middle
thoracic ESCCs are tumors that range from the azygos vein to the
inferior pulmonary vein and its incidence ranks first. Lower
thoracic ESCCs are tumors that range from the inferior pulmonary
vein to the lower esophageal sphincter and its incidence ranks
second (5). In these 431 cases,
middle thoracic ESCC cases (n=279) were greater than lower
thoracic ESCC cases (n=152). This is consistent with the
different incidences of middle and lower thoracic ESCCs (6).
TNFR2 protein is encoded by the tumor necrosis
factor superfamily 1B (TNFRSF1B) gene, which weights 75 kDa
(19). Its high expression and
potential in maintaining the malignant biological behaviors of
cells, metastasis (20), invasion
(21) and proliferation (22) have been reported in several types of
tumors. Moreover, it was found that TNFR2 could promote the
transformation of resident fibroblasts to cancer associated
fibroblasts (CAFs) (23).
Furthermore, it is well-known that CAFs can contribute to
migration, invasion and metastasis, and even prevent
differentiation and induce the stemness of tumor cells (24,25). In
the present study, the investigators attempted to confirm the
clinical significance of TNFR2 in ESCCs. In the total cases, it was
found that TNFR2 was positively correlated with invasion depth,
advanced clinical stage and low differentiation degree. In middle
thoracic ESCC samples, it was found that TNFR2 was also positively
correlated with invasion depth and advanced clinical stage. The
correlation of TNFR2 with differentiation did not reach a
statistical significance, but a positive trend was found. This
supports the results from the study of the total cases. In lower
thoracic ESCC samples, a positive correlation between TNFR2 and low
differentiation was also found, and this supplied further support
to the roles of TNFR2 in differentiation. All these results confirm
the role of TNFR2 in ESCC progression, and are consistent with the
roles of TNFR2 in the malignant biological behaviors of tumor
cells, such as in promoting migration, invasion, preventing
differentiation and inducing stemness, as previously reported
(20–25).
The roles of TNFR2 in prognosis have been reported
by several researchers, but the results were inconsistent. Heemann
C and Nakamura N reported that the circulating level of TNFR2 was
associated with poor outcome of patients with peripheral T-cell
non-Hodgkin lymphoma and diffuse large B-cell lymphoma (26,27).
However, in 2005, Mestiri S reported that the 196R-TNFRII allele
revealed a significant association with increased OS and
disease-free survival in breast carcinoma patients (28). In the present study, regardless of
whether it pertains to the total cases or middle thoracic ESCC
patients, the result revealed that TNFR2 was positively correlated
with poor OS. This is consistent with the reports of Heemann C and
Nakamura N, and was in line with the roles of TNFR2 in the
malignant behaviors of tumor cells, which was previously reported.
However, this was not consistent with the report of Mestiri S. This
can be explained by the differences in tumor origin or races.
Moreover, the difference in OS between the two groups began vary
early, which was nearly from six months. Otherwise, in lower
thoracic ESCC patients, although the prognosis of patients in the
high expression of TNFR2 group was poorer than in the low
expression of TNFR2 group, the difference was not significant.
Particularly at the early period (before 46 months), there was
nearly no difference in OS between the two groups, and the
difference was mainly found at the late period (46 months later).
This suggests that the role of TNFR2 in the prognosis of lower
thoracic ESCC might be weaker and later than that of middle
thoracic ESCC.
ESCC is a rapidly evolving disease, and its
prognosis can be affected by clinical stage, tumor size, lymph node
involvement, and so on. Advanced clinical stage usually leads to
more malignant behaviors in tumor cells or longer time intervals in
cancer progression, and this can often result in poor prognosis
(29,30). Lymph node involvement and deeper
invasion usually imply the stronger invasion ability of tumor
cells, and are commonly used as criteria for evaluating for
clinical stage and progression (31–33). In
the total cases, the univariate COX regression analysis revealed
that clinical stage, lymph node involvement, and invasion depth
could significantly affect the OS of ESCC patients. Moreover, the
multivariate COX regression analysis revealed that both lymph node
involvement and invasion depth could significantly affect the OS of
ESCC patients. This suggests that clinical stage, lymph node
involvement, and invasion depth can be treated as independent
prognostic factors of ESCC. For middle thoracic ESCCs, clinical
stage, lymph node involvement and invasion depth were proven as
independent prognostic factors. However, in lower thoracic ESCCs,
only lymph node involvement could significantly affect the OS of
ESCC patients. This might be attributed to the reason that lower
thoracic ESCC was closely adjacent to esophageal and cardiac lymph
nodes, making it easier for lymph node involvement, or attributed
to the limited specimens in the present study.
