Introduction
Lung cancer is one of the major causes of
cancer-associated mortality, particularly in China (1,2). The
increase in the frequency of cancer, the shortage of curative
treatments and the severe side effects associated with synthetic
drugs (2) has made it important to
investigate novel and more effective molecules. Recently, there has
been an increasing interest in the use of plant-derived natural
products worldwide due to fewer side effects. Natural products have
gained notable importance as anticancer agents due to fewer side
effects. Betulinic acid is a pentacyclic compound plant obtained
from the bark of white-barked birch trees (3–5). Betulinic
acid has been demonstrated to exhibit notable pharmacological
properties. For instance, betulinic acid has been reported to
exhibit antitumor properties against various types of cancer cells,
including breast and liver cancer cells (6). Additionally, Native Americans have used
bark of white birch as a folk medicine for the treatment of cancer.
With the increase in the incidence of drug-resistance, the
treatment and management of cancer has become very difficult
(4). In the present study, the
effects of betulinic acid, a natural product, on human lung cancer
cells were determined. The half-maximal inhibitory concentration
(IC50) of betulinic acid was detected at 50 µM. Of note,
the results of the present study indicated that betulinic acid
exhibited marked anticancer activity. It was observed that
treatment with betulinic acid was able to induce apoptosis in human
lung cancer H460 cells, alter mitochondrial membrane potential
(MMP) and cause cell cycle arrest. Treatment with betulinic acid
was able to downregulate the expression of B-cell lymphoma-2
(Bcl-2) and upregulate the expression of Bcl-2-associated X (Bax).
Collectively, betulinic acid may beauseful drug candidate for the
management of drug-resistant lung cancer.
Materials and methods
Chemicals, reagents and cell culture
conditions
Betulinic acid, propidium iodide (PI), RNase A
Triton X-100 and dimethyl sulfoxide (DMSO) were obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). All primary and
secondary antibodies were purchased from Santa Cruz Biotechnology
Inc. (Dallas, TX, USA). The fluorescent probes
(dichloro-dihydro-fluorescein diacetate DCFH-DA, DiOC6
and DAPI), fetal bovine serum (FBS), RPMI-1640 medium, L-glutamine
and antibiotics were obtained from Invitrogen (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Paclitaxel-resistant human
lung cancer cell line (H460) and non-cancerous FR2 cells were
procured from Cancer Research Institute of Beijing (Beijing,
China), which were maintained in Dulbecco's modified Eagle's medium
and was supplemented with 10% FBS and antibiotics (100 µg/ml
streptomycin and 100 U/ml penicillin G) in incubator at 37°C (5%
CO2 and 95% air).
MTT and colony formation assay
The cytotoxic effect of betulinic acid in
paclitaxel-resistant human lung H460 cancer cells was determined
using an MTT assay. The cells were cultured at 1×106
cells per well in 96-well plates for a time period of 12 h and then
administrated with varying concentrations of betulinic acid (0–500
µM) for 48 h. MTT solution (20 µl) was added to each well. Prior to
the addition of 500 µl DMSO, the media was completely removed and
replaced with fresh media. To solubilize MTT formazan crystals, 500
µl DMSO was added. ELISA plate reader was used for the
determination of optical density at 570 nm. H460 lung cancer cells
were then subjected to 0, 25, 50 and 100 µM betulinic acid for
further experiments. To evaluate colony formation, lung cancer H460
cells at the exponential growth phase were harvested and counted
with a hemocytometer. The cells were plated at 200 cells per well.
This was followed by incubation for a time period of 48 h at 37°C
to allow the cells to adhere and then various doses (0, 25, 50 and
100 µM) of betulinic acid were added. Following administration of
betulinic acid, the cells were again incubated for 6 days at 37°C
and washed with PBS. Methanol was used to fix the colonies at −20°C
for 4 min and then stained with crystal violet at room temperature
for ~30 min prior to analysis under a light microscope (10
fields).
