Introduction
Giant cell tumor of bone (GCTB) is an aggressive
osteolytic tumor that typically originates in the epimetaphyseal
region of a long bone and frequently occurs in the distal end of
the femur or the proximal end of the tibia (1,2). GCTB is
clinically defined as a benign bone tumor, but is characterized by
locally aggressive growth and usually leads to an extensive bone
lesion. Radiographically, the lesion of GCTB is purely lytic,
exhibiting geographic bone destruction (3). Although it rarely causes mortality, the
recurrence rate for GCTB has been reported as 18–50% (4–8).
Histologically, GCTB is composed of three major cell
types: Multinucleated, osteoclast-like giant cells; monocytic
round-shaped, macrophage-like cells; and spindle-shaped,
fibroblast-like stromal cells (9–11). The
stromal cells are considered to be the neoplastic component of GCTB
as they are the only proliferating cell component in long-term
cultures (12,13) and express oncogenes (14–16). The
macrophage-like cells, which are recruited by stromal cells, are
considered to be osteoclast precursors, and are able to fuse into
multinucleated osteoclast-like giant cells, with the latter
eventually causing aggressive bone resorption and skeletal
destruction (17–19). As stable cell lines of the
multinucleated giant cells and monocyte-derived macrophages have
not been established, individual GCTB cell components cannot be
maintained in culture. As such, it has not been possible to
determine the efficacy of antitumor agents on GCTB in vitro
(20,21).
Although several in vivo GCTB models are
available, studies on this tumor are restricted as the development
of the in vivo model is incomplete, associated with a short
survival time or does not induce osteolytic lesion (22–24).
Stromal cells injected subcutaneously into immunocompromised mice
do not produce giant cells (22,24,25).
Furthermore, tumor tissues grown on chick chorioallantoic membranes
do not appear to recruit chicken monocytes to synthesize new giant
cells despite increased vascularization from the membrane, and the
survival time is typically 10 days (23). Thus, it was speculated that GCTB
monocytes do not originate from the circulation, but rather arise
from the bone marrow. Therefore, it is necessary to directly inject
GCTB cells into the bone environment (1).
The intratibial injection method is one of the most
widely used murine models to investigate cancer cell growth within
the bone environment. It has been used to establish orthotopic
models for investigating osteosarcoma biology within the bone
environment (26). It has also been
used to research bone cancer pain and cancer bone metastasis in
prostate (27), and breast cancer
(28,29). In addition, the intratibial injection
method leads to a reproducible and valuable model for drug testing
(27).
Thus far, attempts to grow GCTB in animal models and
derive suitable cell lines from primary tumors have failed. This
has limited research in the pathobiology of GCTB and the
development of specific anti-GCTB agents. In the present report,
this problem was addressed by examining whether it was possible to
establish an orthotopic model of GCTB in nude mice following
intratibial injection of patient-derived tumor cells.
Materials and methods
Ethics statement and patient
samples
The use of all patient-derived tumor specimens was
approved by the Institutional Review Board and the Research Ethics
Committee of Changzheng Hospital (Shanghai, China). Written
informed consent was obtained individually from each patient. The
pathological diagnosis of GCTB was confirmed by biopsy prior to
surgical excision. Specimens were obtained at the time of surgery
from patients undergoing tumor resection, and the diagnosis of GCTB
was verified postoperatively by a bone pathologist. Tissue samples
from 5 cases (2 male: 3 female) of GCTB were used in the present
study, and all experiments were performed with three mice per 5
patient sample. The mean age of the five study patients was
24.4±1.5 years (median age, 25 years; range, 19–28 years). Patients
were underwent pathological examination for pathological diagnosis
and patients with GCTB were selected for the present study.
Reagents and established cell
lines
The human fetal osteoblast hFOB1.19 cell line was
obtained from the Institute of Biochemistry and Cell Biology of
Shanghai (Shanghai, China). Dulbecco's modified Eagle's medium-F12
(DMEM-F12), DMEM, penicillin/streptomycin and fetal bovine serum
(FBS) were purchased from Thermo Fisher Scientific, Inc. (Gibco;
Waltham, MA, USA). Collagenase B was obtained from Roche
Diagnostics GmbH (Mannheim, Germany). DAPI and the
tartrate-resistant acid phosphatase (TRAP) staining kit was
purchased from Merck KGaA (Sigma-Aldrich; Darmstadt, Germany). The
primary antibody against human Mitochondria (HuMi) was obtained
from EMD Millipore (Chemicon; Billerica, MA, USA).