Another TNFR, TNFR1 is not studied in this paper.
Its role in tumor has been reported previously. Zhao Y reported
that downregulating TNFR1 could suppress growth of breast cancer
cells (34). But, You BR reported
that down-regulation of TNFR1 suppressed apoptosis in TNF-α treated
lung cancer cells (35). In view of
different roles of TNFR1 in different types of tumors, it is hard
to predict the clinical significance of TNFR1 in ESCC. The role of
TNFR1 in tumor progression and prognosis still needs our later
study.
Of course, there are also some limitations in our
study. All cases in our study are mainly from nearby areas, so the
data is regional and the result is limited. Multi-center study
would supply more universal results. In addition, we only showed
clinical significance of TNFR2 here, no cell and animal experiments
was performed. Further experiments on cell, animal and molecular
levels would enrich our undersatanding about roles of TNFR2 in
ESCC.
In conclusion, the clinical significance of TNFR2 in
middle and lower thoracic ESCCs was confirmed, which enriched our
knowledge on the roles of TNFR2 in tumors. TNFR2 might be used as a
good prognostic factor of ESCC. Moreover, it can also be used as a
therapeutic target. As our deeper study, more efficient target drug
for TNFR2 is also possible. Of course, this still needs hard
work.
Acknowledgements
Not applicable.
Funding
This study was funded by the Doctor Foundation of
the Affiliated Hospital of Jining Medical University (grant no.
2016-BS-005, 2016-BS-002), the Natural Science foundation of
Shandong Provience, China(ZR2016HQ28).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DY and ZFL designed the study and wrote the
manuscript. RDL, HLW and JYW analyzed the data. YL, HBW and WW
collected the tissue specimens and performed immunohistochemistry
staining.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Jining Medical
University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Feng J, Qi B, Guo L, Chen LY, Wei XF, Liu
YZ and Zhao BS: miR-382 functions as a tumor suppressor against
esophageal squamous cell carcinoma. World J Gastroenterol.
23:4243–4251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han G, Wu Z, Zhao N, Zhou L, Liu F, Niu F,
Xu Y and Zhao X: Overexpression of stathmin plays a pivotal role in
the metastasis of esophageal squamous cell carcinoma. Oncotarget.
8:61742–61760. 2017.PubMed/NCBI
|
|
3
|
Jiang Q, Chen J, Zhang B, Niu J and He Y:
Prognostic Significance of periostin and mammalian target of
rapamycin (mTOR) in locally advanced esophageal squamous cell
carcinoma. Med Sci Monit. 23:3200–3208. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong Q, Fu L, Zhao Y, Liu Y, Li Q, Qiu X
and Wang E: Derlin-1 is a target to improve radiotherapy effect of
esophageal squamous cell carcinoma. Oncotarget. 8:55135–55146.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Zhao L, Zhang W, Yang C, Lian Z,
Wang S, Liu N, Pang Q, Wang P and Yu J: Prognostic value of
supraclavicular nodes and upper abdominal nodes metastasis after
definitive chemoradiotherapy for patients with thoracic esophageal
squamous cell carcinoma. Oncotarget. 8:65171–67185. 2017.PubMed/NCBI
|
|
6
|
Deng W, Wang Q, Xiao Z, Tan L, Yang Z,
Zhou Z, Liu N, Pang Q, Wang P, Yu J, et al: A prognostic nomogram
for overall survival after neoadjuvant radiotherapy or
chemoradiotherapy in thoracic esophageal squamous cell carcinoma: a
retrospective analysis. Oncotarget. 8:41102–41112. 2017.PubMed/NCBI
|
|
7
|
Tachimori Y, Nagai Y, Kanamori N, Hokamura
N and Igaki H: Pattern of lymph node metastases of esophageal
squamous cell carcinoma based on the anatomical lymphatic drainage
system. Dis Esophagus. 24:33–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Merkow RP, Bilimoria KY, Keswani RN, Chung
J, Sherman KL, Knab LM, Posner MC and Bentrem DJ: Treatment trends,
risk of lymph node metastasis and outcomes for localized esophageal
cancer. J Natl Cancer Inst. 106:dju1332014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miao X, Huang Y, Liu TT, Guo R, Wang B,
Wang XL, Chen LH, Zhou Y, Ji RR and Liu T: TNF-α/TNFR1 signaling is
required for the full expression of acute and chronic itch in mice
via peripheral and central mechanisms. Neurosci Bull. 34:42–53.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao YP, Tian QY, Frenkel S and Liu CJ:
The promotion of bone healing by progranulin, a downstream molecule
of BMP-2, through interacting with TNF/TNFR signaling.
