Introduction
Brain metastasis (BM) is one of the important causes
of death in patients with non-small cell lung cancer (NSCLC). The
natural course of the disease is 1–3 months, and the median
survival time is only 4 months (1).
The incidence of BMs has increased in recent years, and is
associated with poor prognosis. Treatment of metastatic brain
tumors for NSCLC presents a particular challenge: the majority of
patients with BMs present multiple lesions. The standard treatment
is whole brain radiotherapy (WBRT), which may help to improve the
local control rate and alleviate the clinical symptoms, and the
median survival time extended to 3–6 months. The recurrence rate of
intracranial tumors following WBRT is 52%, so it is necessary to
give a higher therapeutic dose to the BMs, in order to improve the
local control and survival rate of the tumor (2,3).
Historically, the treatment options of local tumor pushing with BMs
include three-dimensional conformal radiotherapy (3D-CRT),
intensity-modulated radiation therapy (IMRT) and stereotactic
radiosurgery (SRS). In recent years, IMRT has played an important
role in the treatment of radiation therapy, which has attracted
wide attention. Therefore, IMRT has been proposed for the treatment
of BMs. In particular, IMRT was evaluated in several planning
irradiation studies, mainly in order to protect normal brain
tissue, to simultaneously boost the local dose to brain lesions
during WBRT (4–7).
Systemic chemotherapy in the treatment of BMs is
limited and it has been widely controverted. The limited ability of
most chemotherapeutic agents to cross the blood-brain barrier (BBB)
is believed to be one of the principal reasons. The agents that
have difficulty in reaching the central nervous system can not
achieve the effective blood drug concentration, and therefore are
less active against disease in the central nervous system than
against extra-cranial, systemic disease (8). Several chemotherapeutic agents in
combination with WBRT have failed to result in the expected
therapeutic benefit (9). Temozolomide
(TMZ) is an oral, new alkylating agent that has demonstrated a
preclinical activity against a variety of solid tumors. It readily
crosses the BBB, achieving therapeutic concentrations in the brain,
which makes it an attractive agent against BMs (10). It has been reported that the
concomitant treatment of TMZ and WBRT was active and was conducive
to improving quality of life (QoL) with an encouraging objective
response (OR) rate (11). The
combination of TMZ and WBRT may improve the treatment response
(12).
We believe that adding TMZ to IMRT is more efficient
than WBRT alone. The primary target of the study was to evaluate
the recent response to treatment and safety, and the impact on QoL
by using this treatment regimen with BMs from NSCLC.
Patients and methods
Eligibility criteria
Patients with histologically confirmed lung
adenocarcinoma with no more than 4 (≥1 and ≤4) BMs by magnetic
resonance imaging (MRI) and controlled extracranial disease between
2014 and 2015 were recruited. Patients were aged ≤75 years, and had
a World Health Organization Performance Status (PS) of ≤3. Eligible
patients may have received previous radiation therapy to the
primary tumor or systemic metastatic sites but no previous WBRT or
radiosurgery for BMs. Routine blood counts and biochemistry
examination were basically normal. This study was approved by the
Ethics Committee of the Affiliated Hospital of Soochow University
(Suzhou, China). Signed informed consents were obtained from the
patients.
Treatment plan
Every patient was immobilized by using a Medtec
mask. A volumetric computed tomography simulation with a 3 mm slice
thickness was required for planning of the target volume.
Simulation computed tomography and pre-RT brain MRI fusion was
performed for target delineation. The gross tumor volume (GTV) for
BMs was delineated on the contrast-enhanced planning CT scan with
visual comparison to the T1+C-weighed axial diagnostic
gadolinium-enhanced MRI (Gd-MRI) scan. Clinical tumor volume (CTV)
was defined as the whole brain, and a margin of 5 mm was added to
CTV as the planning target volume (PTV). The plan was created to
deliver a dose of 30 Gy in 10 daily fractions to CTV according to
IMRT, then additional dose of 9 Gy in 3 fractions of IMRT was
delivered to GTV only. TMZ was administered orally, daily during RT
at a dose of 75 mg/m2/day from the first day to the end
of day 14. If grade 3/4 toxicity occurred in the study, TMZ was
withheld until the toxicity resolved, and the missed doses were no
longer administered. For the control group, patients were given a
dose of 30 Gy in 10 daily fractions to CTV according to IMRT, then
additional dose of 9 Gy in 3 fractions of IMRT was delivered to GTV
only without oral administration of temozolidomide.