Detection of apoptosis and estimation
of apoptotic populations
Paclitaxel-resistant H460 cells (density,
2×105 cells/well) that were plated in 6-well plates were
administrated with 0, 25, 50 and 100 µM betulinic acid for 48 h at
37°C. The cells were harvested by trypsinization and fixed with
acetic acid and methanol (1:3) for 6 h at −20°C. Following
incubation at −20°C for 6 h, the cells were centrifuged for 10 min
at 8,000 × g at 4°C and pellets were resuspended in methanol:acetic
acid (1:3). The cells were then plated on a chilled glass slide.
DAPI was added for 20–30 min at 25°C in the dark at a concentration
of 1 µg/ml, and the images were captured using fluorescence
microscope (excitation wavelength, 488 nm; 10 different fields).
For Annexin V/PI staining, the cells were harvested and stained
with Annexin V/PI for 15 min in dark at room temperature.
Subsequently, analysis was performed with a flow cytometer (IX-70;
Olympus Corporation, Tokyo, Japan) as previously described
(7).
Cell cycle distribution of H460
cells
The cells were seeded into 6-well plates
(2×105 cells/well), and betulinic was administrated to
the cells at 0, 25, 50 and 100 µM, followed by 24 h of incubation
at 37°C. DMSO was used as a control. For estimation of the DNA
content, PBS was used to wash the cells, which were then fixed in
ethanol at −20°C for 24 h. This was followed by re-suspension in
PBS, containing 40 µg/ml PI, RNase A (0.1 mg/ml) and Triton X-100
(0.1%), for 30 min in a dark room at 37°C. Subsequently, analysis
was conducted with a flow cytometer (IX-70; Olympus Corporation).
The estimated percentage of cells in each phase of the cell cycle
was quantified using WinMDI software version 2.0 (Informer
Technologies, Inc., Los Angeles, CA, USA).
Determination of MMP
H460 cells were seeded at a density of
2×105 cells/well in a 6-well plate, which were
maintained for 24 h and treated with 50 µM betulinic acid for 0,
12, 24 and 48 h at 37°C in 5% CO2 and 95% air.
Thereafter, the cells from all treatment groups were collected,
washed twice with PBS and re-suspended in 500 µl
3,3′-dihexyloxacarbocyanine iodide (1 µmol/l) for MMP at 37°C in a
dark room for 30 min. The samples were then analyzed immediately
using a fluorescence microscope (IX-70; Olympus Corporation) and a
flow cytometer with WinMDI software version 2.0 (Informer
Technologies, Inc.).
Western blot analysis
The betulinic acid-treated cells (concentration, 0,
25, 50 and 100 µM) were harvested and lysed in lysis buffer [20 mM
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid, 350 mM NaCl,
20% glycerol, 1% Nonidet P 40, 1 mM MgCl2, 0.5 mM EDTA,
0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, protease inhibitor cocktail and
phosphatase inhibitor cocktail]. The protein concentrations of the
lysates were quantified by a bicinchoninic acid assay using
specific antibodies. β-actin was used as a control. From each
sample, equal quantities of protein (0.5 µg) were loaded and
separated by electrophoresis on a 12% denaturing SDS gel.
Subsequently, the proteins were transferred onto polyvinylidene
difluoride membranes (pore size, 0.45 µm). Following transfer, the
membranes were blocked with 3% bovine serum albumin (Thermo Fisher
Scientific, Inc.) in tris-phosphate-buffered saline (200 mM Tris/pH
7.0, 1.37 M NaCl and 1% Tween-20) for 1 h at room temperature. The
membranes were then incubated with appropriate primary antibodies
(rabbit polyclonal β-actin; sc-58673, Bax; sc-6236, BCl2; sc-509)
overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated alkaline phosphatase secondary antibody
(sc-2372; dilution 1:1,000) for 1 h at room temperature. Super
Signal West Dura Extended Duration Chemiluminescent substrate was
used for the enhanced chemiluminescence reaction, and the signal
was detected and quantified using the ImageQuantLAS4000 imaging
system (GE Healthcare Life Sciences, Little Chalfont, UK).
