Introduction
Breast cancer is the most common malignant tumor in
women with increasing incidence in China. Breast cancer is a
serious threat to women's health and brings serious economic
burdens to society and families (1).
Main methods of clinical treatment of breast cancer are surgery,
radiotherapy and chemotherapy, among which combination of
adriamycin with other drugs is commonly used in clinical practices
(1). However, for patients with
advanced breast cancer, prognosis of this treatment plan is poor,
and recurrence and metastasis are prone to occur. An important
reason for treatment failure is resistance of cancer cells to
adriamycin (2). miRNAs are
single-stranded, non-coding small RNAs consisting of 18–25
nucleotides, and they exist in eukaryotes and exert their roles in
the development and progression of tumors by up- or downregulating
the expression of certain important proteins (3). Studies suggest that miRNAs can regulate
the occurrence and development of tumor and their resistance to
chemotherapeutic agents (4). For
example, miR100 and miR-367 are upregulated in adriamycin-resistant
tumor cells (5,6). Chu et al showed that expression
of miR-93 in breast cancer cells can affect its resistance to
chemotherapeutic drugs (7).
In this study, the expression of miR-93 in breast
cancer cell line MCF-7 and adriamycin-resistant cell line MCF-7/ADM
was detected. miR-93 was transfected into cancer cells to examine
the effect of miR-93 on the resistance of MCF-7/ADM cells to
adriamycin, and to explore the possible mechanism of action of
miR-93. Our findings provide new insights for the clinical
treatment of adriamycin-resistant breast cancer patients.
Materials and methods
Materials
MCF-7/ADM and MCF-7 cell lines were purchased from
Aolushengwu (https://aolushengwu.biomart.cn/, Shanghai, China).
Human breast cancer azithromycin-resistant cell lines (MCF-7/ADM)
were purchased from Shanghai Zhen Biotechnology (Shanghai, China).
miR-93 mimics, inhibitor, and primers were purchased from Thermo
Fisher Scientific, Inc. Waltham, MA, USA). Adriamycin, fetal bovine
serum, thiazolyl (MTT) and RPMI-1640 medium were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dimethyl sulfoxide (DMSO),
reverse transcription kit, real-time-quantitative PCR (RT-qPCR)
kit, Bcl-2 antibody, P-gp antibody, GAPDH antibody, β-actin were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
TRIzol kit, liposomes (Lipofectamine 2000), U6 snRNA real-time PCR
kit which were purchased from Invitrogen (Thermo Fisher Scientific,
Inc., Carlsbad, CA, USA).
The study was approved by the Ethics Committee of
Shandong Provincial Hospital affiliated to Shandong University
(Jinan, China).
Cell culture
MCF-7/ADM and MCF-7 cells were cultured in RPMI-1640
medium (containing 10% fetal bovine serum) at 37°C with 5%
CO2, and 1 µg/ml of adriamycin was added to MCF-7/ADM
culture medium. Subculture was performed every 3 to 4 days.
Detection of miR-93 expression by
RT-qPCR
U6 snRNA was used as the endogenous control to
measure the relative expression level of miR-93. MCF-7 and
MCF-7/ADM cells were collected, and TRIzol reagent was used to
extract total RNA. Total RNA was reversely transcribed into cDNA
and the reaction conditions were: 37°C for 40 min, 85°C for 40 sec,
using cDNA as a template and using miR-93 primers for PCR
amplification. PCR reaction conditions were: 94°C for 2 min,
followed by 50 cycles of 94°C for 30 sec, 55°C for 30 sec, and 72°C
for 30 sec. Cq values were processed using 2−ΔΔCq method
to calculate the relative expression level of miR-93 (8). miR-93 primer sequences were:
5′-UUCUCCGAACGUGUCACGUTT-3′ (forward) and
5′-ACGUGACACGUUCGGAGAAATT-3′ (reverse).
Cell transfection
MCF-7 and MCF-7/ADM cells were seeded into 6-well
plates and miR-93 mimics, inhibitor, and their negative controls
were transfected into MCF-7 and MCF-7/ADM, respectively, using
Lipofectamine 2000. After 6 h, cells were cultured for 48–96 h
(37°C, 5% CO2) in fresh culture medium and harvested.
RT-qPCR was used to detect mRNA expression, and reduced expression
level of miRNA indicated the successful transfection.
