Introduction
Breast cancer is a significant health problem for
women in China. According to the China Health Statistics Yearbook
published in 2011, approximately 169,452 new patients with breast
cancer were diagnosed and 44,908 breast cancer-associated
mortalities occurred in China in 2008; furthermore, these numbers
accounted for 12.2% of all newly diagnosed breast cancer cases and
9.6% of all mortalities from breast cancer worldwide (1,2).
Therefore, it is important to identify predictive and prognostic
factors for breast cancer, and to assess their potential impact for
treatment selection. The discovery of human epidermal growth factor
receptor 2 (HER-2)/neu gene amplification and its association with
poor prognosis and an aggressive tumor phenotype has improved our
understanding of the prognosis and therapeutic management of
patients with breast cancer (3,4).
The HER-2/neu gene is an oncogene located on
chromosome 17 that encodes HER-2/neu, a transmembrane glycoprotein
with tyrosine kinase activity from the epidermal growth factor
receptor family (5). In ~20% of
patients with invasive breast cancer, the HER-2/neu gene is
amplified or overexpressed (6,7). It can
activate cellular signaling pathways such as PI3K-Akt and Ras-MAPK,
leading to cell proliferation, growth, and survival (8). The amplification of HER-2 is associated
with a poor prognosis, metastasis, chemoresistance, and an
aggressive tumor phenotype (9).
Trastuzumab, a recombinant humanized monoclonal antibody against
HER-2, improves the survival of patients with HER-2-overexpressing
tumors (10,11). However, in clinical practice, the
definition of primary tumor HER-2 overexpression in breast cancer
patients is controversial. In certain patients with primary
trastuzumab resistance, HER-2 expression in the peripheral blood
after primary tumor resection is difficult to detect. In rescue
treatments for metastatic breast cancer, the anti-HER-2 therapies
are ineffective in a proportion of the patients. In addition,
biopsy results for certain types of metastasis are often
unavailable, and the HER-2 status cannot be determined.
Furthermore, the use of anti-HER-2 treatments based on primary
tumor HER-2 status is not adequate because of the treatment delay
(12,13). Therefore, the identification of an
indicator for the real-time monitoring of HER-2 status is urgently
required.
In clinical practice, the main methods currently
used to determine tissue HER-2 status are immunohistochemistry
(IHC) and fluorescence in situ hybridization (FISH)
(14). Only tumors with scores of 2+
or 3+ with a FISH ratio ≥2.0 are defined as HER-2-positive
(15). In addition to determining
HER-2 status in tissue specimens, there has been a high level of
interest in liquid biopsy to determine the level of circulating
HER-2, due to its accessibility and the possibility for the serial
monitoring of the tumor response to therapy (16). The detection of HER-2 mRNA-positive
circulating tumor cells (CTCs) in peripheral blood is considered a
useful tool in the early diagnosis of breast cancer, and an
independent prognostic factor for disease-free survival (DFS)
(17,18). A number of previous studies have
indicated that HER-2 mRNA in the peripheral blood of patients with
breast cancer can be detected by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR), and that the detected levels
were consistent with HER-2 status determined by IHC (19–21). To
the best of our knowledge, a cutoff value for HER-2 mRNA as a
marker of breast cancer has not been determined to date. Changes in
the HER-2 mRNA level in peripheral blood may provide information
for the selection of adequate therapeutic regimens, especially in
patients exhibiting a poor response to chemotherapy.
In the present study, one-step RT-qPCR was used to
detect circulating HER-2 mRNA, in order to determine its efficacy
in indicating HER-2 expression status in breast cancer. The main
aim of the present study was to determine the HER-2 mRNA status in
the peripheral blood of patients with breast cancer prior to
surgery and healthy individuals, to assess its potential diagnostic
value in patients with breast cancer. For this purpose, we
established an exact cutoff for HER-2 mRNA as a marker of breast
cancer. In addition, the present study investigated whether the
HER-2 mRNA level in the peripheral blood could predict the efficacy
of neoadjuvant chemotherapy without trastuzumab.
Materials and methods
Patients
Peripheral blood was obtained from 70 patients with
breast cancer without distant metastases, and 35 healthy control
subjects (median age is 52 years, age range: 27 to 82 years,
female, with no history of breast cancer at the Second Affiliated
Hospital of Soochow University (Suzhou, China) between August 2016
and August 2017. All patients who participated in this study signed
a document of informed consent. Study approval was obtained from
the independent ethics committee at the Second Affiliated Hospital
of Soochow University (Suzhou, China). The privacy of the patients
involved was protected.
Patient characteristics
This study included 70 women with breast cancer,
with a median age of 52 years (range: 27–82 years). The pathology
type was invasive ductal carcinoma for all patients. The
distribution of tumor sizes (T) was as follows: 42.9% (n=30) T1 (≤2
cm), 54.3% (n=38) T2 (>2 cm and <5 cm), and 2.8% (n=2) T3 (≥5
cm). Lymph node status was negative in 41.4% (n=29), positive in
50% (n=35), and unknown in 8.6% (n=6) of the patients. According to
the World Health Organization grading system (22,23), 74.3%
(n=52) of tumors were well differentiated (grade I) and/or
moderately differentiated (grade II), 10% (n=7) were poorly
differentiated (grade III), and 15.7% (n=11) were of unknown
differentiation. None of the patients had distant metastases; 24.3%
(n=17) had stage I cancer, 41.4% (n=29) had stage II, 21.4% (n=15)
had stage III, and 12.9% (n=9) had an unknown cancer status.
Clinical and pathological characteristics of the patients are
listed in Table I. Among the 95 blood
samples isolated from the patients with breast cancer, 70 (73.7%)
were from preoperative patients, 13 (13.7%) were from postoperative
patients without adjuvant therapy, and 12 (12.6%) were from
patients receiving neoadjuvant therapy.
 | Table I.Patient clinical and pathological
characteristics. |
Table I.
