Introduction
Non-small cell lung cancer (NSCLC) is one of the
most common types of cancer worldwide, and has become the leading
cause of cancer-associated mortality in China within the previous 5
years (1). NSCLC includes a variety
of cancer types, including large cell carcinoma, squamous cell
carcinoma and adenocarcinoma. Compared with SCLC, NSCLC
proliferates more slowly and metastasizes later (2,3). NSCLC
accounts for ~85% of total lung cancers and >80% of patients
diagnosed with NSCLC are in middle- or late-stage disease (4). Therefore, the 5-year survival rate of
NSCLC is relatively low (1). As NSCLC
seriously threatens the lives of patients, investigation is
required to develop a therapeutic method for the treatment of NSCLC
(5,6).
As with other types of cancer, chemotherapy,
radiotherapy and surgery serve critical roles in the treatment of
NSCLC. However, the aforementioned methods are associated with
various disadvantages and insufficiencies (7–9).
Radiotherapy has been demonstrated to have the best curative effect
for NSCLC (10). Radiotherapy mainly
targets lymphatic metastasis and primary tumors, with chemotherapy
serving an auxiliary role (11,12).
Chemotherapy presents marked curative effects on NSCLC; however,
bleeding and other side effects have been reported (13). Surgery has the greatest limitation, as
it is not applicable for patients with complications or those
>70 years old (14,15). Therefore, investigations into the
molecular mechanism of NSCLC occurrence and development are
required, and may provide valuable information for clinical
practice.
Gefitinib exhibits significant curative effects on
NSCLC, but its detailed molecular mechanism remains poorly
understood (16). The present study
aimed to investigate the effects of gefitinib on NSCLC H1650 cell
viability and apoptosis. Gefitinib may be used alone or in
combination with other chemotherapy drugs for the treatment of
NSCLC (17–19). Gefitinib is an antagonist of the
tyrosine protein kinase of epidermal growth factor receptor (EGFR).
Generally, gefitinib serves an antitumor role by inhibiting EGFR
tyrosine protein kinase activity (20). Gefitinib significantly inhibited the
proliferation of tumor cells, and also reduced lung cancer tumor
growth, invasion and metastasis in a rat model (21,22).
However, the knockdown or overexpression of EGFR in vitro or
in vivo failed to alter NSCLC cell sensitivity to gefitinib,
indicating that gefitinib may have a novel target or molecular
mechanism underlying NSCLC (23,24).
Tumor necrosis factor-related apoptotic inducing
ligand (TRAIL) is a novel member of the tumor necrosis factor
family (25). TRAIL is highly
expressed within activated T lymphocytes and may induce apoptosis
via interaction with its ligands, including death receptor 4 (DR4)
and DR5 (26). By contrast, TRAIL may
inhibit apoptosis if not combined with DR4/DR5. A recent study
indicated that TRAIL expression levels in NSCLC tissue were
altered, indicating that TRAIL may be associated with NSCLC
occurrence and development (27).
The present study aimed to investigate the
regulatory role and associated mechanism of gefitinib on NSCLC
H1650 cell viability and apoptosis in vitro, and may provide
valuable information for NSCLC treatment in the clinical
setting.
Materials and methods
Reagents
High-glucose Dulbecco's modified Eagle's medium
(DMEM) was purchased from Gibco; Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). Trypsin, EDTA, poly-l-lysine, Hanks buffer,
penicillin and streptomycin were obtained from Sigma-Aldrich; Merck
KGaA (Darmstadt, Germany). Phosphate-buffered saline (PBS) and
dimethyl sulfoxide (DMSO) were purchased from Beijing Dingguo
Changsheng Biotechnology Co., Ltd. (Beijing, China). MTT reagent,
fluorescein isothiocyanate (FITC)-Annexin V and Caspase 3 Activity
Assay kit were obtained from Beyotime Institute of Biotechnology
(Haimen, China). TRAIL small interfering RNA (siRNA) and control
siRNA were synthetized by Sangon Biotech Co., Ltd. (Shanghai,
China). TRAIL plasmids were produced in-house. TRAIL and β-actin
antibodies were obtained from Sigma-Aldrich; Merck KGaA.
Cell culture
The H1650 cell line was purchased from the American
Type Culture Collection (Manassas, VA, USA) and cultured in high
glucose DMEM medium at 37°C and 5% CO2.