Treatment evaluation and
follow-up
Physical examination, neurological examination, QoL
score and enhancement of the brain MRI were included in the
pre-treatment evaluation within 28 days prior to treatment.
Standard laboratory tests were obtained before treatment. All
patients were monitored weekly during RT, including functional
status assessment, neurologic examination, blood counts,
biochemical functions and QoL score. Radiographic tumor response
was assessed by Gd-MRI. Imaging was performed 3, 6 and 12 months
following RT completion.
Patients were evaluated in the third month after
treatment, including neurologic examination, functional status,
toxicity assessment, QoL score and Gd-MRI of the brain. The primary
endpoint was efficacy as measured by OR, including complete
response (CR) and partial response (PR). CR was defined as a
complete disappearance of all evidence of disease in the brain. PR
was defined as response >50% in all BMs. Responses in tumor
lesions <50% or increase in size <25% was defined as stable
disease (SD). A progressive disease (PD) was defined as either the
occurrence of new lesion(s) or an increase in size of >25%.
Tumor response for patients who died before the 3-month follow-up
was defined as PD. PFS and overall survival (OS) were
monitored.
Definition of progression and PFS
PFS was defined as the time from the date of the
first dose of TMZ to the date of progression. Tumor progression was
defined as an increment of 25% of the cross-product area
measurement of nodular contrast enhancement compared with previous
examination or any new enhancing tumor. Alternately, a clinical
neurological performance change of two when causes other than tumor
progression have been ruled out, was considered as progression.
Definition of OS
OS was defined as the time from the date of first
dose of TMZ to date of death. Cause of death and its relationship
with either the systemic or CNS disease was also recorded. Toxicity
was evaluated according to the Common Terminology Criteria for
Adverse Events (CTCAE) v3.0, divided into 1–5 degrees.
Evaluation of QoL
QoL was measured by using subject-completed
Functional Assessment of Cancer Therapy (FACT) instrument. Twelve
most relevant questions were selected in FACT questionnaire. The
QoL was assessed around 3 months after radiotherapy. FACT for QoL
score of cancer patients (1990 edition) includes appetite, spirit,
sleep, fatigue, pain, family understanding and coordination,
understanding and coordination of colleagues, understanding of
cancer, attitude to treatment, daily life, side-effects of
treatment, facial expression. It is divided into 5 levels, 1–5 the
degree of expression is gradually reduced. QoL classification: the
QoL full score is 60 points, poor QoL is <20 points, the
difference is 21–30 points, general is 31–40 points, better is
41–50 points, and good is 51–60 points (13).
Statistical analysis
The analysis of treatment response and toxicity were
evaluated in a descriptive manner. The descriptive method used in
this report is to evaluate the short-term efficacy: according to
the criteria for evaluating the curative effect of solid tumor,
RECIST was used to evaluate the short-term curative effect, which
was divided into CR, PR, SD and PD. CR: all the target lesions
disappeared; PR: 30% reduction of the sum of the longest diameter
of the target lesion compared with the baseline state; PD: the sum
of the longest diameter of the target lesion increased by 20%, or
one or more new lesions, compared with the sum of the longest
diameter of the target lesion recorded after the beginning of
treatment; SD: between PR and PD. Objective response rate (ORR)
includes CR and PR. The descriptive method used in adverse
reactions was classified into 0–4 grades according to the commonly
used drug toxicity standard CTCAE v3.0. Kaplan-Meier estimates were
used to assess PFS and OS. Log-rank test was used for survival
analysis. The QoL data were analysed by ANOVA test and the post hoc
test was SNK. SPSS software (version 19.00; IBM Corp., Armonk, NY,
USA) was used for statistical analysis.
Results
Between 2014 and 2016, 18 patients were enrolled.