Patient-derived GCTB cells collection
and cells culture
Patient-derived GCTB cells were isolated from tumor
samples from tumor resections at the Changzheng Hospital as
aforementioned. The tissue was mechanically cut into small pieces,
and digested with 1.5 mg/ml collagenase B for 3 h at 37°C in DMEM
containing 4.5 g/l glucose supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were collected by
filtration (100-µm diameter filter), then centrifuged at 200 × g
for 5 min at room temperature and washed twice in PBS. The cells
were counted using a hemocytometer and resuspended at a density of
5×105 cells/20 µl PBS. For morphological observation and
TRAP staining, 1×105 cells were seeded in 6-well plate
and cultured in DMEM supplemented with 10% FBS, 100 U/ml penicillin
and 100 µg/ml streptomycin in a 37°C humidified incubator with 5%
CO2 (30).
The hFOB1.19 cells were maintained in DMEM-F12
supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin in a humidified atmosphere with 5% CO2 at
34°C. The medium was replenished every 2–3 days. After reaching
70–80% confluence, cells were passaged by treatment with 0.25%
trypsin. Prior to intratibial injection, the cells were detached
from the petri dish with 0.25% trypsin and centrifuged at 200 × g
for 5 min at room temperature. Cells were resuspended into a
5×105 cell/20 µl cell suspension in PBS.
Morphological observations
Cells (2×105 cells/well) were incubated
for 48 h in 24-well plates. Following incubation, the medium was
removed and the cells were washed once with PBS. Morphology was
observed using a phase contrast inverted microscope (Leica
Microsystems GmbH, Wetzlar, Germany). For detection of osteoclasts,
cells (2×105 cells/well) were incubated for 48 h in
24-well plates. Following incubation, the medium was removed and
cells were washed twice with PBS. Cultures were fixed for 10 min at
room temperature with 4% paraformaldehyde in PBS and then washed
four times with PBS. Fixed cells were stained for TRAP using the
Acid Phosphatase Leukocyte (TRAP) kit, according to the
manufacturer's protocol (cat. no. 387A-1KT; Sigma-Aldrich; Merck
KGaA). Staining was observed using a phase contrast inverted
microscope at a ×400 magnification (Olympus Corporation, Tokyo,
Japan).
Experimental animals
A total of fifteen male 4–5-week-old BALB/c nu/nu
mice (weighing 18–20 g) were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). The animals were
housed in the specific pathogen-free animal facilities with free
access to food and water. The temperature was maintained at 20–26°C
and a relative humidity of 40–70%. Lighting conditions, 12 h a day
fluorescent light and 12 h of darkness.
Intratibial injection of
patient-derived GCTB cells
Patient-derived GCTB cells and hFOB1.19 cells were
resuspended in PBS at a working concentration of 5×105
cells/20 µl. Patient-derived GCTB cells were prepared from the
fresh giant cell tumor of bone specimens without any culture. The
intratibial bone injection was performed in 4- to 5-week-old
BALB/c, nu/nu mice anesthetized with pentobarbital (50 mg/kg) via a
percutaneous approach (27). Briefly,
the proximal end of the left tibia was exposed surgically, the knee
was maintained in a flexed position, and 20 µl of PBS-containing
patient-derived GCTB cells were injected into the bone marrow space
with a 26-gauge needle. Control mice were injected with hFOB1.19
cells or PBS in the same manner. Four months after injection, nude
mice were sacrificed and the injected tibial samples were fixed,
and further analyzed as described below. Procedures involving
animals and their care were conducted in conformity with the
National Institutes of Health guidelines (31), and were approved by the Animal Care
and Use Committee of the Second Military Medical University.
Detection of bone lesions by
radiography
Animals were anesthetized and radiographs were
obtained using a Kodak DXS 4000 Pro system (Kodak, Rochester, NY,
USA) for 1 min at 35 kV on day 0, and weekly following intratibial
injection. The radiographs were analyzed for the presence and type
of bony lesion (osteolytic, osteoblastic or mixed
lytic/blastic).