MTT assay to detect the resistance of
cancer cells to adriamycin
Transfected tumor cell suspension was inoculated
into the microtiter wells of the test plates. After cell adherence,
adriamycin was added to final concentrations of 5, 10, 20, 40, and
80 µg/ml, and control and blank control wells were set. Cells were
cultured at 37°C with 5% CO2 for 72 h, then 20 µl MTT
solution was added to each well, followed by cell culture for
another 4 h. Finally, 100 µl DMSO was added to each well, and
crystals were dissolved by shaking for 15 min. Absorbance of each
well at a wavelength of 490 nm was measured with a microplate
reader (Bio-Rad, Hercules, CA, USA). Cell growth inhibition rate =
(absorbance of the control group - absorbance of the experimental
group)/absorbance of the control group ×100%. IC50 value
of adriamycin was calculated.
Western blot analysis to detect Bcl-2
and P-gp protein expression
MCF-7 and MCF-7/ADM cells were seeded into 6-well
plates. MCF-7/ADM cells were transfected with miR-93 mimics and
negative control groups, and total protein was extracted after 72 h
of cell culture. Protein concentration was measured by BCA method.
Then, 10% PAGE was performed to separate proteins, followed
transmembrane to PVDE membrane. Blocking was performed using 5%
skimmed milk powder (BSA). After washing with TBST (TBS + Tween) 3
times, rabbit polyclonal Bcl-2 antibody (dilution: 1/500; cat. no.
ab59348) and rabbit polyclonal P-gp antibody (dilution: 1/500; cat.
no. ab103477) were added and incubated for 12 h at 4°C. After
washing with TBST 3 times, secondary goat anti-rabbit (HRP) IgG
antibody (dilution: 1/2,000; cat. no. ab6721) was added and
incubated for 2 h at room temperature. All the antibodies were
purchased from Abcam (Cambridge, MA, USA) After washing with TBST 3
times, ECL reagent was added to develop signals, and ImageJ
software was used to normalize the expression level of each protein
to endogenous control GAPDH.
Statistical analysis
SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Quantitative data were analyzed
by t-test, and results were expressed as mean ± standard deviation.
P-values indicate a two-sided probability, and test standard α was
0.05.
Results
Expression of miR-93 in MCF-7 and
MCF-7/ADM cells
Results showed that expression level of miR-93
decreased in MCF-7/ADM cells, and the expression level was 40%
(0.39±0.04) of that in MCF-7 cells, and the difference was
statistically significant (p<0.05, Fig. 1).
Effect of miR-93 transfection on
adriamycin resistance of MCF-7 and MCF-7/ADM cells