The demographics and baseline disease characteristics of the
assessable patients are listed in Table
I.
 | Table I.Patient characteristics and efficacy
data evaluation after therapy. |
Table I.
Patient characteristics and efficacy
data evaluation after therapy.
|
| No. of patients
(%) |
|---|
|
|
|
|---|
| Characteristics | R+T | R |
|---|
| Sex |
| Male | 7 (77.8) | 6 (66.7) |
|
Female | 2 (22.2) | 3 (33.3) |
| Age (years) |
|
<40 | 0 (0) | 0 (0) |
|
40–60 | 3 (33.3) | 2 (22.2) |
|
>60 | 6 (66.7) | 7 (77.8) |
| ECOG score |
| 0 | 5 (55.6) | 4 (44.4) |
| 1 | 4 (44.4) | 5 (55.6) |
| No. of
metastases |
| 1 | 3 (33.3) | 3 (33.3) |
| 2 | 2 (22.2) | 2 (22.2) |
| 3 | 0 (0) | 2 (22.2) |
| 4 | 4 (44.4) | 2 (22.2) |
| Year of
recruitment |
| 2014 | 3 (33.3) | 1 (11.1) |
| 2015 | 6 (66.7) | 8 (88.9) |
| Time course of
disease (months) |
|
<1 | 0 (0) | 0 (0) |
|
1–3 | 0 (0) | 1 (11.1) |
|
3–6 | 1 (11.1) | 2 (22.2) |
|
6–12 | 4 (44.4) | 3 (33.3) |
|
≥12 | 4 (44.4) | 3 (33.3) |
| Response |
| CR | 1 (11.1) | 0 (0) |
| PR | 6 (66.7) | 4 (44.4) |
| Objective response
(CR+PR) | 7 (77.8) | 4 (44.4) |
| SD | 2 (22.2) | 3 (33.3) |
| Local control
(CR+PR+SD) | 9 (100) | 7 (77.8) |
| PD | 0 (0) | 2 (22.2) |
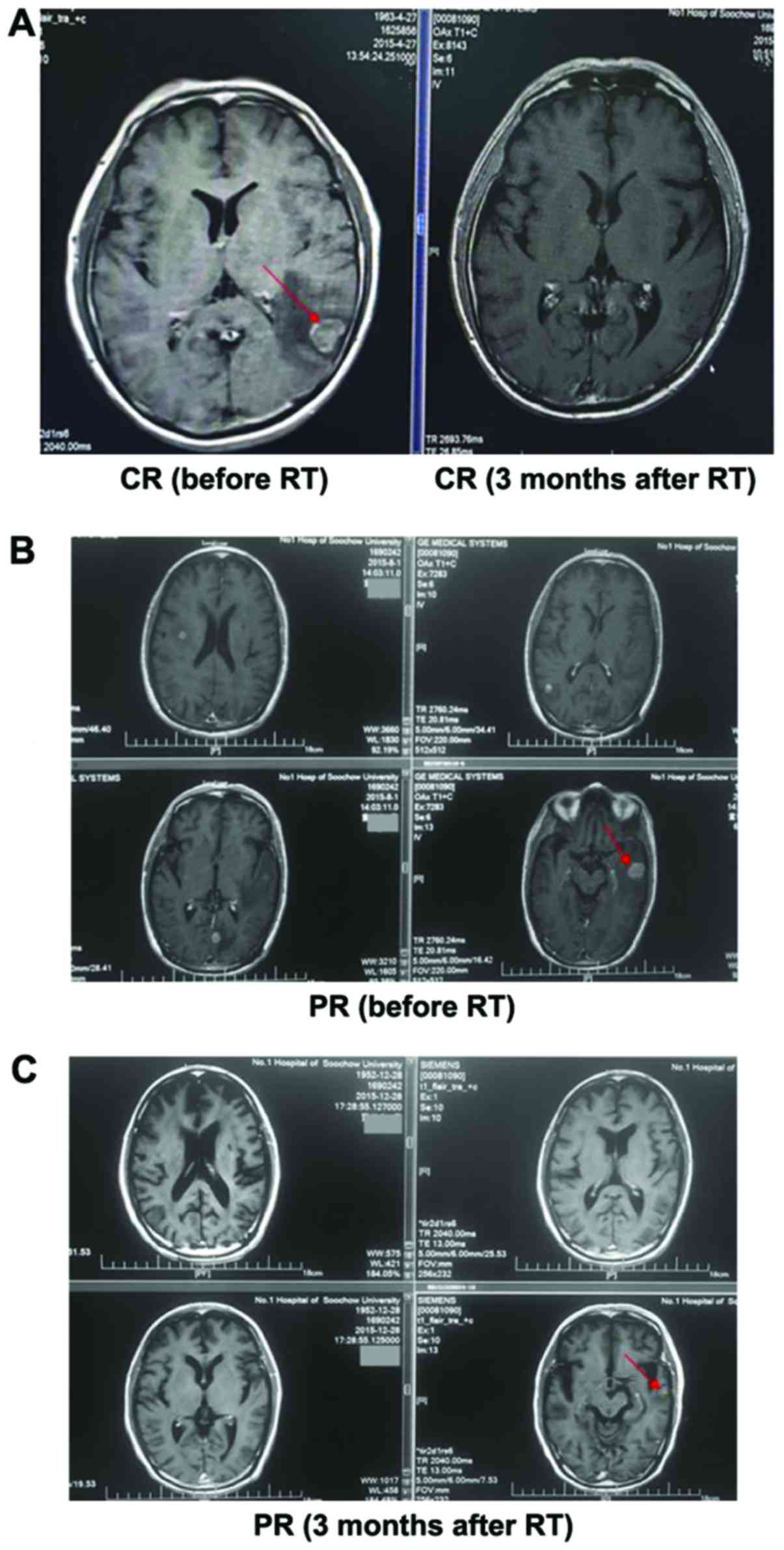
Response of BMs after therapy
Objective tumor response was evaluated after 3
months. For the combined treatment group, 1 patient achieved CR
(11.1%), 6 patients obtained PR (66.7%), and there were no patients
in progression. Therefore, as shown in Table I, OR reached 77.8%. While the disease
local control (CR+PR+SD) rate obtained 100%. However, the other 9