Three-dimensional, micro-computed
tomography (micro-CT)
Micro-CT was performed using a SkyScan 1076 Desktop
X-ray microtomographer (Bruker microCT, Kontich, Belgium). The
excised mouse legs were secured in polystyrene imaging tubes and
scanned [x50 magnification; 5.86 µm resolution; 7.5 sec exposure
time (40 kV and 258UA); 0.45 rotation step; 180°; and a 1 mm
Aluminum filter]. Image reconstruction was performed with
volumetric reconstruction software (version 2.4.0.0) and 3D image
analysis was performed with CT Analyzer software (version 1.8.1.2)
and 3DVisualization software (version 2.0.0.4) (all from Bruker
microCT).
The bone volume (trabecular bone volume/tissue
volume, BV/TV), and the bone lesion area (bone lesion/normal bone,
BL/NB) were determined by three-dimensional structural parameters
analysis.
Histological analysis of bone
destruction
Following X-ray analysis, the tibial specimens were
processed by fixation with 4% paraformaldehyde in PBS for 24 h at
4°C, followed by decalcification in 0.5 M EDTA. Paraffin-embedded
sections were cut with a microtome into 4-µm thick sections, then
stained with hematoxylin and eosin (H&E) for histological
examination of tumor growth, and general form of the tibia. For
H&E staining, tissue slices underwent standard rehydration and
deparaffinization procedures (32)
and were then stained with hematoxylin for 10 min and eosin for 3
min at room temperature. The H&E stained sections were imaged
using the Leica DM 4000B microscope at ×400 magnification (Leica
Microsystems GmbH).
Detection of TRAP activity
Unstained tibia sections were deparaffinized and
rehydrated for detection of TRAP activity as an osteoclast marker.
Briefly, specimens were equilibrated for 20 min at room temperature
in 0.2 M sodium acetate buffer containing 50 mM L-(+)-tartaric acid
in deionized water, pH 5.0. The sections were incubated in the TRAP
staining reagent for 1 h at 37°C and then at room temperature for
20–30 min until the color reaction was complete. The sections were
air-dried and mounted with Eukitt medium. TRAP stained sections
were analyzed using the Olympus DP71 phase contrast inverted
microscope at ×400 magnification (Olympus Corporation).
Cell tracking using anti-HuMi
immunofluorescence
Unstained tibia sections were deparaffinized,
rehydrated and processed for human specific antigens (mouse
anti-human mitochondria; cat. no. MAB 1273; HuMi, 1:50; Chemicon,
Temecula, CA, USA) to determine the presence of human cells.
Sections were incubated overnight at 4°C with the aforementioned
primary antibodies diluted in PBS containing 10% normal goat serum.
The following day, sections were treated with the appropriate
secondary antibodies (goat anti-mouse IgG; cat no. ab150113;
1:1,000; Abcam, Cambridge, MA, USA) for 1 h at room temperature.
Negative controls involved the same procedures with the omission of
the primary antibody. DAPI was used for counterstaining nuclei for
5 min at room temperature. The immunostained sections were analyzed
using the Olympus BX60 and IX71 epiflourescence microscopes with
Olympus DP-70 digital acquisition system at ×400 magnification
(Olympus Corporation). Images were obtained using 40X objectives
and processed with Adobe Photoshop CS3 version 10.0.1 software
(Adobe Systems, Inc., San Jose, CA, USA).
Statistical analysis
All of the quantitative experiments were performed
more than three times. A Student's t-test was used to determine
significant differences between various groups. P≤0.05 was
considered to indicate a statistically significant difference. All
statistical analysis was carried out using SPSS version 18.0 (SPSS,
Inc., Chicago, IL, USA).
Results
Clinical characteristics and cellular
analysis
The clinical characteristics of the 5 patients
included in the present study are presented in Table I. The H&E and TRAP staining of
patient tumor tissues are presented in Fig. 1 (Fig.
1A-E and F-J, respectively). Patient-derived GCTB cells were
isolated from patient samples using the collagenase B digestion
method. They contained the three major cell types, namely
mesenchymal stromal cells, round monocytes of macrophage lineage,
and multinucleated giant cells (Fig.