Transfection
H1650 cells were cultured at 50% density 1 day prior
to transfection.
N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium
methyl-sulfate (DOTAP) Liposomal Transfection Reagent
(Sigma-Aldrich; Merck KGaA) was used for transfection. TRAIL siRNA
(cat. no. 1027423; Qiagen Sciences, Inc., Gaithersburg, MD, USA) or
control siRNA (cat. no. 1027310; Qiagen Sciences, Inc.) (1 µg) were
cloned into a TRAIL plasmid with the DOTAP liposomal transfection
reagent (Sigma-Aldrich; Merck KGaA) at room temperature for 3 min.
The mixture was then added to the cells and maintained for 12 h at
room temperature. The cells were cultured for a further 24 h
following the replacement of medium and subsequent
experimentation.
MTT assay
H1650 cell viability was investigated by colorimetry
as previously described (28).
Un-transfected H1650 cells were washed in DMEM and treated with 7
µl MTT solution (0.1 M, pH 7.2). The cells were cultured at 37°C
and 5% CO2 for 4 h and subsequently washed in DMEM,
followed by the addition of 50 µl DMSO for 15 min to dissolve the
purple formazan. The plate was analyzed at 540 nm.
Flow cytometry
Flow cytometry was employed to investigate
phosphatidylserine expression on the H1650 cell surface as
previously described (29). A total
of 1×104 H1650 cells were incubated with FITC-Annexin V
dye and Annexin Binding Buffer (Thermo Fisher Scientific, Inc.) for
16 min at room temperature. Subsequently, the cells were analyzed
using a flow cytometer (BD FACSCalibur™) at 465 (emitted light) and
630 nm (absorbed light) and data were analyzed by BD Cell Quest Pro
software, version 5.1 (BD Biosciences, Franklin, NJ, USA).
Caspase-3 activity detection
As previously described, H1650 cell caspase-3
activity was determined using a microplate reader (9). A total of 1×104 H1650 cells
were treated with Cell Lysis Buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA) for 30 min on ice and incubated with a
chromophore p-nitroaniline (pNA) (Included in the caspase-3
activity assay kit) at room temperature for 18 min. Finally, the
cells were read on microplate reader at 490 nm.
Western blot analysis
TRAIL expression levels in H1650 cells were analyzed
via western blotting as described previously (17). The cells were lysed on ice for 30 min
using Cell Lysis Buffer (Cell Signaling Technology). Following
centrifugation at 10,000 × g for 5 min at 4°C, the protein was
quantified using Pierce BCA Protein Assay kit (Thermo Fisher
Scientific, Inc.) and separated by 10% SDS-PAGE electrophoresis (10
µg/lane). Then the protein was transferred to a polyvinylidene
fluoride membrane and blocked with 5% skimmed milk at room
temperature for 1 h. Subsequently, the membrane was incubated with
a TRAIL primary antibody (cat. no., T9191) (dilution, 1:1,000) and
β-actin primary antibody (cat. no., SAB5500001) (dilution, 1:2,000)
for 2 h at room temperature. The membrane was then incubated with
HRP-conjugated goat anti-rabbit secondary antibody (cat. no.,
AP187P; dilution, 1:1,000; Sigma-Aldrich; Merck KGaA) for 2 h at
room temperature and washed with PBST three times. Finally, the
membrane was treated with an enhanced chemiluminescence agent
(Thermo Fisher Scientific, Inc.) and analyzed. ImageJ software
version 1.51 (National Institutes of Health, Bethesda, MD, USA) was
used for data analysis.
Statistical analysis
All statistical analyses were performed using SPSS
software and data were presented as mean ± standard deviation (SD)
A Levene's test was first performed to detect normal distribution.
One-way analysis of variance with Student-Newman-Keuls multiple
comparison post-hoc analysis was used for comparison of the means
(16). P<0.05 was considered to
indicate a statistically significant difference.
Results
Gefitinib inhibits H1650 cell
viability
As presented in Fig.
1, an MTT assay revealed that H1650 cell viability was markedly
declined following treatment with 1 µg/ml gefitinib (P=0.0067).