Patient clinical and pathological
characteristics.
|
| All patients | HER-2 mRNA
positive | HER-2 mRNA
negative | HER-2 value |
|---|
|
|
|
|
|
|
|---|
| Parameter | n | % | n | % | n | % | P-value |
|---|
| Patients
enrolled | 70 | 100% | 22 | 31.4 | 48 | 68.6 |
|
| Age years (median)
range) | 52 (27–82) | 51 (30–66) | 53 (27–82) | 0.131 |
| Menopausal
status |
|
|
|
|
|
| 0.114 |
|
Premenopausal | 36 | 51.4 | 12 | 17.1 | 24 | 34.3 |
|
|
Postmenopausal | 34 | 48.6 | 10 | 14.3 | 24 | 34.3 |
|
| Stage |
|
|
|
|
|
| 0.367 |
| I | 17 | 24.3 | 9 | 12.9 | 8 | 11.4 |
|
| II | 29 | 41.4 | 5 | 7.1 | 24 | 34.3 |
|
|
III | 15 | 21.4 | 5 | 7.1 | 10 | 14.3 |
|
|
Unknown | 9 | 12.9 | 3 | 4.3 | 6 | 8.6 |
|
| Tumor grade |
|
|
|
|
|
| 0.666 |
|
I/II | 52 | 74.3 | 16 | 22.9 | 36 | 51.4 |
|
|
III | 7 | 10.0 | 2 | 2.9 | 5 | 7.1 |
|
|
Unknown | 11 | 15.7 | 4 | 5.7 | 7 | 10.0 |
|
| Tumor size
(cm) |
|
|
|
|
|
| 0.663 |
| 1
(2≥T1) | 30 | 42.9 | 10 | 14.3 | 20 | 28.6 |
|
| 2
(2<T2<5) | 38 | 54.3 | 11 | 15.7 | 27 | 38.6 |
|
| 3
(5≤T3) | 2 | 2.8 | 1 | 1.4 | 1 | 1.4 |
|
| Lymph node
status |
|
|
|
|
|
| 0.860 |
| 0 | 29 | 41.4 | 10 | 14.3 | 19 | 27.1 |
|
|
1–3 | 20 | 28.6 | 5 | 7.1 | 15 | 21.4 |
|
|
4–9 | 11 | 15.7 | 3 | 4.3 | 8 | 11.4 |
|
|
≥10 | 4 | 5.7 | 2 | 2.9 | 2 | 2.9 |
|
|
Unknown | 6 | 8.6 | 2 | 2.9 | 4 | 5.7 |
|
| Lymphovascular
Invasion |
|
|
|
|
|
| 0.035a |
| No | 37 | 52.9 | 9 | 12.9 | 28 | 40.0 |
|
|
Yes | 19 | 27.1 | 8 | 11.5 | 11 | 15.7 |
|
|
Unknown | 14 | 20.0 | 5 | 7.1 | 9 | 12.8 |
|
| Perineural
Invasion |
|
|
|
|
|
| 0.506 |
| No | 43 | 61.4 | 9 | 12.8 | 34 | 48.7 |
|
|
Yes | 7 | 10.0 | 4 | 5.7 | 3 | 4.3 |
|
|
Unknown | 20 | 28.6 | 9 | 12.8 | 11 | 15.7 |
|
| ER |
|
|
|
|
|
| 0.526 |
|
Negative | 21 | 30.0 | 5 | 7.1 | 16 | 22.9 |
|
|
Positive | 49 | 70.0 | 17 | 24.3 | 32 | 45.7 |
|
| PR |
|
|
|
|
|
| 0.748 |
|
Negative | 32 | 45.7 | 6 | 8.6 | 26 | 37.1 |
|
|
Positive | 38 | 54.3 | 16 | 22.9 | 22 | 31.4 |
|
| Ki-67 |
|
|
|
|
|
| 0.007a |
|
≤14 | 16 | 22.9 | 5 | 7.1 | 11 | 15.7 |
|
|
>14 | 54 | 77.1 | 17 | 24.3 | 37 | 52.9 |
|
| Sub-type |
|
|
|
|
|
| 0.148 |
| Luminal
A | 10 | 14.3 | 5 | 7.1 | 5 | 7.1 |
|
| Luminal
B | 39 | 55.7 | 12 | 17.1 | 27 | 38.6 |
|
| ERBB
2+ | 10 | 14.3 | 0 | 0.0 | 10 | 14.3 |
|
|
Basal-like | 11 | 15.7 | 5 | 7.1 | 6 | 8.6 |
|
| HER-2 |
|
|
|
|
|
| 0.039a |
|
Negative | 50 | 71.4 | 21 | 30.0 | 29 | 41.4 |
|
|
Positive | 20 | 28.6 | 1 | 1.4 | 19 | 21.7 |
|
Neoadjuvant therapy
Three cycles of docetaxel (75 mg/m2 on
day 1 every 3 weeks), followed by three cycles of FEC
(5′-fluorouracil 500 mg/m2, epirubicin 75
mg/m2, and cyclophosphamide 600 mg/m2 on day
1 every 3 weeks).
MRI assessment
A total of 5 patients with confirmed breast cancer
underwent MRI examinations at GE Signa Excite HD 3.0T scanner. All
breast MRI scans were confirmed by two consultant radiologists, and
where there was discordance the images were reviewed by a third
consultant radiologist. The schedule of imaging was: Prior to
antitumor treatment (time point zero; TP0), after three treatment
cycles (TP3), and after six treatment cycles (TP6). However, due to
the small sample of biopsy samples, the tissue sub-type could not
be determined.
Sample collection
Peripheral blood (5 ml) was collected in vacuum
blood collection tubes with EDTA (0.05 M). The whole blood samples
were stored at 4°C.