1K-O). The multinucleated giant cells were positively stained
with TRAP to confirm this characteristic osteoclast feature
(Fig. 1P-T). The components,
morphology and characteristics of patient-derived GCTB cells were
consistent with the clinical pathology reports and H&E
staining.
 | Table I.Epidemiological and pathological
characteristics of 5 patients with GCTB. |
Table I.
Epidemiological and pathological
characteristics of 5 patients with GCTB.
| Case | Age (years) | Gender | Site | Grade | Remarks |
|---|
| GCTB-1 | 26 | Female | Tibia | I | Primary |
| GCTB-2 | 19 | Male | Radial | II | Primary |
| GCTB-3 | 24 | Male | Tibia | III | Primary |
| GCTB-4 | 28 | Female | Femur | I | Primary |
| GCTB-5 | 25 | Female | Sacrum | II | Primary |
X-ray and micro-CT analysis of bone
lesions
The sites of intratibial injections in mice were
visible on weekly X-rays. Radiographic data revealed the appearance
of osteolytic bone lesions 2 months following intratibial injection
of patient-derived GCTB cells (Fig.
2). The intensity of osteolysis gradually increased as time
passed (Fig. 2A). Notably, the BL/NB
was significantly increased in GCTB mice (Fig. 2C). In control mice that received
injections with hFOB1.19 or PBS, there was no evidence of bone
damage in the proximal end of the tibia (Fig. 2A and C).
Micro-CT analysis confirmed marked osteolytic
destruction in GCTB mice (Fig. 2B).
The analysis revealed that GCTB tumors had almost completely
destroyed the tibial bone architecture 4 months after the
introduction of primary tumor cells, whereas the tibial bone
architecture was intact in PBS alone or hFOB1.19 control mice. The
ratio of BV/TV was significantly reduced in GCTB mice compared with
the control group (Fig. 2D).
Histological analysis and TRAP
staining
Paraffin histology revealed a cavity within the
tibias of mice injected with primary GCTB cells. The visible
cavities were surrounded with tumor cells arranged in a barrier
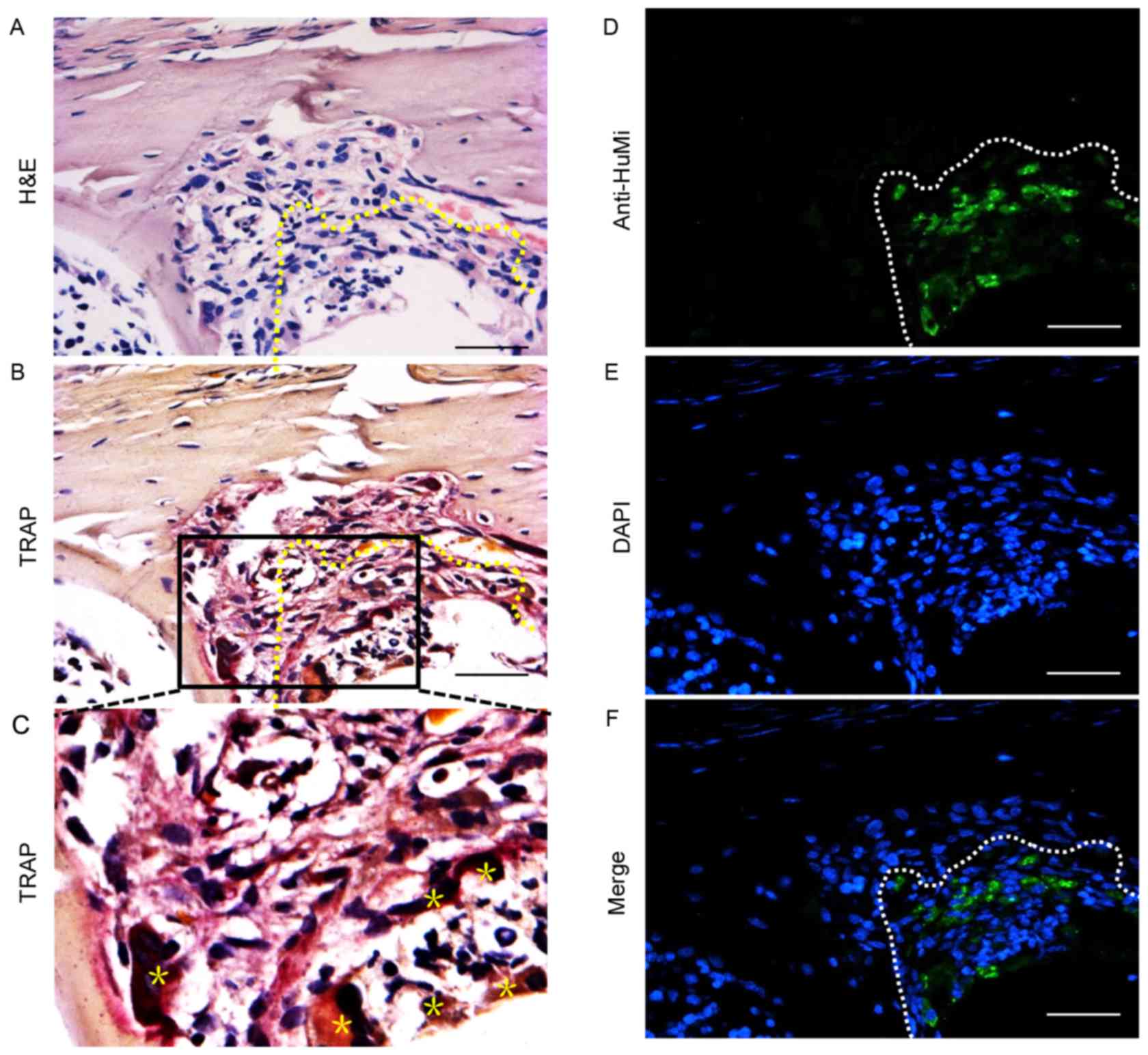
around the opening (Fig. 3A).
Multinucleated TRAP-positive cells, which are osteoclast-like
cells, were clearly visible within the bone matrix (Fig. 3B and C).
GCTB cells survival at the lesion
site
Four months after transplantation, human GCTB cells
were detected with an antibody against HuMi at the bone lesion
site, indicating the presence of transplanted cells amongst a
background of unstained mouse cells (Fig.
3D-F).
Discussion
The recent development of in vitro culture of
human GCTB cells has contributed to the understanding of the
phenotypes and physiology of GCTB. Although the stromal cell
subcutaneous injection model and chick chorioallantoic membrane