patients who received intensity-modulated radiation only had much
worse results. The CR was 0, PR rate was 44.4%, OR rate was 44.4%
(Table I). The MRI of a patient is
shown in Fig. 1.
Safety and tolerability
Acute adverse effects are shown in Table II. Compared to control group, adding
TMZ to IMRT was generally well tolerated. The most common
side-effect was anemia. The most frequent adverse events include
neutropenia, anemia, nausea, fatigue, vomiting, anorexia and
dizziness. Most side-effects can be alleviated and controlled by
supporting therapy.
 | Table II.Adverse events during treatment. |
Table II.
Adverse events during treatment.
| Toxicity | Grade 1–2 | Grade 3 | Grade ≥4 | No. of patients
(R+T) | Grade 1–2 | Grade 3 | Grade ≥4 | No. of patients
(R) |
|---|
| Neutropenia | 3 | 0 | 0 | 3 (33.3%) | 2 | 0 | 0 | 2 (22.2%) |
|
Thrombocytopenia | 2 | 0 | 0 | 2 (22.2%) | 2 | 0 | 0 | 2 (22.2%) |
| Anemia | 4 | 0 | 0 | 4 (44.4%) | 3 | 0 | 0 | 3 (33.3%) |
| Nausea | 1 | 1 | 0 | 2 (22.2%) | 1 | 1 | 0 | 2 (22.2%) |
| Vomiting | 2 | 1 | 0 | 3 (33.3%) | 2 | 0 | 0 | 2 (22.2%) |
| Fatigue | 3 | 0 | 0 | 3 (33.3%) | 2 | 0 | 0 | 2 (22.2%) |
| Anorexia | 2 | 0 | 0 | 2 (22.2%) | 2 | 0 | 0 | 2 (22.2%) |
| Dizziness | 2 | 0 | 0 | 2 (22.2%) | 1 | 0 | 0 | 1 (11.1%) |
Impact on QoL
Eighteen patients completed the assessment of QoL
questionnaire. The mean QoL-score after 3 months was significantly
improved, and there were significant differences (p≤0.05). The
change of the mean QoL-score is shown before treatment, during
treatment, after treatment in Fig.