Biomaterials. 34:6412–6421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ,
Liu GY, Syed NM, Lai Y, Lin EA, Kong L, et al: The growth factor
progranulin binds to TNF receptors and is therapeutic against
inflammatory arthritis in mice. Science. 332:478–484. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin EM, Remke A, Pfeifer E, Polz J,
Pietryga-Krieger A, Steffens-Weber D, Freudenberg MA, Mostböck S
and Männel DN: TNFR2 maintains adequate IL-12 production by
dendritic cells in inflammatory responses by regulating endogenous
TNF levels. Innate Immun. 20:712–720. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrarelli LK: Locking TNFR2 to kill
ovarian cancer. Science. 355:257–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X and Oppenheim JJ: Targeting TNFR2,
an immune checkpoint stimulator and oncoprotein, is a promising
treatment for cancer. Sci Signal. 10:eaal23282017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang F, Zhao N and Wu N: TNFR2 promotes
Adriamycin resistance in breast cancer cells by repairing DNA
damage. Mol Med Rep. 16:2962–2968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spreafico A, Coate L, Zhai R, Xu W, Chen
ZF, Chen Z, Patel D, Tse B, Brown MC, Heist RS, et al: Early
adulthood body mass index, cumulative smoking and esophageal
adenocarcinoma survival. Cancer Epidemiol. 47:28–34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi YJ, Lee DH, Han K, Yoon H, Shin CM,
Park YS and Kim N: Joint effects of low body mass index and alcohol
consumption on developing esophageal squamous cell cancer: A korean
nationwide population-based cohort study. Asian Pac J Cancer Preve.
18:1881–1887. 2017.
|
|
18
|
Short MW, Burgers KG and Fry VT:
Esophageal cancer. Am Fam Physician. 95:22–28. 2017.PubMed/NCBI
|
|
19
|
Xu F, Zhou G, Han S, Yuan W, Chen S, Fu Z,
Li D, Zhang H, Li D and Pang D: Association of TNF-α, TNFRSF1A and
TNFRSF1B gene polymorphisms with the risk of sporadic breast cancer
in northeast Chinese Han women. PLoS One. 9:e1011382014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jöhrer K, Janke K, Krugmann J, Fiegl M and
Greil R: Transendothelial migration of myeloma cells is increased
by tumor necrosis factor (TNF)-α via TNF receptor 2 and autocrine
up-regulation of mcp-1. Clin Cancer Res. 10:1901–1910. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tanimura Y, Kokuryo T, Tsunoda N, Yamazaki
Y, Oda K, Nimura Y, Mon Naing N, Huang P, Nakanuma Y, Chen MF, et
al: Tumor necrosis factor α promotes invasiveness of
cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett.