RNA isolation
Total RNA was collected using an RNA extraction kit
for whole blood (Ezol kit, Suzhou GenePharma Co., Ltd.) according
to the manufacturer's protocol. RNA purity was determined through
the measurement of absorbance using a 96-well plate (Corning
Incorporated, Corning, NY, USA) (A) at 260, 280, and 230 nm with
NanoDrop 2000 (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
(24). RNA concentration was
determined from the A260. Qualifying samples were used to detect
HER-2 expression by one-step RT-qPCR.
One-step RT-qPCR
HER-2 levels were measured using one-step HER-2
TaqMan RT-qPCR kits (Suzhou GenePharma Co., Ltd.), the fluorophore
(SYBR Green) was purchased from Thermo Fisher Scientific, Inc.
Reactions contained 2.5 µl 10× PCR buffer, 2.5 µl 5× RT buffer,
0.375 µl of each primer, 0.5 µl of each probe, 0.5 µl enzyme mix,
and 8 µl blood RNA extract in 20 µl. Total RNA from MDA-MB-231
cells (from the American Type Culture Collection, Manassas, VA,
USA) were used as the negative control. RT-qPCR cycling was
performed on an ABI-Step One Plus system (Thermo Fisher Scientific,
Inc.) as follows: 45°C for 5 min, 95°C for 30 sec, and 40 cycles of
5 sec at 95°C and 50 sec at 62°C. Fluorogenic signals were detected
at the end of the annealing-extension steps. A threshold was
automatically set and the threshold cycle value (Cq) was determined
(25,26). Two replicate assays within and between
runs were performed. The sequences of the primers used for HER-2
were as follows: Forward, 5′-CCAGCTGGCTCTCACACTG-3′; and reverse,
5′-AGCCCTTACACATCGGAGAAC-3′; probe,
5′-FAM/AGGCCCGAGAGCGGTTGGTGT/BHQ1-3′. Sequences of the primers used
for β-actin were as follows: Forward,
5′-GACCCAGATCATGTTTGAGACCTT-3′; and reverse,
5′-CCATCACGATGCCAGTGGTA-3′; probe,
5′-FAMCCATGTACGTTGCTATCCAGGCTGTGCBHQ1-3′.
Sensitivity evaluation of HER-2 mRNA
RT-qPCR
To test the sensitivity of HER-2 detection by
RT-qPCR on blood samples, the SKBR-3 cell line (ATCC), which
expresses high levels of HER-2, was 10-fold serially diluted with
PBS (pH 7.4) from 1×105 cells/ml to 1 cell/ml using
fluorescence-activated cell sorting (FACS; BD FACSARIA II; BD
Biosciences, Franklin Lakes, NJ, USA) and spiked into 5 ml normal
blood (27). Following RNA
extraction, HER-2 mRNA RT-qPCR was performed in duplicate.
Immunohistochemistry (IHC) and
fluorescence in situ hybridization (FISH)
Immunohistochemistry was performed according to
previously described methods using a normal light microscope
(magnification, ×400) (28).
Paraffin-embedded tissues from surgery were deparaffinized and
pretreated in a microwave. The slides were incubated using
monoclonal mouse anti-HER2 antibodies (1:200, Proteintech
60311-1-lg, China) for 1 h at 37°C. After rinsing with PBS (pH
7.4), sections were treated with a horseradish peroxidase
conjugated-goat-anti-mouse secondary antibody (1:2,000, Jackson
ImmunoResearch, Inc., West Grove, PA, USA; cat no. 115-035-003) at
room temperature for 1 h. Then, the slides were incubated with
3-diaminobenzidine solution for 20 min at room temperature.
Patients with HER2+ breast cancer was diagnosed by FISH in the
Second Affiliated Hospital of Soochow University as described
previously (22).
Cutoff value determination
To determine the cutoff value for HER-2, a total of
35 normal blood and 70 blood samples from breast cancer patients
prior to surgery were collected and sorted by FACS (27). MB-MDA-231 and SKBR-3 cells (1×10°,
1×101, 1×102, 1×103, and
104) were spiked into 5 ml normal blood. Samples were
analyzed using the TaqMan HER-2 RT-qPCR kits (Suzhou GenePharma
Co., Ltd). For each sample, Ct values for HER-2 were normalized to
the Ct values of β-actin as an endogenous control to yield ∆Ct
data. For all of the 70 breast cancer blood samples and cell
line-spiked samples, ∆Ct values were normalized to the median ∆Ct
values of the 35 normal blood samples to obtain ∆∆Ct data. HER-2
relative expression for each sample was calculated using the
2−∆∆Ct formula (26,28).
Statistical analysis
Statistical analyses were performed using
Statistical Package for Social Sciences software version 22 for
Windows (IBM Corp., Armonk, NY, USA) and GraphPad Prism 6.01
(GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of
variance (ANOVA), followed by Tukey's multiple comparison test by
using GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA,
USA) to compare the HER-2 mRNA levels in cell lines, peripheral
blood and tissues. The Pearson χ2 test was used to
assess the associations between blood HER-2 status and clinical
features. The Wilcoxon signed rank test was performed to examine
the mean changes in paired samples. Receiver operating
characteristic (ROC) curve analysis was performed to determine the
relationship between the HER-2 mRNA level in blood samples, and the
HER-2 FISH and IHC status of primary tumor tissues. Sensitivity,
specificity, and Youden index values were calculated to assess the
diagnostic performance of RT-qPCR for determining HER-2 status.
HER-2 expression curves representative of measurements taken during
neoadjuvant chemotherapy were compared with changes in tumor size.