Statistical analysis
All experiments were conducted in triplicate and
expressed as the mean ± standard deviation. Statistical analysis
was carried out using a Student's t-test and one-way analysis of
variance followed by Tukey's test. GraphPad prism software (version
5.0; GraphPad Software, Inc., La Jolla, CA, USA) was used.
P<0.01 was considered to indicate a statistically significant
difference.
Results
Cytotoxic potential of betulinic acid
on paclitaxel-resistant lung H460 cancer cells
The chemical structure of betulinic acid is
displayed in Fig. 1A. The
cytotoxicity of betulinic acid on the paclitaxel-resistant human
lung cancer cell line H460 was evaluated. The results of the MTT
assay revealed that betulinic acid exhibited
concentration-dependent anti-proliferative activity on H460 cells.
The IC50 of betulinic acid on paclitaxel-resistant lung
H460 cells was detected to be 50 µM (Fig.
1B). Betulinic acid exhibited a lower cytoxicity on normal
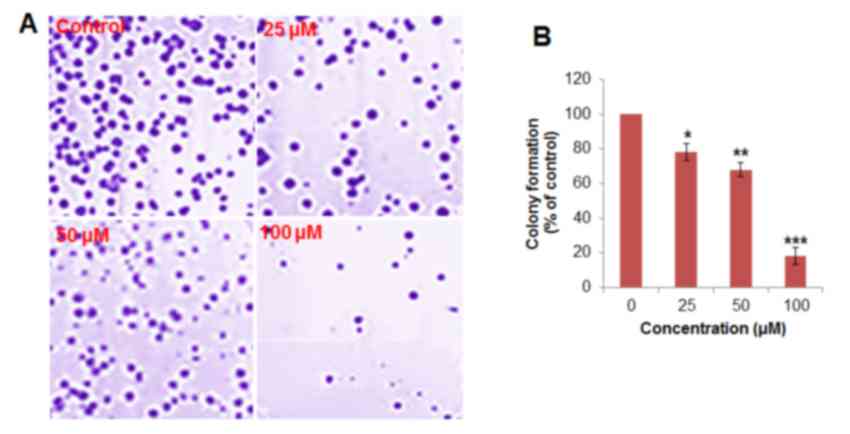
human epithelial FR2 cells compared with H460 cells (Fig. 1C). In the colony formation assay, the
cells were treated with 0, 25, 50 and 100 µM betulinic acid,
respectively. It was observed that the administration of betulinic
acid was able to reduce the number of colonies in a dose-dependent
manner (Fig. 2). Colony formation was
reduced by ≤78% at 100 µM vs. untreated control.
Betulinic acid induces apoptosis in
paclitaxel-resistant human H460 lung cancer cells
DAPI staining indicated that betulinic acid was able
to induce apoptosis in H460 paclitaxel-resistant cancer cells in a
dose-dependent manner (Fig. 3). At 50
and 100 µM of betulinic acid, apoptotic cells were notably visible.
In order to confirm apoptotic cell death that was induced by
betulinic acid, Annexin V/PI staining was conducted following the
treatment of H460 cells with 0, 25, 50 and 100 µM. Flow cytometric
results revealed that the percentage of apoptotic cell population
increased to 7.82, 17.05 and 36.07% in paclitaxel-resistant human
H460 lung cancer cells after 48 h, compared with the untreated
control at 25, 50 and 100 µM, respectively (Fig. 4). Therefore, the results indicated
that treatment with betulinic acid resulted in apoptotic cell death
in paclitaxel-resistant human H460 cancer cells in a
concentration-dependent manner.