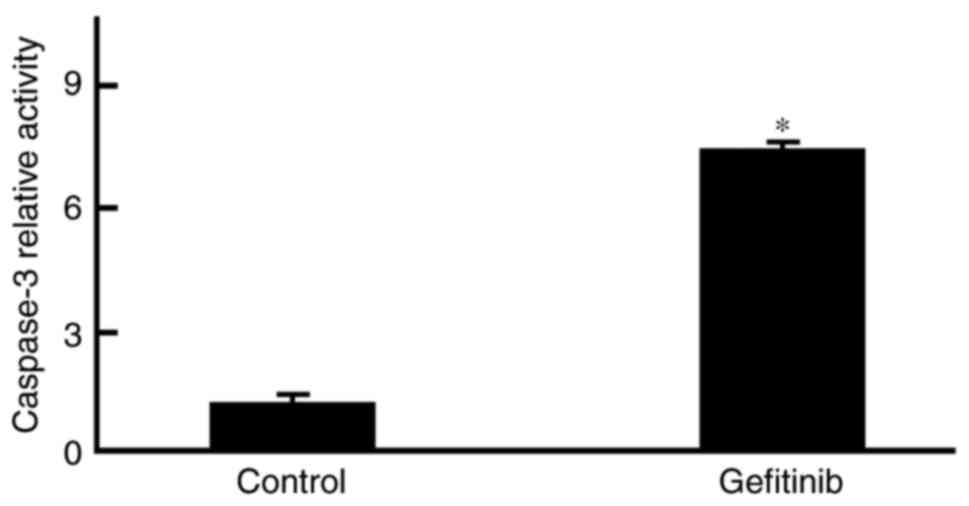
Gefitinib induces H1650 cell
apoptosis
Flow cytometry demonstrated that the
phosphatidylserine levels on the H1650 cell surface were
significantly increased compared with levels in the control
(P=0.023; Figs. 2 and 3).
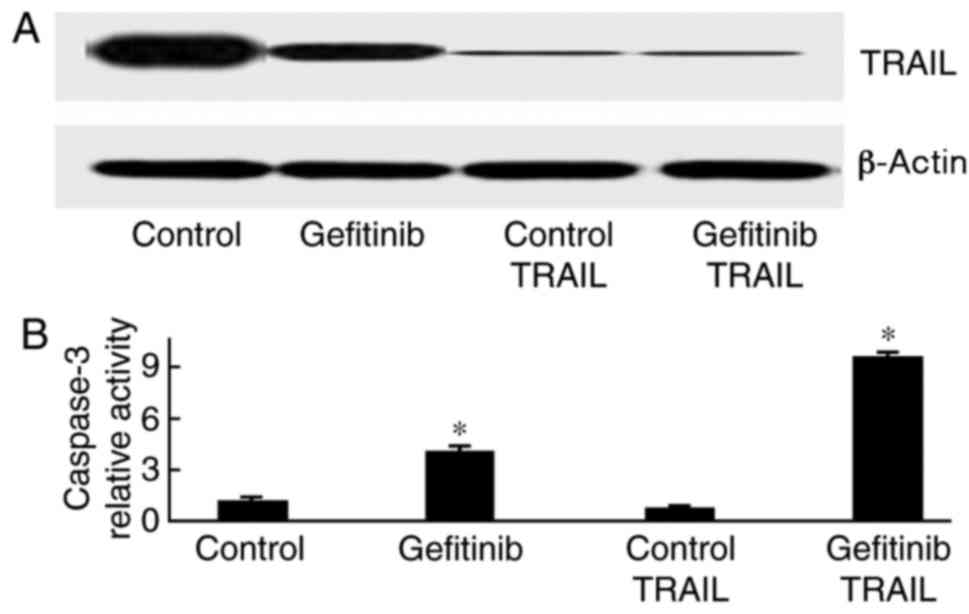
Gefitinib decreases TRAIL protein
expression levels
Western blot analysis demonstrated that, compared
with the control, TRAIL protein expression levels were markedly
declined following treatment with 1 µg/ml gefitinib (P<0.05;
Fig. 4).
TRAIL knockdown enhances
gefitinib-induced H1650 cell apoptosis
H1650 cells were transfected with TRAIL siRNA and
treated with gefitinib. As presented in Fig. 5, caspase-3 activity was markedly
increased in response to gefitinib treatment (P=0.011).