Cohen's Kappa coefficient and 95% confidence intervals (CI) were
calculated to assess the agreement in HER-2 status between primary
tumors and peripheral blood. All data are presented as the mean ±
standard error. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sensitivity evaluation of RT-qPCR
detection of HER-2 mRNA
The relative values obtained using serially diluted
SKBR-3 cells are shown in Fig. 1. The
mean relative RT-qPCR values obtained using 1×104,
1×103, 1×102, 1×101, and 1×10°
cells/ml were 5.32, 1.84, 0.89, 0.84, and 0.70, respectively; the
results indicated that the relative Ct values of RT-qPCR decreased
with a decreasing cell number. Therefore, it was determined that
RT-qPCR demonstrated good sensitivity for the detection of HER-2
mRNA. Ct values from blank control reactions were negative for all
experiments.
Peripheral blood HER-2 mRNA in
preoperative patients and controls
Peripheral blood HER-2 mRNA status in samples from
70 preoperative patients and 35 healthy controls were determined by
RT-qPCR. Tumor samples were HER-2-positive in 20 (28.6%) patients
and negative in 50 (71.4%) patients when analyzed by IHC and/or
FISH (14,22,28–30).
The upper 95% CI value for normal blood was 1.512,
which was set as the cutoff for the system analysis based on the
2−∆∆Ct method (19,26,31).
The system could distinguish tumor blood from normal blood, and
tumor cells with high HER-2 expression from cells with low
expression. The median values for the relative HER-2 mRNA level
were 4.52 (0.39–29.92) for HER-2-positive, 1.89 (0.14–26.71) for
HER-2-negative, and 1.12 (0.48–4.41) for healthy control samples
(P<0.0001, one-way ANOVA; Fig. 2).
Significant differences were observed between the peripheral blood
samples with positive HER-2 mRNA expression from healthy controls
and that from samples patients with from HER-2-positive (n=22,
P<0.001, one-way ANOVA) or HER-2-negative (n=48, P<0.001,
one-way ANOVA) tumor samples. Significant differences were observed
between the patients with HER-2 mRNA-positive peripheral blood and
those with HER-2-positive tissue (P<0.001), and between the
peripheral blood from subjects with HER-2 negative tissue or
samples before operation from preoperative patients with breast
cancer (P<0.05). No significant differences were observed
between HER-2 mRNA-positive and HER-2 mRNA-negative peripheral
blood from patients prior to surgery (P>0.05).
Comparison with HER-2 status
determined by IHC/FISH in tumor tissue samples as a standard
HER-2 mRNA level in blood was correlated with HER-2
status in primary tumor tissue samples (P<0.05, Pearson
χ2 test). Blood HER-2 mRNA level in patients with breast
cancer and HER-2 status determined by IHC/FISH in tumor tissues was
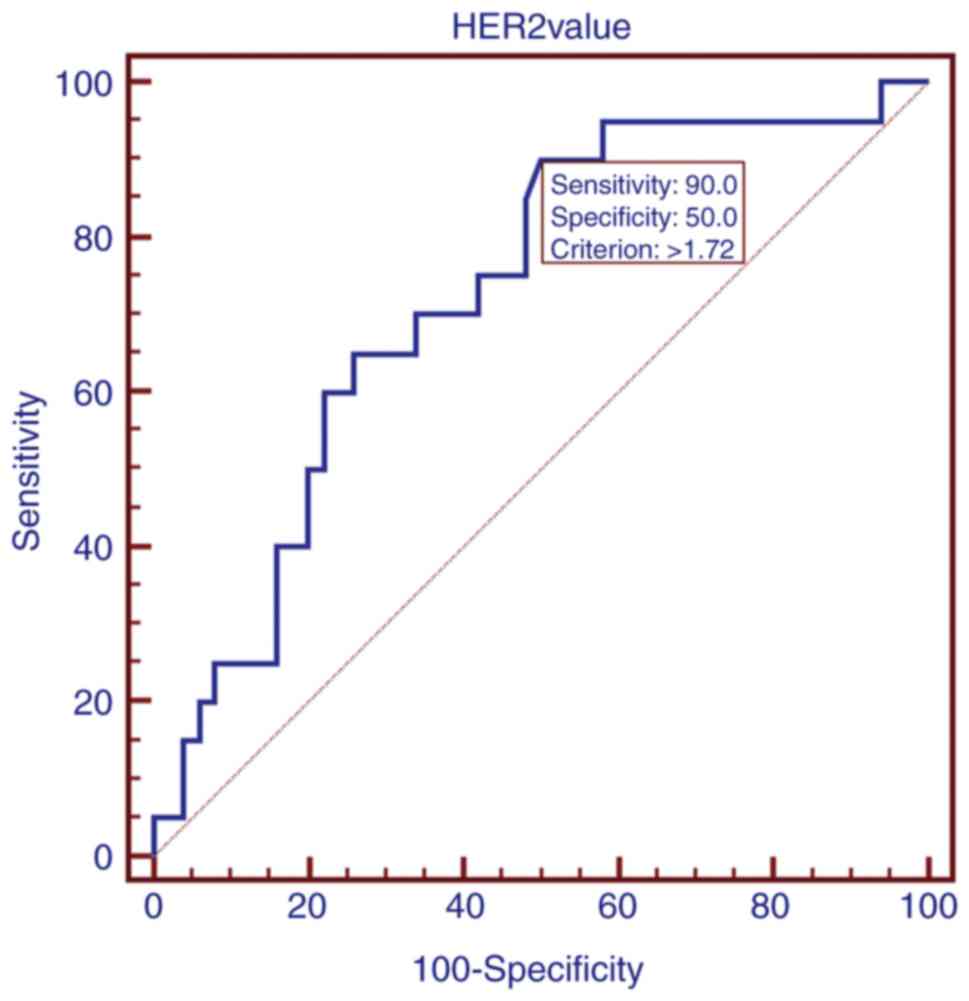
used to produce an ROC curve (Fig.