2.
Survival analysis
Compared with the control group, the combined
treatment group had better OS rate and no progression-free survival
time, however, the difference was not statistically significant due
to the small number of cases (p=0.390, 0.281) (Fig. 3).
Discussion
BMs are a major cause of mortality in patients with
NSCLC. In general, the results of a previous analysis had confirmed
the advantages of IMRT. On the one hand, IMRT boosted greater
biological anticancer effectiveness, due to the accelerated
treatment at GTV. On the other hand, it also improved sparing of
healthy brain tissue, due to the integration of the boost within
the WBRT treatment. A case of a patient with 8 BMs was published
(14). By IMRT and IMRT-simultaneous
integrated boost (SIB), maximum doses were <105% of prescribed
dose, 95% prescribed dose contained the volume of for all BMs and
dose homogeneity is within 3%. Moreover, maximum doses to eyes,
lens, optic nerves and optic chiasma were limited. It contributed
to the protection of normal organs. From a clinical point of view,
subsequent MRI brain controls showed a complete clinical response.
After 40 months of treatment, the patients had no PD and showed no
late brain toxicity. A decision was reached that the use of
concomitant boost treatment by IMRT during routine WBRT improved
overall local control and had no adverse effects without resort to
Gamma Knife SRS (15). Some studies
(16,17) thought that IMRT could conformally
avoid the hippocampus, which is an important part of the brain
memory function. In the study we report 18 patients with a limited
number (≤4) of BMs in whom an IMRT facility has been used to
sequentially boost the GTV of bulky brain metastatic disease to (39
Gy/13 F/3 W) after a (30 Gy/10 F/2 W). The volume for all lesions
receiving 95% of prescribed dose was boosted. IMRT could guarantee
a better CI and HI. Moreover, IMRT plan can significantly reduce
the irradiation dose of eyes, lens, optic, nerves, and parotid
glands, and especially the middle ear.
Chemotherapy undoubtedly remains the primary
therapeutic approach for disseminated systemic tumor, and therefore
it is likely to be a reasonable choice for BMs of NSCLC as well.
Radiotherapy combined with chemotherapy turned to primary research
tendency in recent years. Some clinical trials (18–22) failed
to explore effective drug to improve OR rate and prolong survival
for BMs of NSCLC, for example carboplatin, cisplatin, 5-FU,
topotecan, and vinorelbine. One cause involved the ability of
chemotherapeutic agents to cross the BBB, therefore limited the
delivery of chemotherapeutic agents into the brain and could not
achieve therapeutic blood concentration. However, several
chemotherapeutic agents has been developed with the ability to
cross BBB, but only a few reached the clinical phase of
development. TMZ was the best used and studied in clinic, because
TMZ easily penetrates BBB, and oral, secure, and low toxic profile.
TMZ is an alkylating agent prodrug, delivering a methyl group to
purine bases of DNA (O6-guanine; N7-guanine and N3-adenine). During
DNA replication, the methylated guanines or adenines would mispair
with thymine or cytosine, which would induce DNA damage and toxic
lesion, then cell apoptosis (23). It
was reported that (24) 32 cases of
NSCLC with BM were treated with WBRT followed by IMBRT with
concomitant TMZ. The results showed that the OR rate was 37.5%, the
median survival time was 8 months, and the median PFS was 5.5
months. Toxic reaction was well tolerated.
In 2007, study showed that (11) 59 patients were enrolled and received
30 Gy WBRT with concomitant TMZ (75 mg/m2/day) for 10
days, and subsequently continued to take TMZ (150
mg/m2/day) for six cycles. Five patients achieved a CR,
21 patients obtained a PR, while 18 patients had SD. The overall
response rate (45%) exceeded the target activity per study design.