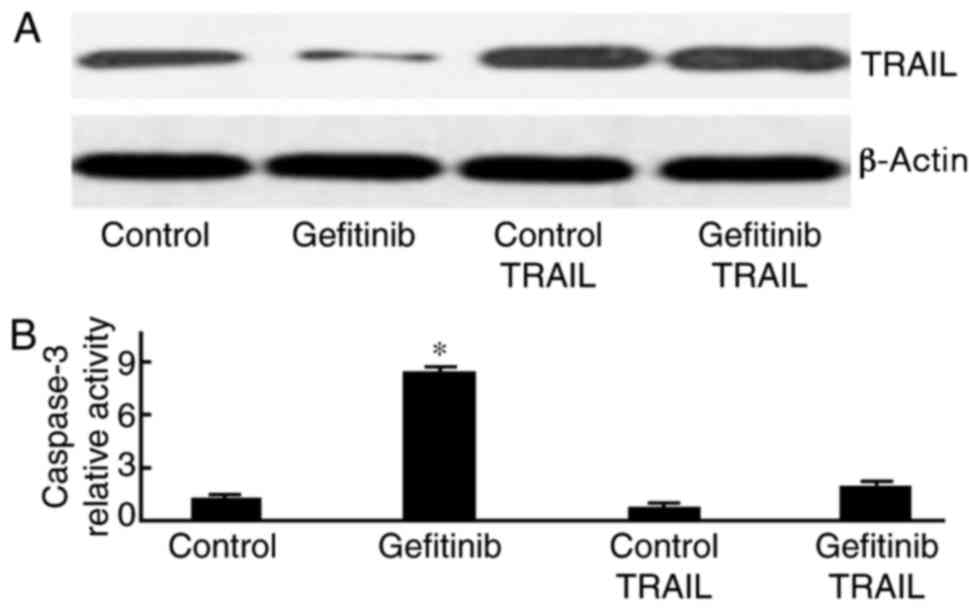
TRAIL plasmid transfection reduces
gefitinib-induced cell apoptosis
H1650 cells were initially transfected with a
TRAIL-containing plasmid and were subsequently treated with
gefitinib. As presented in Fig. 6,
caspase-3 activity was significantly weakened following gefitinib
treatment (P=0.013).
Discussion
NSCLC severely threatens the lives of patients, but
the molecular mechanism of NSCLC remains to be further investigated
(1). A recent study suggested that
microRNAs may serve a regulatory role in NSCLC cell proliferation
and survival (29,30). The present study aimed to investigate
the effects of gefitinib on H1650 cells. The results suggested that
gefitinib significantly reduced H1650 cell viability and may have
caused H1650 cell apoptosis.
To further analyze the molecular mechanism of
gefitinib on NSCLC H1650 cells, various doses of gefitinib (1, 2, 5
and 10 µm) were applied for varying durations (12, 18, 24, 30 and
36 h) to H1650 cells (data not shown). Gefitinib may induce H1650
cell apoptosis in a dose- and time-dependent manner, which is
highly consistent with other reports on different types of cancer
cells. Additionally, gefitinib exhibited an antitumor effect by
inducing apoptosis (3,15,19).
Single TRAIL knockdown may not cause apoptosis; however, TRAIL is
an important member of the anti-apoptotic proteins, which exhibit
anti-apoptotic effects under apoptotic conditions. Its single
knockdown did not cause apoptosis, which was the same as the
mechanism of other anti-apoptosis proteins, including B-cell
lymphoma 2 (Bcl-2) and Bcl-extra large.
There were three main observations of the present
study: i) Gefitinib suppressed H1650 cell viability, induced H1650
cell apoptosis and downregulated TRAIL protein expression without
affecting the genetic level; ii) TRAIL interference enhanced H1650
apoptosis induced by Gefitinib; and iii) TRAIL overexpression
inhibited gefitinib-induced H1650 apoptosis. These results
suggested that gefitinib induced the apoptosis of NSCLC H1650 cells
by reducing TRAIL expression levels.
The present study also had several drawbacks and
limitations: i) How gefitinib regulated TRAIL expression levels was
not confirmed; ii) gefitinib-induced H1650 cell apoptosis via the
downregulation of TRAIL expression levels was not investigated in
an animal model; and iii) clinical cancer and para-carcinoma
tissues were not obtained to investigate the association between
gefitinib treatment, TRAIL expression levels and potential curative
effects. Taken together, the results of the present study confirmed
that gefitinib induced the apoptosis of NSCLC H1650 cells by
decreasing TRAIL expression levels.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed are included in this
published article.