219:205–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang D, Wang LL, Dong TT, Shen YH, Guo XS,
Liu CY, Liu J, Zhang P, Li J and Sun YP: Progranulin promotes
colorectal cancer proliferation and angiogenesis through TNFR2 Akt
and ERK signaling pathways. Am J Cancer Res. 5:3085–3097.
2015.PubMed/NCBI
|
|
23
|
Wang L, Yang D, Tian J, Gao A, Shen Y, Ren
X, Li X, Jiang G and Dong T: Tumor necrosis factor receptor 2/AKT
and ERK signaling pathways contribute to the switch from
fibroblasts to CAFs by progranulin in microenvironment of
colorectal cancer. Oncotarget. 8:26323–26333. 2017.PubMed/NCBI
|
|
24
|
Geary LA, Nash KA, Adisetiyo H, Liang M,
Liao CP, Jeong JH, Zandi E and Roy-Burman P: CAF-secreted annexin
A1 induces prostate cancer cells to gain stem cell-like features.
Mol Cancer Res. 12:607–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kinugasa Y, Matsui T and Takakura N: CD44
expressed on cancer-associated fibroblasts is a functional molecule
supporting the stemness and drug resistance of malignant cancer
cells in the tumor microenvironment. Stem Cells. 32:145–156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heemann C, Kreuz M, Stoller I, Schoof N,
von Bonin F, Ziepert M, Löffler M, Jung W, Pfreundschuh M, Trümper
L and Kube D: Circulating levels of TNF receptor II are prognostic
for patients with peripheral T-cell non-Hodgkin lymphoma. Clin
Cancer Res. 18:3637–3647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakamura N, Goto N, Tsurumi H, Takemura M,
Kanemura N, Kasahara S, Hara T, Yasuda I, Shimizu M, Sawada M, et
al: Serum level of soluble tumor necrosis factor receptor 2 is
associated with the outcome of patients with diffuse large B-cell
lymphoma treated with the R-CHOP regimen. Eur J Haematol.
91:322–331. 2013.PubMed/NCBI
|
|
28
|
Mestiri S, Bouaouina N, Ben Ahmed S and
Chouchane L: A functional polymorphism of the tumor necrosis factor
receptor-II gene associated with the survival and relapse
prediction of breast carcinoma. Cytokine. 30:182–187. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chai P, Tian J, Zhao D, Zhang H, Cui J,
Ding K and Liu B: GSE1 negative regulation by miR-489-5p promotes
breast cancer cell proliferation and invasion. Biochem Biophys Res
Commun. 471:123–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Richards P, Ward S, Morgan J, Lagord C,
Reed M, Collins K and Wyld L: The use of surgery in the treatment
of ER+ early stage breast cancer in England: Variation by time, age
and patient characteristics. Eur J Surg Oncol. 42:489–496. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J, Long Q, Li H, Lv Q, Tan Q and Yang
X: The value of positive lymph nodes ratio combined with negative
lymph node count in prediction of breast cancer survival. J Thorac
Dis. 9:1531–1537. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jutric Z, Grendar J, Hoen HM, Cho SW,
Cassera MA, Newell PH, Hammill CW, Hansen PD and Wolf RF: Regional
metastatic behavior of nonfunctional pancreatic neuroendocrine
tumors: Impact of lymph node positivity on survival. Pancreas.
46:898–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okura M, Yanamoto S, Umeda M, Otsuru M,
Ota Y, Kurita H, Kamata T, Kirita T, Yamakawa N, Yamashita T, et
al: Prognostic and staging implications of mandibular canal
invasion in lower gingival squamous cell carcinoma. Cancer Med.
5:3378–3385. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao Y, Yang F, Li W, Xu C, Li L, Chen L,
Liu Y and Sun P: miR-29a suppresses MCF-7 cell growth by
downregulating tumor necrosis factor receptor 1. Tumour Biol.
39:10104283176922642017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
You BR, Han BR and Park WH:
Suberoylanilide hydroxamic acid increases anti-cancer effect of
tumor necrosis factor-α through up-regulation of TNF receptor 1 in
lung cancer cells. Oncotarget. 8:17726–17737. 2017. View Article : Google Scholar : PubMed/NCBI
|