Betulinic acid causes alterations in
cell cycle distribution of H460 cancer cells
The results of the present study indicated that
betulinic acid may have induced cell cycle arrest of H460 lung
cancer cells. It was observed that the percentage of cells was
notably increased at G2. G2 arrest was detected following treatment
with 25, 50 and 100 µM betulinic acid (Fig. 5). Additionally, the population of G2
H460 cells was slightly increased at a dose of 25 µM, markedly
elevated at 50 µM and highly elevated at 100 µM compared with the
control. The effect of betulinic acid on the proportion of H460
cells at G2 was observed to be dose-dependent.
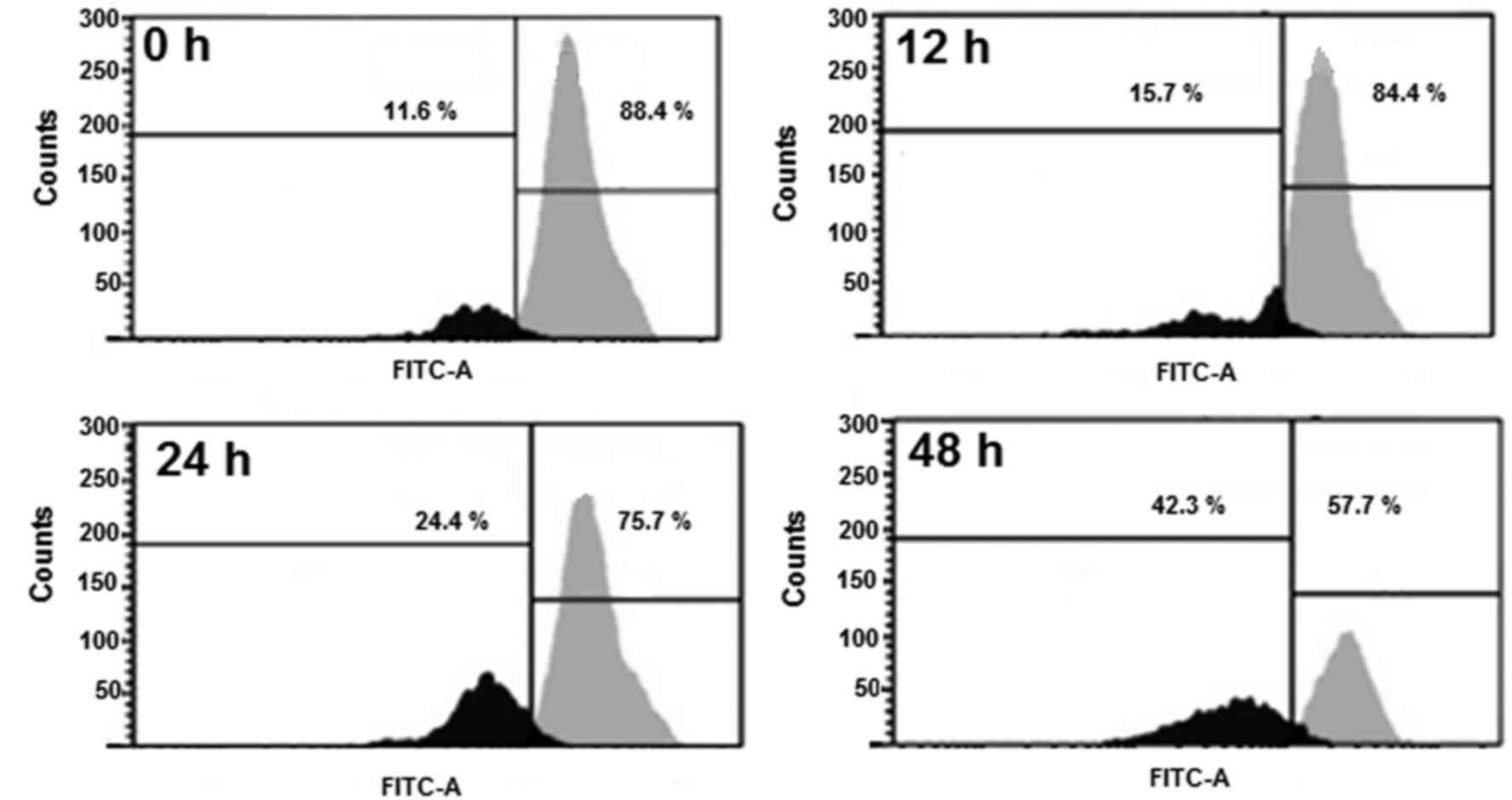
Betulinic acid induces MMP loss in
human H460 lung cancer cells
TheH460 cells were administrated with 50 µM
betulinic acid for various time intervals, and the levels of MMP
were evaluated. A marked reduction in the level of MMP (Fig. 6) was detected in the betulinic
acid-treated H460 cells compared with the control. After 12, 24 and
48 h, the MMP was observed to be 84.10, 75.26 and 57.14% compared
with the untreated paclitaxel-resistant human H460 lung cancer
cells.