Authors' contributions
HY, SL, HL, PW and HZ performed the experiments and
analyzed the data. XC designed the study and wrote the
manuscript.
Ethics statement and consent to
participate
All experimental procedures involving animals were
approved by the Ethnic Committee of Yinzhou Affiliated Hospital to
Medical School of Ningbo University (Ningbo, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cao W, Liu Y, Zhang R, Zhang B, Wang T,
Zhu X, Mei L, Chen H, Zhang H, Ming P and Huang L:
Homoharringtonine induces apoptosis and inhibits STAT3 via
IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer
cells. Sci Rep. 5:84772015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang J, Guo F, Du Y, Liu X, Qin Q, Liu X,
Yin T, Jiang L and Wang Y: Continuous exposure of non-small cell
lung cancer cells with wild-type EGFR to an inhibitor of EGFR
tyrosine kinase induces chemoresistance by activating STAT3. Int J
Oncol. 46:2083–2095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sudo M, Mori S, Madan V, Yang H, Leong G
and Koeffler HP: Short-hairpin RNA library: Identification of
therapeutic partners for gefitinib-resistant non-small cell lung
cancer. Oncotarget. 6:814–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garofalo M, Romano G, Di Leva G, Nuovo G,
Jeon YJ, Ngankeu A, Sun J, Lovat F, Alder H, Condorelli G, et al:
EGFR and MET receptor tyrosine kinase-altered microRNA expression
induces tumorigenesis and gefitinib resistance in lung cancers. Nat
Med. 18:74–82. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ahn SH, Jeong EH, Lee TG, Kim SY, Kim HR
and Kim CH: Gefitinib induces cytoplasmic translocation of the CDK
inhibitor p27 and its binding to a cleaved intermediate of caspase
8 in non-small cell lung cancer cells. Cell Oncol (Dordr).
37:377–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chao TT, Wang CY, Lai CC, Chen YL, Tsai
YT, Chen PT, Lin HI, Huang YC, Shiau CW, Yu CJ and Chen KF: TD-19,
an erlotinib derivative, induces epidermal growth factor receptor
wild-type nonsmall-cell lung cancer apoptosis through
CIP2A-mediated pathway. J Pharmacol Exp Ther. 351:352–358. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bokobza SM, Jiang Y, Weber AM, Devery AM
and Ryan AJ: Combining AKT inhibition with chloroquine and
gefitinib prevents compensatory autophagy and induces cell death in
EGFR mutated NSCLC cells. Oncotarget. 5:4765–4778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong FM, Zhao J, Wang J and Faivre-Finn C:
Radiation dose effect in locally advanced non-small cell lung
cancer. J Thorac Dis. 6:336–347. 2014.PubMed/NCBI
|
|
11
|
Lee JY, Lee YM, Chang GC, Yu SL, Hsieh WY,
Chen JJ, Chen HW and Yang PC: Curcumin induces EGFR degradation in
lung adenocarcinoma and modulates p38 activation in intestine: The
versatile adjuvant for gefitinib therapy. PLoS One. 6:e237562011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao W, Bao P, Qi H and You H: Resveratrol
down-regulates survivin and induces apoptosis in human
multidrug-resistant SPC-A-1/CDDP cells. Oncol Rep. 23:279–286.