Before transfection, IC50 value of MCF-7
cells to adriamycin (11.02±0.95) was significantly lower than that
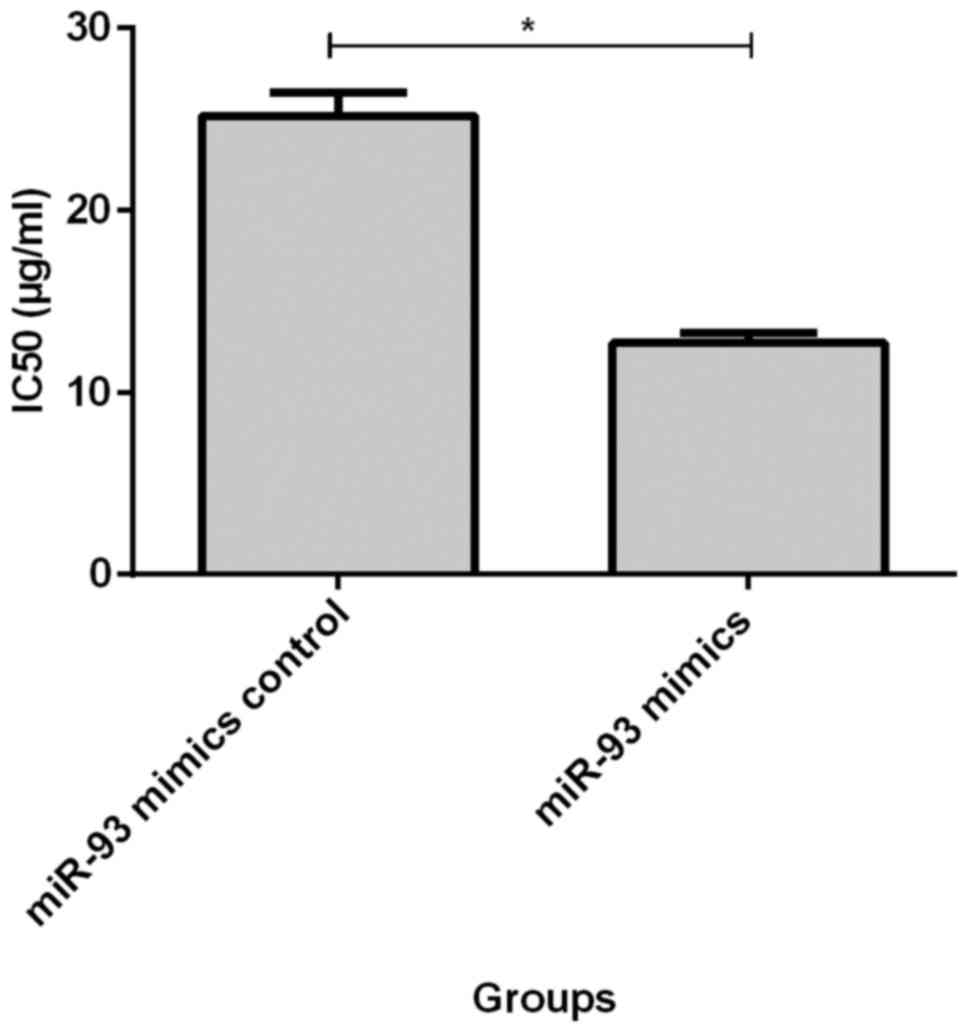
of MCF-7/ADM cells (21.29±1.83, p<0.05, Fig. 2). IC50 value of MCF-7/ADM
cells at 72 h after transfection of miR-93 mimics (12.71±0.54) was
significantly lower than that of the negative control group
(25.16±1.28, p<0.05, Fig. 3).
IC50 was significantly higher in MCF-7 cells
(19.88±1.28) at 72 h after transfection with miR-93 inhibitor than
in negative control group (11.02±0.95, p<0.05, Fig. 4).
Effect of miR-93 transfection on the
expression of Bcl-2 and P-gp proteins in MCF-7 and MCF-7/ADM
cells
Before transfection, expression levels of Bcl-2 and
P-gp proteins in MCF-7/ADM cells were 1.63±0.24 and 1.76±0.22 times
that of MCF-7 cells, respectively. The differences were
statistically significant (p<0.05, Fig. 5). At 72 h after transfection of miR-93
mimics, the expression levels of Bcl-2 and P-gp protein in
MCF-7/ADM cells were 0.27±0.06 and 0.39±0.05 times of those in the
negative control group, and the differences were statistically
significant (p<0.05, Fig. 6). At
72 h after transfection with miR-93 inhibitor, expression levels of
Bcl-2 and P-gp protein in MCF-7 cells was 1.48±0.10 and 1.56±0.11
times of that of the negative control group, respectively, and the
differences were statistically significant (p<0.05, Fig. 7).
Discussion
The combinations of adriamycin and other drugs are
widely used in the clinical treatment of breast cancer (1). Adriamycin is a kind of chemotherapeutic
drug widely used in clinical practices (9). Due to the emergence of adriamycin
resistance in breast cancer cells, patients with advanced breast
cancer are prone to recurrence or metastasis after treatment, which
reduces the therapeutic effect. Mechanism of development of drug
resistance in tumor cells is complex and may be related to the
expression and dysfunction of multiple genes (10).
miRNA is a non-coding RNA and plays an important
role in the occurrence and development of tumors (5). Studies have shown that dysfunction of
different miRNAs is closely related to tumor recurrence,
metastasis, and drug resistance, suggesting that miRNAs can be used
as gene targets for cancer therapy (7). Li et al (10) found that miR-106b can reverse the
resistance of breast cancer cell lines. miR-93 expression in
non-small cell lung cancer, gastric cancer, esophageal cancer,
colon cancer tissue exist down phenomenon (11–14), in
breast cancer cells can affect its resistance to chemotherapeutic
drugs (7).