Betulinic acid targets the Bcl-2/Bax
signaling pathway
The expression levels of Bcl-2and Bax were
determined to confirm if betulinic acid induced-apoptosis followed
the mitochondrial apoptotic pathway. The findings are presented in
Fig. 7. It was observed that the
expression of Baxincreased and Bcl-2 decreased following the
treatment with betulinic acid, which may have resulted in
apoptosis. Compared with the untreated control cells, betulinic
acid-treated cells demonstrated a concentration-dependent increase
in the Bax/Bcl-2 ratio.
Discussion
Lung cancer is one of the most lethal types of
cancer detected worldwide and thousands of patients are diagnosed
with this disease annually (1).
Existing treatment options exhibit effective clinical results, yet
numerous cancer-associated mortalities are attributable to lung
cancer. In addition, existing treatment options have severe side
effects, which negatively affect the quality of life (2). Furthermore, the development of drug
resistance has made cancer very difficult to treat (4). Against this backdrop, molecules from
natural sources with limited side effects may prove useful.
In the present study, betulinic acid revealed
potential growth-inhibiting activity against paclitaxel-resistant
lung H460 cancer cells as evident from the proliferation assay. As
reported previously, numerous drugs exhibit antiproliferative
effects via the induction of apoptosis. For instance, several
chemotherapeutic drugs, including cisplatin, taxol and
5-fluorouracil (8–16) have been reported to alter specific
apoptotic pathways. Additionally, resistance to drug may be
partially explained by the ability of cancer cells to evade
apoptosis (17). To assess whether
treatment with betulinic acid induces apoptosis in H460 cells, DAPI
staining of betulinic acid-treated cells was performed. It was
observed that treatment with betulinic acid was able to induce
apoptosis in a concentration-dependent manner. Furthermore, a
marked reduction in MMP was observed following treatment with
betulinic acid and a concentration-dependent pattern was detected.
The results of the present study are in agreement with studies that
were conducted previously (16). The
results of the present study suggested that treatment with
betulinic acid may induce apoptosis by reducing MMP. Several
anticancer drugs target cancer cells partly by accumulating high
levels of ROS and reducing MMP (17).
MMP serves an important role in the induction of apoptosis. For
example, capsaicin disrupts MMP and mediates oxidative stress
resulting in the apoptosis of pancreatic cancer cells (10–16).
Flow cytometry using PI as a probe was used to
analyze the effects of betulinic acid on cell cycle progression in
the present study. Treatment with betulinic acid induced G2/M cell
cycle arrest and led to a marked increase of G2/M cells in a
dose-dependent manner. Furthermore, it was reported that betulinic
acid may inhibit H460 cancer cells in a concentration-dependent
manner. These findings are promising as it is well established that
paclitaxel-resistant lung cancer is one of the cancer types with a
high mortality rate, and betulinic acid may a potential compound
for treatment (17).
The effects of betulinic acid on Bcl-2/Bax signaling
were investigated using western blot analysis. A
concentration-dependent downregulation of Bcl-2 and upregulation of
Bax were observed in betulinic acid-treated cells, which may
ultimately induce apoptosis.