3), for which the area under the curve (AUC) was 0.723
(P<0.001). Based on the ROC curves, the optimized cutoff for
peripheral blood HER-2 mRNA positivity in preoperative patients was
1.72, with 90% sensitivity, 50% specificity, and a Youden index
value of 0.40 for one-step RT-qPCR distinguishing HER-2-negative
and -positive tumors compared with IHC/FISH.
Combined pre- and postoperative
analysis of HER-2 mRNA
The cutoff value for HER-2 mRNA positivity in
circulating blood was 1.512 (P<0.0001). Five patients exhibited
a positive peripheral blood HER-2 mRNA status prior to and
following surgery (pre+/post+), while 2 were pre+/post-, 4 were
pre-/post+, and 2 were pre-/post- (Table
II). Thus, only 2 patients demonstrated a postoperative
decrease in peripheral blood HER-2 mRNA, whereas 11 exhibited an
HER-2 mRNA level that did not decrease.
 | Table II.Change of HER-2 mRNA level in
patients pre- and postoperative based on cutoff of 1.512. |
Table II.
Change of HER-2 mRNA level in
patients pre- and postoperative based on cutoff of 1.512.
|
| HER-2 mRNA
(Postoperative) |
|---|
|
|
|
|---|
|
| Negative | Positive |
|---|
| HER-2 mRNA
(Preoperative) | n | % | n | % |
|---|
| Negative (n=6) | 2 | 33.3 | 4 | 66.7 |
| Positive (n=7) | 2 | 28.6 | 5 | 71.4 |
Circulating HER-2 mRNA is a prognostic
biomarker in patients receiving neoadjuvant chemotherapy
To analyze the effect of neoadjuvant chemotherapy on
HER-2 mRNA expression in peripheral blood, the blood from five
breast cancer patients was monitored during neoadjuvant therapy
(n=5). All patients received three cycles of
5-FU/epirubicin/cyclophosphamide with three sequential cycles of
docetaxel (22,32). According to the treatment cycles,
blood samples were divided into groups as follows: prior to
antitumor treatment (time point zero; TP0), three treatment cycles
(TP3), and six treatment cycles (TP6). Patients receiving
neoadjuvant therapy were regularly monitored by MRI to evaluate
treatment efficacy (32). In all
patients who received neoadjuvant treatment, peripheral blood HER-2
mRNA and the original tumor size decreased (Fig. 4). This result suggests that the change
in circulating HER-2 mRNA following neoadjuvant therapy was
consistent with the results of imaging evaluation.
Discussion
Breast cancer is a highly heterogeneous malignant
tumor, and HER-2 status changes during tumor progression, though
these changes may not be detected in primary tumor histological
examinations (33). Postoperative
chemotherapy, endocrine therapy, and targeted therapy have been
demonstrated to affect HER-2 expression (34). In clinical practice, the principal
methods currently used to determine HER-2 tissue status are IHC and
FISH, which can be used to assess HER-2 status at the time of
diagnosis (35). In patients with
metastatic breast cancer, biopsy samples for the metastases are not
always available; furthermore, as breast cancer is highly
heterogeneous and core needle biopsy (CNB) assesses only part of
the tumor tissue, it may provide incomplete information for the
diagnosis of metastatic breast cancer (36). Liquid biopsies can detect the HER-2
released into the peripheral blood from tumor tissues, even in
patients with multiple tumor foci. Liquid biopsies also allow the
detection of cancer-associated alleles in the blood and provide a
genetic landscape for primary and metastatic tumors (16,37). A
liquid biopsy detects all circulating HER-2 mRNA released by breast
tumors, and can be used for quantification by RT-qPCR (31,38).
Therefore, liquid biopsy results may be useful complementary
information to the information obtained by histology. Furthermore,
liquid biopsies can dynamically monitor changes in HER-2 levels in
breast cancer following chemotherapy or targeted therapy, which
provides prognostic information for clinical decisions (39).
HER-2 status in the peripheral blood can be
converted from positive to negative following anti-HER-2 treatment
in patients presenting with HER-2 positive primary tumor tissue,
and these changes determine whether anti-HER-2 treatment should be
continued (40). Koumarianou et
al (41) demonstrated that
certain patients with breast cancer with HER-2 negative tissue
benefited from treatment with trastuzumab. Therefore, liquid
biopsies may be more convenient and accurate than CNB biopsy for
tumor monitoring (42).
In the present study, a suitable cutoff for
circulating HER-2 mRNA was established based on the circulating
HER-2 mRNA levels in healthy controls (22 positive and 48
negative). The cutoff for circulating blood HER-2 was 1.512
(19). In previous studies, Savino
et al (19) and Korantizis
et al (20) have described
that the circulating levels of HER-2 were associated with the HER-2
mRNA level of tissues. The results of the RT-qPCR analysis were
correlated with those of tissue HER-2 status determined by IHC
and/or FISH, though there was a deviation of ~4%. Compared with
previous study, Savino et al (19) performed qPCR to detect peripheral
blood HER-2 expression from 30 HER-2 positve breast cancer patients
(IHC) (19,43). After establishing a cut-off value, 18
out of the 30 HER-2 positive patients were scored, indicating ~40%
deviation (14). These two results
indicated circulating HER-2 is a potential diagnostic marker
although there were some differences observed, which may be derived
from using a different patient cohort. It is established that
although IHC pathology is conveys a high degree of accuracy, the
results are not definitive and are open to interpretation (44,45).