model have been utilized (22,23,25),
little is known regarding the orthotopic behavior of human GCTB
cells due to the difficulties in establishing a stable cell line
that allows for detailed investigation of their biology. In the
present study, patient-derived tumor cells isolated from GCTB were
injected into the bone marrow of nude mice in order to establish an
orthotopic murine model for human GCTB, a method proven to aid
engraftment and develop bone lesions in the murine bone marrow
microenvironment. Successful engraftment of GCTB-originating cells
and subsequent bone lesions were observed in bone environment of
mice.
In the current study, histological analysis of GCTB
xenograft tissue sections were performed using anti-HuMi, H&E
and TRAP staining. Histological analysis of H&E and TRAP
staining revealed that the patient-derived GCTB cells survived, and
caused an osteolytic reaction in the bone environment. Furthermore,
previous studies have reported that stromal cells can differentiate
into mature osteoblasts, adipocytes and chondrocytes (11,12,33–36).
Observed differences between the results of the present study and
others may indicate that stromal cells undergo multidirectional
differentiation, which may be influenced by the tissue
microenvironment. As such, different environmental conditions may
produce varied differentiation features and functions.
The X-ray and micro-CT features of bone destruction
in the present model were similar to the clinical imaging
characteristics, revealing pure lytic lesions without any degree of
matrix calcification or ossification and surrounding reactive
sclerosis (3). However, biopsies of
nude mice tibia did not exhibit the same clinicopathological
features as the chick chorioallantoic membrane model, which is
characterized by the presence of numerous multinucleated giant
cells that are uniformly distributed amongst mononuclear
spindle-like stromal cells and other monocytes (19). In the present study, a large number of
TRAP-positive cells were identified surrounded the surviving
stromal cells, and were distributed at the surface of the tibia
trabecular and matrix bone. As reported in the literature, stromal
cells are capable of secreting a variety of cytokines, including
stromal cell-derived factor-1 (37),
macrophage colony-stimulating factor (38) and monocyte chemotactic protein-1
(39). The release of these cytokines
results in the recruitment of monocytes and further secretion of
interleukin (IL)-6 (40), matrix
metalloproteinases (41,42), and parathyroid hormone-related protein
(43) to promote osteoclast fusion
and bone resorption.