In this study, the differential expression of miR-93
in breast cancer cell lines MCF-7 and MCF-7/ADM was detected.
miR-93 mimics and inhibitor were transfected into cancer cells to
analyze the effect of miR-93 on the resistance of MCF-7/ADM cells
to adriamycin, and to detect the expression of Bcl-2 and P-gp
proteins before and after transfection, so as to explore the
possible mechanism of the act of miR-93. All experiments were
performed according to the manufacturer's instructions, so our data
are reliable and accurate.
Results showed that the expression level of miR-93
decreased in MCF-7/ADM cells, indicating that miR-93 expression was
downregulated in adriamycin-resistant breast cancer cells, which is
consistent with the findings of Chu et al (7). Before transfection, IC50
values of MCF-7 cells to adriamycin were lower than MCF-7/ADM
cells. After transfection of miR-93 mimics, IC50 value
of MCF-7/ADM cells was lower than that before transfection.
IC50 value of MCF-7 cells at 72 h after transfection
with miR-93 inhibitor were higher than that before transfection,
suggesting that transfection of miR-93 could increase the
resistance of drug-resistant cells to adriamycin, which were
consistent with the findings reported by previous studies (15–17).
P-gp is an expression product of multidrug
resistance gene MDR. By combining with tumor chemotherapeutic
drugs, P-gp pumped drugs out of the cell to reduce the
concentration of drug in cells to produce drug resistance (18). In this study, expression level of P-gp
in MCF-7/ADM cells was significantly higher than that in MCF-7
cells, so upregulated expression of P-gp may be related to the
mechanism of drug resistance of tumor cells to adriamycin. Compared
with negative control cells, tranfection with miR-93 mimics
significantly reduced the expression level of P-gp in MCF-7/ADM
cells. In addition, expression level of Bcl-2 protein in MCF-7/ADM
cells before transfection was significantly higher than that in
MCF-7 cells, and the expression level of Bcl-2 protein in MCF-7/ADM
cells after transfection of miR-93 mimics was lower than that of
negative control group. After transfection of miR-93 inhibitor into
MCF-7 cells, expression levels of P-gp and Bcl-2 protein were
higher than those of negative control group, indicating that the
transfection of miR-93 can downregulate the expression of Bcl-2 and
P-gp proteins, so as to reduce adriamycin resistance. Bcl-2 related
anti-apoptotic gene (BAG) family is associated with the occurrence
and prognosis of various tumors, and the expressed proteins have
the functions of inhibiting apoptosis, promoting tumor cell
proliferation and metastasis (19).
The binding of BAG3 protein to anti-apoptotic protein Bcl-2 can
regulate the apoptosis process and play an important role in
development of drug resistance in malignant tumor cells (20,21).
This study shows that miR-93 is downregulated in
adriamycin-resistant breast cancer cell lines. Upregulation of
miR-93 can reduce the resistance of cells to adriamycin, and the
mechanism of action may be related to the downregulated Bcl-2 and
P-gp proteins expression. Therefore, our findings provide new
insights for the treatment of breast cancer, and miR-93 may serve
as a potential target for gene therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
QW drafted this manuscript. QW and CS were mainly
devoted to cell transfection and MTT assay. CW and JL contributed
to western blot analysis. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Shandong Provincial Hospital affiliated to Shandong University
(Jinan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Turati F, Carioli G, Bravi F, Ferraroni M,
Serraino D, Montella M, Giacosa A, Toffolutti F, Negri E, Levi F,
et al: Mediterranean diet and breast cancer risk. Nutrients.
10:E3262018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang F, Zhao N and Wu N: TNFR2 promotes
adriamycin resistance in breast cancer cells by repairing DNA
damage. Mol Med Rep. 16:2962–2968. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fang L, Li H, Wang L, Hu J, Jin T, Wang J
and Yang BB: MicroRNA-17-5p promotes chemotherapeutic drug
resistance and tumour metastasis of colorectal cancer by repressing
PTEN expression. Oncotarget. 10:2974–2987. 2014.
|
|
5
|
Danesh H, Hashemi M, Bizhani F, Hashemi SM
and Bahari G: Association study of miR-100, miR-124-1, miR-218-2,
miR-301b, miR-605, and miR-4293 polymorphisms and the risk of
breast cancer in a sample of Iranian population. Gene. 647:73–78.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang GC, He QY, Tong DK, Wang CF, Liu K,
Ding C, Ji F and Zhang H: MiR-367 negatively regulates apoptosis
induced by adriamycin in osteosarcoma cells by targeting KLF4. J
Bone Oncol. 5:51–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu S, Liu G, Xia P, Chen G, Shi F, Yi T
and Zhou H: miR-93 and PTEN: Key regulators of
doxorubicin-resistance and EMT in breast cancer. Oncol Rep.