Therefore, the present study highlighted that HER-2 mRNA in
peripheral blood may be an effective complementary assay in
clinical practice. The prospective detection of HER-2 mRNA using
one-step RT-qPCR on peripheral blood samples from patients with
breast cancer prior to surgery or during treatment detected a
significant difference in peripheral blood HER-2 mRNA level between
normal samples and samples from breast cancer patients
(P<0.0001). Xu et al (46)
previously identified that ~43.3% of patients with breast cancer
were positive for plasma HER-2 mRNA, whereas only 10% were positive
in the control group (P<0.001). Oloomi et al (47) obtained similar results, with HER-2
positivity detected in 36.7% of patients with breast cancer, and
reported significant differences between patients and healthy
controls (P<0.05). However, Owrangi et al (21), indicated that there were no
differences in the expression of HER2 in patients with cancer
compared with healthy individuals, which may be a result of a
different patient sample. Collectively these studies indicate that
peripheral blood HER-2 mRNA is higher in breast cancer samples than
in samples from patients without cancer, indicating that HER-2 mRNA
in blood may be a dependable biomarker for identifying patients
with breast cancer irrespective of the HER-2 status of the primary
tumor. However, the exact level of circulating HER-2 mRNA in
peripheral blood that identifies breast cancer, especially
HER-2-positive cancer, was not determined in these studies.
The present study also identified an association
between the level of HER-2 mRNA in blood and HER-2 tissue status
(P<0.05) with high sensitivity and low specificity; the
agreement between blood HER-2 mRNA detected by one-step RT-qPCR and
tissue HER-2 status determined by IHC/FISH was 30.4% (P<0.01,
Kappa coefficient). These results suggest that the detection of
circulating HER-2 mRNA may be a useful predictive method for breast
cancer diagnosis, complementary to tissue analysis. However, a
previous study identified no correlation between blood HER-2 mRNA
level and tissue HER-2 status (48).
Additionally, no associations were observed between the blood HER-2
mRNA level, and the lymph node status, tumor grade, tumor stage,
tumor size, patient age, menopausal status, or ER or PR status of
the primary tumor in a previous study (49). Furthermore, the association between
the level of HER-2 mRNA in blood and Ki-67 expression or the
lymphovascular invasion status of primary tumors (P<0.01 and
P<0.05, respectively) indicated a poor prognosis. The
discrepancy in HER-2 status between peripheral blood and tissue
could be attributed to differences in the two techniques, and the
heterogeneity of breast cancer. No association was observed between
peripheral blood HER-2 mRNA and other prognostic factors.
Information on peripheral HER-2 mRNA levels for
predicting the efficacy of antitumor treatment in breast cancer is
limited. In the present study, changes in HER-2 mRNA during
neoadjuvant therapy were evaluated. Peripheral blood HER-2 mRNA in
breast cancer patients was monitored during neoadjuvant therapy to
correlate circulating HER-2 mRNA levels with therapeutic efficacy.
The changes in circulating HER-2 mRNA during treatment were
consistent with the results of MRI evaluation. As depicted in
Fig. 4, all patients receiving
neoadjuvant chemotherapy exhibited a decrease in circulating HER-2
mRNA after antitumor treatment, excluding patient 5. However, these
results were opposite with the prognostic value of peripheral blood
HER-2 mRNA detection during antitumor treatment using Docetaxel
which may resulted from different patient cohort (20). The discrepancy between peripheral
blood HER-2 mRNA level and tumor size after antitumor treatment in
these patients may be attributed to differences in therapeutic
responses and the heterogeneity of breast cancer. The detection of
circulating HER-2 mRNA in patients receiving neoadjuvant
chemotherapy suggested that patients benefited from neoadjuvant
therapy for the treatment of breast cancer, and that circulating
HER-2 mRNA could be used to predict breast cancer progression.
Thus, the results demonstrated that HER-2 mRNA in peripheral blood
could be used as a prognostic biomarker during neoadjuvant or
adjuvant treatment.
To conclude, the present study has provided evidence
from a patient cohort for the diagnostic value of circulating HER-2
for breast cancer; meanwhile, the cut-off value of 1.512 (mean of
2−∆∆Ct) was established which may be useful in clinical
applications. Finally, five patients were analyzed and identified
that circulating HER-2 was associated with the outcome of
neoadjuvant chemotherapy, which may serve as a novel prognostic
biomarker.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Suzhou
Health Planning Commission Key Clinical Diagnosis and Treatment
Program (grant no. LCZX201606), the Soochow Science and Technology
Project (grant no. SYS201631), and the Science, Education and
Health Foundation of Soochow City (grant no. KJXW2014011). The
project was also supported by the Second Affiliated Hospital of
Soochow University's Preponderant Clinic Discipline Group Project
Funding (grant no. XKQ2015008).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YW and QM performed the experiments, analyzed the
data and wrote the manuscript. ZY, LS, JW, JR, BL, DX, RH, PZ
provided technical assistance, analyzed the data and modified the
manuscript. GJ designed and supervised the study. All authors are
in agreement with the content of the manuscript.
Ethics approval and consent to
participate
Study approval was obtained from the independent
ethics committee at the Second Affiliated Hospital of Soochow
University (Suzhou, China). The privacy of the patients involved
was protected. Patients provided written informed consent.
Patient consent for publication
Study participants provided consent for the
publication of the data and any associated images.
Competing interests
This study uses equipment from Shanghai GenePharma
Co., Ltd. (Shanghai, China).