A limitation of the current study was the use of
patient-derived GCTB cells containing all types of cells from GCTB
tissue. Isolation of the different subtypes of GCTB primary cells
using flow cytometry to compare the survival and tumorigenicity of
different cell populations within the bone environment was not
performed. Therefore, additional studies are required to evaluate
the survival and tumorigenicity of the various cell types or
combinations. Previously, it has been reported that GCTB stromal
cells transfected with green fluorescence protein survived 52 weeks
in vivo (24). However,
isolation of stromal cells from GCTB in vitro results in the
rapid loss of expression of receptor activator of nuclear factor-κB
ligand (44). This suggests that the
pathogenesis of GCTB requires cellular interactions, which provide
a suitable microenvironment for tumor growth.
In conclusion, the present study described the
establishment of a novel xenograft model of GCTB using
patient-derived tumor cells. This murine xenograft GCTB model
produced visible osteolytic damage in the proximal tibias of nude
mice. Anti-HuMi immunofluorescence staining confirmed that the
surviving cells in the osteolytic destruction were of human GCTB
cell origin. These findings may contribute to the understanding of
the pathogenesis of GCTB, and thus aid in the improvement of
diagnosis, treatment and patient outcome.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Planning Projects of Xiamen Science & Technology
Bureau, China (grant no. 3502Z20164039) and the Science and
Technology Commission of Shanghai Municipality (grant no.
09411950500 and 12R21418300).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LX conducted the experiment studies. ZW and ZZ
performed the statistical analysis and drafted the manuscript. JX
and XY made substantial contributions to conception and design of
this work and critically revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The use of all patient-derived tumor specimens was
approved by the Institutional Review Board and the research ethics
committee of Shanghai Changzheng Hospital (approval no. 2011/081),
which appeared in the proceedings of the meeting of the Ethics
Committee on November 18, 2011.
Patient consent to participate
Written informed consent was obtained individually
from each patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cowan RW and Singh G: Giant cell tumor of
bone: A basic science perspective. Bone. 52:238–246. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alsulaimani SA and Turcotte RE; Canadian
Orthopaedic Oncology Society (CANOOS) collaborators, : Iterative
curettage is associated with local control in giant cell tumors
involving the distal tibia. Clin Orthop Relat Res. 471:2668–2674.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stacy GS, Peabody TD and Dixon LB: Mimics
on radiography of giant cell tumor of bone. AJR Am J Roentgenol.
181:1583–1589. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prosser GH, Baloch KG, Tillman RM, Carter
SR and Grimer RJ: Does curettage without adjuvant therapy provide
low recurrence rates in giant-cell tumors of bone? Clin Orthop
Relat Res. 435:211–218. 2005. View Article : Google Scholar
|
|
5
|
Kremen TJ Jr, Bernthal NM, Eckardt MA and
Eckardt JJ: Giant cell tumor of bone: Are we stratifying results
appropriately? Clin Orthop Relat Res. 470:677–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin WH, Lan TY, Chen CY, Wu K and Yang RS:
Similar local control between phenol- and ethanol-treated giant
cell tumors of bone. Clin Orthop Relat Res. 469:3200–3208. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Klenke FM, Wenger DE, Inwards CY, Rose PS
and Sim FH: Recurrent giant cell tumor of long bones: Analysis of
surgical management. Clin Orthop Relat Res. 469:1181–1187. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klenke FM, Wenger DE, Inwards CY, Rose PS
and Sim FH: Giant cell tumor of bone: Risk factors for recurrence.