2010.PubMed/NCBI
|
|
13
|
Zappa C and Mousa SA: Non-small cell lung
cancer: Current treatment and future advances. Transl Lung Cancer
Res. 5:288–300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Viallet J and Haura EB: A small
molecule pan-Bcl-2 family inhibitor, GX15-070, induces apoptosis
and enhances cisplatin-induced apoptosis in non-small cell lung
cancer cells. Cancer Chemother Pharmacol. 61:525–534. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morgillo F, Kim WY, Kim ES, Ciardiello F,
Hong WK and Lee HY: Implication of the insulin-like growth
factor-IR pathway in the resistance of non-small cell lung cancer
cells to treatment with gefitinib. Clin Cancer Res. 13:2795–2803.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hotta K, Tabata M, Kiura K, Kozuki T,
Hisamoto A, Katayama H, Takigawa N, Fujimoto N, Fujiwara K, Ueoka H
and Tanimoto M: Gefitinib induces premature senescence in non-small
cell lung cancer cells with or without EGFR gene mutation. Oncol
Rep. 17:313–317. 2007.PubMed/NCBI
|
|
17
|
Fan XX, Li N, Wu JL, Zhou YL, He JX, Liu L
and Leung EL: Celastrol induces apoptosis in gefitinib-resistant
non-small cell lung cancer cells via caspases-dependent pathways
and Hsp90 client protein degradation. Molecules. 19:3508–3522.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li B, Ren S, Li X, Wang Y, Garfield D,
Zhou S, Chen X, Su C, Chen M, Kuang P, et al: MiR-21 overexpression
is associated with acquired resistance of EGFR-TKI in non-small
cell lung cancer. Lung Cancer. 83:146–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song JY, Kim CS, Lee JH, Jang SJ, Lee SW,
Hwang JJ, Lim C, Lee G, Seo J, Cho SY and Choi J: Dual inhibition
of MEK1/2 and EGFR synergistically induces caspase-3-dependent
apoptosis in EGFR inhibitor-resistant lung cancer cells via BIM
upregulation. Invest New Drugs. 31:1458–1465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ansari J, Palmer DH, Rea DW and Hussain
SA: Role of tyrosine kinase inhibitors in lung cancer. Anticancer
Agents Med Chem. 9:569–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zou M, Xia S, Zhuang L, Han N, Chu Q, Chao
T, Peng P, Chen Y, Gui Q and Yu S: Knockdown of the Bcl-2 gene
increases sensitivity to EGFR tyrosine kinase inhibitors in the
H1975 lung cancer cell line harboring T790M mutation. Int J Oncol.
42:2094–2102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Grève J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee YC, Lee LM, Yang CH, Lin AM, Huang YC,
Hsu CC, Chen MS, Chi CW, Yin PH, Kuo CD, et al: Norcantharidin
suppresses cell growth and migration with enhanced anticancer
activity of gefitinib and cisplatin in human non-small cell lung
cancer cells. Oncol Rep. 29:237–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang B, Jiao J, Liu Y, Guo LX, Zhou B, Li
GQ, Yao ZJ and Zhou GB: Gefitinib analogue V1801 induces apoptosis
of T790M EGFR-harboring lung cancer cells by up-regulation of the
BH-3 only protein Noxa. PLoS One. 7:e487482012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan XX, Yao XJ, Xu SW, Wong VK, He JX,
Ding J, Xue WW, Mujtaba T, Michelangeli F, Huang M, et al:
(Z)3,4,5,4′-trans-tetramethoxystilbene, a new analogue of
resveratrol, inhibits gefitinb-resistant non-small cell lung cancer
via selectively elevating intracellular calcium level. Sci Rep.
5:163482015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu T, Wang Z, Liu Y, Mei Z, Wang G, Liang
Z, Cui A, Hu X, Cui L, Yang Y and Liu CY: Interleukin 22 protects
colorectal cancer cells from chemotherapy by activating the STAT3
pathway and inducing autocrine expression of interleukin 8. Clin
Immunol. 154:116–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imamura Y, Wang PL, Masuno K and Sogawa N:
Salivary protein histatin 3 regulates cell proliferation by
enhancing p27(Kip1) and heat shock cognate protein 70
ubiquitination. Biochem Biophys Res Commun. 470:269–274. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Alam MM, Sohoni S, Kalainayakan SP,
Garrossian M and Zhang L: Cyclopamine tartrate, an inhibitor of
Hedgehog signaling, strongly interferes with mitochondrial function
and suppresses aerobic respiration in lung cancer cells. BMC
Cancer. 16:1502016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li LH, Wu P, Lee JY, Li PR, Hsieh WY, Ho
CC, Ho CL, Chen WJ, Wang CC, Yen MY, et al: Hinokitiol induces DNA
damage and autophagy followed by cell cycle arrest and senescence
in gefitinib-resistant lung adenocarcinoma cells. PLoS One.
9:e1042032014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Inamura K and Ishikawa Y: MicroRNA in lung
cancer: Novel biomarkers and potential tools for treatment. J Clin
Med. 5(pii): E362016. View Article : Google Scholar : PubMed/NCBI
|