Collectively, betulinic acid may be a potential
candidate for the treatment of lung cancer by regulating the
Bcl-2/Bax signaling pathway. With limited drug options available
and limited toxicity associated with naturally occurring betulinic
acid, this molecule appears to be a viable option, but further
investigation is required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ and JL conceived and designed the experiments;
XZ, JL, SZ, PX and MF performed the experiments; JL and MF analysed
the data; XZ, JL and SZ drafted the manuscript. All authors
reviewed and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J and
Ward E: Global cancer statistics. CA Cancer J Clin. 61:69–90. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Curr Cancer Drug Targets: Targeting
apoptosis pathways in cancer therapy. Curr Cancer Drug Targets.
4:569–576. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Degterev A, Boyce M, Yuan JA, Walczak H
and Krammer PH: The CD95 (APO-1/Fas) and the TRAIL (APO-2L)
apoptosis systems. Exp Cell Res. 256:58–66. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Romashkova JA and Makarov SS: NF-kappaB is
a target of AKT in anti-apoptotic PDGF signalling. Nature.
401:86–90. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fulda S: Betulinic acid: A natural product
with anticancer activity. Mol Nutr Food Res. 53:140–146. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chiang JH, Yang JS, Ma CY, Yang MD, Huang
HY, Hsia TC, Kuo HM, Wu PP, Lee TH and Chung JG: Danthron, an
anthraquinone derivative, induces dna damage and caspase
cascades-mediated apoptosis in snu-1 human gastric cancer cells
through mitochondrial permeability transition pores and
bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun SY, Hail N Jr and Lotan R: Apoptosis
as a novel target for cancer chemoprevention. J Natl Cancer Inst.
96:662–672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maitra R, Porter MA, Huang S and Gilmour
BP: Inhibition of NFkappaB by the natural product Withaferin A in
cellular models of Cystic Fibrosis inflammation. J Inflamm.
6:152009. View Article : Google Scholar
|
|
10
|
Hissin PJ and Hilf R: A fluorometric
method for determination of oxidized and reduced glutathione in
tissues. Anal biochem. 74:214–226. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chipuk JE, Bouchier-Hayes L and Green DR:
Mitochondrial outer membrane permeabilization during apoptosis: The
innocent bystander scenario. Cell Death Diff. 13:1396–1402. 2006.
View Article : Google Scholar
|
|
12
|
Azuma M, Tamatani T, Ashida Y, Takashima
R, Harada K and Sato M: Cisplatin induces apoptosis in oral
squamous carcinoma cells by the mitochondria-mediated but not the
NF-kappaB-suppressed pathway. Oral Oncol. 39:282–289. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoneda K, Yamamoto T and Osaki T: p53- and
p21-independent apoptosis of squamous cell carcinoma cells induced
by 5-fluorouracil and radiation. Oral Oncol. 34:529–537. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abal M, Andreu JM and Barasoain I:
Taxanes: Microtubule and centrosome targets and cell cycle
dependent mechanisms of action. Curr Canc Drug Targs. 3:193–203.
2003. View Article : Google Scholar
|
|
15
|
Ferreira CG, Epping M, Kruyt FA and
Giaccone G: Apoptosis target of cancer therapy. Clin Cancer Res.
8:2024–2034. 2002.PubMed/NCBI
|
|
16
|
Malaguarnera L: Implications of apoptosis
regulators in tumorigenesis. Cancer Met Rev. 23:367–387. 2004.
View Article : Google Scholar
|
|
17
|
Ding H, Han C, Guo D, Chin YW, Ding Y,
Kinghorn AD and D'Ambrosio SM: Selectiveinduction of apoptosis of
human oral cancer cell lines by avocado extracts via a
ROS-mediated-mechanism. Nutr Cancer. 61:348–356. 2009. View Article : Google Scholar : PubMed/NCBI
|