Clin Orthop Relat Res. 469:591–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldring SR, Schiller AL, Mankin HJ, Dayer
JM and Krane SM: Characterization of cells from human giant cell
tumors of bone. Clin Orthop Relat Res. 1–75. 1986.
|
|
10
|
Komiya S, Sasaguri Y, Inoue A, Nakashima
M, Yamamoto S, Yanagida I and Morimatsu M: Characterization of
cells cultured from human giant-cell tumors of bone. Phenotypic
relationship to the monocyte-macrophage and osteoclast. Clin Orthop
Relat Res. 258:304–309. 1990.
|
|
11
|
Salerno M, Avnet S, Alberghini M, Giunti A
and Baldini N: Histogenetic characterization of giant cell tumor of
bone. Clin Orthop Relat Res. 466:2081–2091. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wülling M, Delling G and Kaiser E: The
origin of the neoplastic stromal cell in giant cell tumor of bone.
Hum Pathol. 34:983–993. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lau CP, Ng PK, Li MS, Tsui SK, Huang L and
Kumta SM: p63 regulates cell proliferation and cell cycle
progressionassociated genes in stromal cells of giant cell tumor of
the bone. Int J Oncol. 42:437–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wuelling M, Delling G and Kaiser E:
Differential gene expression in stromal cells of human giant cell
tumor of bone. Virchows Arch. 445:621–630. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Babeto E, Conceição AL, Valsechi MC, Peitl
Junior P, de Campos Zuccari DA, de Lima LG, Bonilha JL, de Freitas
Calmon M, Cordeiro JA and Rahal P: Differentially expressed genes
in giant cell tumor of bone. Virchows Arch. 458:467–476. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen S, Li C, Wu B, Zhang C, Liu C, Lin X,
Wu X, Sun L, Liu C, Chen B, et al: Identification of differentially
expressed genes and their subpathways in recurrent versus primary
bone giant cell tumors. Int J Oncol. 45:1133–1142. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujikawa Y, Quinn JM, Sabokbar A, McGee JO
and Athanasou NA: The human osteoclast precursor circulates in the
monocyte fraction. Endocrinology. 137:4058–4060. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Massey HM and Flanagan AM: Human
osteoclasts derive from CD14-positive monocytes. Br J Haematol.
106:167–170. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lau YS, Sabokbar A, Gibbons CL, Giele H
and Athanasou N: Phenotypic and molecular studies of giant-cell
tumors of bone and soft tissue. Hum Pathol. 36:945–954. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Haque AU and Moatasim A: Giant cell tumor
of bone: A neoplasm or a reactive condition? Int J Clin Exp Pathol.
1:489–501. 2008.PubMed/NCBI
|
|
21
|
Werner M: Giant cell tumour of bone:
Morphological, biological and histogenetical aspects. Int Orthop.
30:484–489. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
James IE, Dodds RA, Olivera DL, Nuttall ME
and Gowen M: Human osteoclastoma-derived stromal cells: Correlation
of the ability to form mineralized nodules in vitro with formation
of bone in vivo. J Bone Miner Res. 11:1453–1460. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balke M, Neumann A, Szuhai K, Agelopoulos
K, August C, Gosheger G, Hogendoorn PC, Athanasou N, Buerger H and
Hagedorn M: A short-term in vivo model for giant cell tumor of
bone. BMC Cancer. 11:2412011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Singh S, Singh M, Mak I and Ghert M:
Expressional analysis of GFP-tagged cells in an in vivo mouse model
of giant cell tumor of bone. Open Orthop J. 7:109–113. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oreffo RO, Marshall GJ, Kirchen M, Garcia
C, Gallwitz WE, Chavez J, Mundy GR and Bonewald LF:
Characterization of a cell line derived from a human giant cell
tumor that stimulates osteoclastic bone resorption. Clin Orthop
Relat Res. 229–241. 1993.PubMed/NCBI
|
|
26
|
Long F, Cai X, Luo W, Chen L and Li K:
Role of aldolase A in osteosarcoma progression and metastasis: In
vitro and in vivo evidence. Oncol Rep. 32:2031–2037. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park SI, Kim SJ, McCauley LK and Gallick
GE: Pre-clinical mouse models of human prostate cancer and their
utility in drug discovery. Curr Protoc Pharmacol Chapter. 14:Unit
14.15. 2010. View Article : Google Scholar
|
|
28
|
Hoff BA, Chughtai K, Jeon YH, Kozloff K,
Galbán S, Rehemtulla A, Ross BD and Galbán CJ: Multimodality
imaging of tumor and bone response in a mouse model of bony
metastasis. Transl Oncol. 5:415–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Futakuchi M and Singh RK: Animal model for
mammary tumor growth in the bone microenvironment. Breast Cancer
(Tokyo, Japan). 20:195–203. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Balla P, Moskovszky L, Sapi Z, Forsyth R,
Knowles H, Athanasou NA, Szendroi M, Kopper L, Rajnai H, Pinter F,
et al: Epidermal growth factor receptor signalling contributes to
osteoblastic stromal cell proliferation, osteoclastogenesis and
disease progression in giant cell tumour of bone. Histopathology.