38:2401–2407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li BH, Yuan L, Shi RR and Wang JG:
Reversal of adriamycin resistance by digoxin in human breast cancer
cell line MCF-7/adriamycin and its mechanism. Sheng Li Xue Bao.
67:611–617. 2015.(In Chinese). PubMed/NCBI
|
|
9
|
Cao B, Li M, Zha W, Zhao Q, Gu R, Liu L,
Shi J, Zhou J, Zhou F, Wu X, et al: Metabolomic approach to
evaluating adriamycin pharmacodynamics and resistance in breast
cancer cells. Metabolomics. 9:960–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li N, Miao Y, Shan Y, Liu B, Li Y, Zhao L
and Jia L: MiR-106b and miR-93 regulate cell progression by
suppression of PTEN via PI3K/Akt pathway in breast cancer. Cell
Death Dis. 8:e27962017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang W, Bai J, Liu D, Wang S, Zhao N, Che
R and Zhang H: MiR-93-5p up-regulation is involved in non-small
cell lung cancer cells proliferation and migration and poor
prognosis. Gene. 647:13–20. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma DH, Li BS, Liu JJ, Xiao YF, Yong X,
Wang SM, Wu YY, Zhu HB, Wang DX and Yang SM: miR-93-5p/IFNAR1 axis
promotes gastric cancer metastasis through activating the STAT3
signaling pathway. Cancer Lett. 408:23–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ansari MH, Irani S, Edalat H, Amin R and
Roushandeh Mohammadi A: Deregulation of miR-93 and miR-143 in human
esophageal cancer. Tumour Biol. 37:3097–3103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu XF, Zou J, Bao ZJ and Dong J: miR-93
suppresses proliferation and colony formation of human colon cancer
stem cells. World J Gastroenterol. 17:4711–4717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang Y, Liao XH, Yu CX, Yao A, Qin H, Li
JP, Hu P, Li H, Guo W, Gu CJ, et al: MiR-93-5p inhibits the EMT of
breast cancer cells via targeting MKL-1 and STAT3. Exp Cell Res.
357:135–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shyamasundar S, Lim JP and Bay BH: miR-93
inhibits the invasive potential of triple-negative breast cancer
cells in vitro via protein kinase WNK1. Int J Oncol.
49:2629–2636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng ZQ, Qian J, Liu FQ, Lin J, Shao R,
Yin JY, Tang Q, Zhang M and He L: Expression level of miR-93 in
formalin-fixed paraffin-embedded tissues of breast cancer patients.
Genet Test Mol Biomarkers. 18:366–370. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kawami M, Yamada Y, Issarachot O,
Junyaprasert VB, Yumoto R and Takano M: P-gp modulating effect of
Azadirachta indica extract in multidrug-resistant cancer
cell lines. Pharmazie. 73:104–109. 2018.PubMed/NCBI
|
|
19
|
McCollum AK, Casagrande G and Kohn EC:
Caught in the middle: The role of Bag3 in disease. Biochem J.
425:e1–3. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang HQ, Liu BQ, Gao YY, Meng X, Guan Y,
Zhang HY and Du ZX: Inhibition of the JNK signalling pathway
enhances proteasome inhibitor-induced apoptosis of kidney cancer
cells by suppression of BAG3 expression. Br J Pharmacol.
158:1405–1412. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chiappetta G, Ammirante M, Basile A,
Rosati A, Festa M, Monaco M, Vuttariello E, Pasquinelli R, Arra C,
Zerilli M, et al: The antiapoptotic protein BAG3 is expressed in
thyroid carcinomas and modulates apoptosis mediated by tumor
necrosis factor-related apoptosis-inducing ligand. J Clin
Endocrinol Metab. 92:1159–1163. 2007. View Article : Google Scholar : PubMed/NCBI
|