References
|
1
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeSantis CE, Ma J, Sauer Goding A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slamon D, Clark G, Wong S, Levin W,
Ullrich A and McGuire W: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Press MF, Bernstein L, Thomas PA, Meisner
LF, Zhou JY, Ma Y, Hung G, Robinson RA, Harris C and El-Naggar A:
HER-2/neu gene amplification characterized by fluorescence in situ
hybridization: poor prognosis in node-negative breast carcinomas. J
Clin Oncol. 15:2894–2904. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olayioye MA, Neve RM, Lane HA and Hynes
NE: The ErbB signaling network: Receptor heterodimerization in
development and cancer. EMBO J. 19:3159–3167. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dawood S, Broglio K, Buzdar AU, Hortobagyi
GN and Giordano SH: Prognosis of women with metastatic breast
cancer by HER2 status and trastuzumab treatment: An
institutional-based review. J Clin Oncol. 28:92–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ross JS, Slodkowska EA, Symmans WF,
Pusztai L, Ravdin PM and Hortobagyi GN: The HER-2 receptor and
breast cancer: Ten years of targeted anti-HER-2 therapy and
personalized medicine. Oncologist. 14:320–368. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akiyama T, Sudo C, Ogawara H, Toyoshima K
and Yamamoto T: The product of the human c-erbB-2 gene: A
185-kilodalton glycoprotein with tyrosine kinase activity. Science.
232:1644–1646. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belengeanu A, Muresan A, Stoicanescu D and
Lazar E: Amplification of HER-2 gene in breast cancer:
Immunohistochemical and FISH assessment. Rom J Morphol Embryol.
51:321–326. 2010.PubMed/NCBI
|
|
10
|
Piccart-Gebhart MJ, Procter M,
Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga
J, Bell R, Jackisch C, et al: Trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer. N Engl J Med.
353:1659–1672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith I, Procter M, Gelber RD, Guillaume
S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G,
Baselga J, et al: 2-year follow-up of trastuzumab after adjuvant
chemotherapy in HER2-positive breast cancer: A randomised
controlled trial. Lancet. 369:29–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rugo HS, Li H and Gui X: Strategies and
progress of endocrine therapy for patients with metastatic breast
cancer. Adv Exp Med Biol. 1026:403–418. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gianni L, Bisagni G, Colleoni M, Del
Mastro L, Zamagni C, Mansutti M, Zambetti M, Frassoldati A, De Fato
R, Valagussa P and Viale G: Neoadjuvant treatment with trastuzumab
and pertuzumab plus palbociclib and fulvestrant in HER2-positive,
ER-positive breast cancer (NA-PHER2): An exploratory, open-label,
phase 2 study. Lancet Oncol. 19:249–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brugmann A, Lelkaitis G, Nielsen S, Jensen
KG and Jensen V: Testing HER2 in breast cancer: A comparative study
on BRISH, FISH and IHC. Appl Immunohistochem Mol Morphol.
19:203–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
De Mattos-Arruda L: Liquid biopsy for
HER2-positive breast cancer brain metastasis: The role of the
cerebrospinal fluid. ESMO Open. 2:e0002702017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Apostolaki S, Perraki M, Kallergi G,
Kafousi M, Papadopoulos S, Kotsakis A, Pallis A, Xenidis N,
Kalmanti L, Kalbakis K, et al: Detection of occult HER2
mRNA-positive tumor cells in the peripheral blood of patients with
operable breast cancer: Evaluation of their prognostic relevance.
Breast Cancer Res Treat. 117:525–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Apostolaki S, Perraki M, Pallis A,
Bozionelou V, Agelaki S, Kanellou P, Kotsakis A, Politaki E,
Kalbakis K, Kalykaki A, et al: Circulating HER2 mRNA-positive cells
in the peripheral blood of patients with stage I and II breast
cancer after the administration of adjuvant chemotherapy:
Evaluation of their clinical relevance. Ann Oncol. 18:851–858.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savino M, Garrubba M, Parrella P, Baorda
F, Copetti M, Murgo R, Zelante L, Carella M, Valori VM and Santini
SA: Development of real-time quantitative reverse transcription-PCR
for Her2 detection in peripheral blood from patients with breast
cancer. Clin Chim Acta. 384:52–56. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Korantzis I, Kalogeras KT, Papaxoinis G,
Kotoula V, Koutras A, Soupos N, Papakostas P, Dionysopoulos D,
Samantas E, Christodoulou C, et al: Expression of angiogenic
markers in the peripheral blood of patients with advanced breast
cancer treated with weekly docetaxel. Anticancer Res. 32:4569–4580.
2012.PubMed/NCBI
|
|
21
|
Owrangi B, Habibagahi M, Hosseini A,
Haghighi NF, Mardani M, Talei A, Ghaderi A and Jaberipour M: MDM2,
E-cadherin, Survivin and Her2 mRNA status in peripheral blood of
patients with breast cancer. Mid East J Cancer. 4:2013.
|
|
22
|
Press MF, Sauter G, Buyse M, Fourmanoir H,
Quinaux E, Tsao-Wei DD, Eiermann W, Robert N, Pienkowski T, Crown
J, et al: HER2 gene amplification testing by fluorescent in situ
hybridization (FISH): Comparison of the ASCO-College of American
Pathologists Guidelines With fish scores used for enrollment in
breast cancer international research group clinical trials. J Clin
Oncol. 34:3518–3528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malzkorn B and Reifenberger G: Practical
implications of integrated glioma classification according to the
World Health Organization classification of tumors of the central
nervous system. Curr Opin Oncol. 28(494–501): 20162016.
|
|
24
|
Fleige S and Pfaffl MW: RNA integrity and
the effect on the real-time qRT-PCR performance. Mol Aspects Med.
27:126–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
El Hadi H, Abdellaoui-Maane I, Kottwitz D,
El Amrani M, Bouchoutrouch N, Qmichou Z, Karkouri M, ElAttar H,
Errihani H, Fernandez PL, et al: Development and evaluation of a
novel RT-qPCR based test for the quantification of HER2 gene
expression in breast cancer. Gene. 605:114–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leslie DS, Johnston WW, Daly L, Ring DB,
Shpall EJ, Peters WP and Bast RC Jr: Detection of breast carcinoma
cells in human bone marrow using fluorescence-activated cell
sorting and conventional cytology. Ame J Clin Pathol. 94:8–13.