59:376–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|
|
32
|
Tang X, Jin R, Qu G, Wang X, Li Z, Yuan Z,
Zhao C, Siwko S, Shi T, Wang P, et al: GPR116, an adhesion
G-protein-coupled receptor, promotes breast cancer metastasis via
the Gαq-p63RhoGEF-Rho GTPase pathway. Cancer Res. 73:6206–6218.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang L, Teng XY, Cheng YY, Lee KM and
Kumta SM: Expression of preosteoblast markers and Cbfa-1 and
Osterix gene transcripts in stromal tumour cells of giant cell
tumour of bone. Bone. 34:393–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murata A, Fujita T, Kawahara N, Tsuchiya H
and Tomita K: Osteoblast lineage properties in giant cell tumors of
bone. J Orthop Sci. 10:581–588. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ghert M, Simunovic N, Cowan RW, Colterjohn
N and Singh G: Properties of the stromal cell in giant cell tumor
of bone. Clin Orthop Relat Res. 459:8–13. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang T, Zheng XF, Li M, Lin X and Yin QS:
Stimulation of osteogenic differentiation in stromal cells of giant
cell tumour of bone by zoledronic acid. Asian Pac J Cancer Prev.
14:5379–5383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McQuibban GA, Butler GS, Gong JH, Bendall
L, Power C, Clark-Lewis I and Overall CM: Matrix metalloproteinase
activity inactivates the CXC chemokine stromal cell-derived
factor-1. J Biol Chem. 276:43503–43508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Atkins GJ, Haynes DR, Graves SE, Evdokiou
A, Hay S, Bouralexis S and Findlay DM: Expression of osteoclast
differentiation signals by stromal elements of giant cell tumors. J
Bone Miner Res. 15:640–649. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McQuibban GA, Gong JH, Wong JP, Wallace
JL, Clark-Lewis I and Overall CM: Matrix metalloproteinase
processing of monocyte chemoattractant proteins generates CC
chemokine receptor antagonists with anti-inflammatory properties in
vivo. Blood. 100:1160–1167. 2002.PubMed/NCBI
|
|
40
|
Ohsaki Y, Takahashi S, Scarcez T, Demulder
A, Nishihara T, Williams R and Roodman GD: Evidence for an
autocrine/paracrine role for interleukin-6 in bone resorption by
giant cells from giant cell tumors of bone. Endocrinology.
131:2229–2234. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sasaguri Y, Komiya S, Sugama K, Suzuki K,
Inoue A, Morimatsu M and Nagase H: Production of matrix
metalloproteinases 2 and 3 (stromelysin) by stromal cells of giant
cell tumor of bone. Am J Pathol. 141:611–621. 1992.PubMed/NCBI
|
|
42
|
Ueda Y, Imai K, Tsuchiya H, Fujimoto N,
Nakanishi I, Katsuda S, Seiki M and Okada Y: Matrix
metalloproteinase 9 (gelatinase B) is expressed in multinucleated
giant cells of human giant cell tumor of bone and is associated
with vascular invasion. Am J Pathol. 148:611–622. 1996.PubMed/NCBI
|
|
43
|
Cowan RW, Singh G and Ghert M: PTHrP
increases RANKL expression by stromal cells from giant cell tumor
of bone. J Orthop Res. 30:877–884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Morgan T, Atkins GJ, Trivett MK, Johnson
SA, Kansara M, Schlicht SL, Slavin JL, Simmons P, Dickinson I,
Powell G, et al: Molecular profiling of giant cell tumor of bone
and the osteoclastic localization of ligand for receptor activator
of nuclear factor kappaB. Am J Pathol. 167:117–128. 2005.
View Article : Google Scholar : PubMed/NCBI
|