1990. View Article : Google Scholar
|
|
28
|
Catenacci DV, Liao WL, Zhao L, Whitcomb E,
Henderson L, O'Day E, Xu P, Thyparambil S, Krizman D, Bengali K, et
al: Mass-spectrometry-based quantitation of Her2 in
gastroesophageal tumor tissue: Comparison to IHC and FISH. Gastric
Cancer. 19:1066–1079. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Check W: IHC, FISH still sharing HER2
spotlight. CAP Today. 19(1): 4042 passim. 2005.
|
|
30
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Caraguel CG, Stryhn H, Gagne N, Dohoo IR
and Hammell KL: Selection of a cutoff value for real-time
polymerase chain reaction results to fit a diagnostic purpose:
Analytical and epidemiologic approaches. J Vet Diagn Invest.
23:2–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jensen LR, Huuse EM, Bathen TF, Goa PE,
Bofin AM, Pedersen TB, Lundgren S and Gribbestad IS: Assessment of
early docetaxel response in an experimental model of human breast
cancer using DCE-MRI, ex vivo HR MAS and in vivo 1H MRS. NMR
Biomed. 23:56–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao X, Rodland EA, Tibshirani R and
Plevritis S: Molecular subtyping for clinically defined breast
cancer subgroups. Breast Cancer Res. 17:292015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yan J, Liu XL, Han LZ, Xiao G, Li NL, Deng
YN, Yin LC, Ling LJ, Yu XY, Tan CL, et al: Relation between Ki-67,
ER, PR, Her2/neu, p21, EGFR and TOP II-α expression in invasive
ductal breast cancer patients and correlations with prognosis.
Asian Pac J Cancer Prev. 16:823–829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsuda H: HER-2 (c-erbB-2) test update:
Present status and problems. Breast Cancer. 13:236–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hall C, Valad L and Lucci A: Circulating
tumor cells in breast cancer patients. Crit Rev Oncog. 21:125–139.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang HY, Ahn S, Kim S, Park S, Jung D,
Park S, Han H, Sohn J, Kim S and Lee H: Detection of circulating
tumor cell-specific markers in breast cancer patients using the
quantitative RT-PCR assay. Int J Clin Oncol. 20:878–890. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Janjigian YY, Riches JC, Ku GY, Imatiaz T,
Capanu M and Chou JF: Loss of human epidermal growth factor
receptor 2 (HER2) expression in HER2-overexpressing esophagogastric
(EG) tumors treated with trastuzumab. Gastrointesti Cancers
Symposium. 2015.
|
|
39
|
Crowley E, Di Nicolantonio F, Loupakis F
and Bardelli A: Liquid biopsy: Monitoring cancer-genetics in the
blood. Nat Rev Clin Oncol. 10:472–484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Raitoharju E, Seppala I, Oksala N,
Lyytikäinen LP, Raitakari O, Viikari J, Ala-Korpela M, Soininen P,
Kangas AJ, Waldenberger M, et al: Blood microRNA profile associates
with the levels of serum lipids and metabolites associated with
glucose metabolism and insulin resistance and pinpoints pathways
underlying metabolic syndrome: The cardiovascular risk in Young
Finns Study. Mol Cell Endocrinol. 391:41–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Koumarianou A, Karayannopoulou G,
Gourgioti G, Batistatou A, Bobos M, Efstratiou I, Miliaras D,
Galani E, Pentheroudakis G, Pectasides D, et al: PAI-1 and HER2
interaction in advanced breast cancer disease: Evidence for added
benefit from trastuzumab in HER2-negative patients. Cancer
Chemother Pharmacol. 75:1289–1301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hudis CA: Biology before anatomy in early
breast cancer-precisely the point. N Engl J Med. 373:2079–2080.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Savino M, Parrella P, Copetti M, Barbano
R, Murgo R, Fazio VM, Valori VM, Carella M, Garrubba M and Santini
SA: Comparison between real-time quantitative PCR detection of HER2
mRNA copy number in peripheral blood and ELISA of serum HER2
protein for determining HER2 status in breast cancer patients. Cell
Oncol. 31:203–211. 2009.PubMed/NCBI
|
|
44
|
Al Diffalha S, Shaar M, Barkan GA, Wojcik
EM, Picken MM and Pambuccian SE: Immunohistochemistry in the workup
of prostate biopsies: Frequency, variation and appropriateness of
use among pathologists practicing at an academic center. Ann Diagn
Pathol. 27:34–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Allison KH: Molecular pathology of breast
cancer: What a pathologist needs to know. Am J Clin Pathol.
138:770–780. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xu Y, Yao L, Li H, Ouyang T, Li J, Wang T,
Fan Z, Lin B, Lu Y, Larsson O and Xie Y: Presence of erbB2 mRNA in
the plasma of breast cancer patients is associated with circulating
tumor cells and negative estrogen and progesterone receptor status.
Breast Cancer Res Treat. 97:49–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Oloomi M, Bouzari S, Mohagheghi MA and
Khodayaran-Tehrani H: Molecular markers in peripheral blood of
Iranian women with breast cancer. Cancer Microenviron. 6:109–116.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Moazzezy N, Ebrahimi F, Sisakht MM,
Yahyazadeh H, Bouzari S and Oloomi M: Relationship between erb-B2
mRNA expression in blood and tissue of invasive ductal carcinoma
breast cancer patients and clinicopathological characteristics of
the tumors. Asian Pac J Cancer Prev. 17:249–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tse C, Brault D, Gligorov J, Antoine M,
Neumann R, Lotz JP and Capeau J: Evaluation of the quantitative
analytical methods real-time PCR for HER-2 gene quantification and
ELISA of serum HER-2 protein and comparison with fluorescence in
situ hybridization and immunohistochemistry for determining HER-2
status in breast cancer patients. Clin Chem. 51:1093–1101. 2005.
View Article : Google Scholar : PubMed/NCBI
|