The median OS was 13 months, and the median time to progression was
9 months. The treatment is well tolerated, with an remarkable OR
rate, and a significant improvement in QoL (p<0.0001). A
single-institution phase II clinical trial was reported (25). Twenty-seven cases were treated with
WBRT (30 Gy/10 F/2 W) with concomitant TMZ (75
mg/m2/day) for 10 days, and then continued to take low
dose TMZ (75 mg/m2/day) for 21 days/month, for up to 12
cycles. Two patients obtained CR (7.4%), and 11 patients had a PR
(40.7%). The overall median survival time and median
progression-free time, respectively, were 8.8 and 6 months, and the
treatment was well tolerated. It could be concluded that WBRT
combined with long-term low dose TMZ appeared to be an effective,
well-tolerated regimen.
A randomised phase II study evaluated the use of TMZ
concomitant with WBRT (30 Gy/10 F/2 W) and WBRT alone in patients
with BMs (26). The OR of the TMZ
plus WBRT was 78.6%, and only 48.1% for WBRT alone. The median PFS
in combination group and WBRT alone was 11.8 and 5.6 months,
respectively. However, there was no significant difference in OS
between the two groups.
Moreover, two randomized phase II studies compared
TMZ given concurrently with WBRT and WBRT alone in patients with
BMs. Both studies showed an improved overall response rate in the
combination arm, resulting in a benefit in progression-free
survival in only one study (27,28).
However, some reports (29,30) also indicate that TMZ and concurrent
treatment with radiation did not produce better outcomes, including
response rates and longer-term tumor control. Besides, from the
very recent study results, memantine, MAP-kinase inhibitors and Rac
inhibitors also showed very positive data in the trials, which
remind us to replace TMZ with these drugs for further combination
treatment study (16,31,32). Due
to the difference in the time and position during examination, it
is unlikely to provide equivalent slice levels to allow comparison
of pre- and post-therapy response, we will try to make improvement
in the following study.
Overall evidence and future directions: the reported
trial demonstrated that concomitant treatment with IMRT and TMZ (at
a dose of 75 mg/m2/day for 14 days) was well tolerated
and active, achieving a CR rate of 29% and an objective rate of
100%. We demonstrated a positive impact of this therapy on the QoL
of patients with BMs after 3 months from the initiation of
treatment with TMZ (p<0.05). However, this study has limitations
because of the sample size, and further case study will be
needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, JW and YW conceived and designed the study. JL,
XC, YC, XH and HZ were responsible for the collection and analysis
of the patient data. JL, XC, JW and YW interpreted the data and
drafted the manuscript. YW revised the manuscript critically for
important intellectual content. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Affiliated Hospital of Soochow University (Suzhou, China).
Signed informed consents were obtained from the patients or the
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Carbone DP, Ding K, Roder H, Grigorieva J,
Roder J, Tsao MS, Seymour L and Shepherd FA: Prognostic and
predictive role of the VeriStrat plasma test in patients with
advanced non-small-cell lung cancer treated with erlotinib or
placebo in the NCIC Clinical Trials Group BR.21 trial. J Thorac
Oncol. 7:1653–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gaspar LE, Mehta MP, Patchell RA, Burri
SH, Robinson PD, Morris RE, Ammirati M, Andrews DW, Asher AL, Cobbs
CS, et al: The role of whole brain radiation therapy in the
management of newly diagnosed brain metastases: A systematic review
and evidence-based clinical practice guideline. J Neurooncol.
96:17–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhatnagar AK, Flickinger JC, Kondziolka D
and Lunsford LD: Stereotactic radiosurgery for four or more
intracranial metastases. Int J Radiat Oncol Biol Phys. 64:898–903.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kirby N, Chuang C, Pouliot J, Hwang A and
Barani IJ: Physics strategies for sparing neural stem cells during
whole-brain radiation treatments. Med Phys. 38:5338–5344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tarnawski R, Michalecki L, Blamek S,
Hawrylewicz L, Piotrowski T, Slosarek K, Kulik R and
Bobek-Billewicz B: Feasibility of reducing the irradiation dose in
regions of active neurogenesis for prophylactic cranial irradiation
in patients with small-cell lung cancer. Neoplasma. 58:507–515.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gondi V, Tolakanahalli R, Mehta MP,
Tewatia D, Rowley H, Kuo JS, Khuntia D and Tomé WA:
Hippocampal-sparing whole-brain radiotherapy: A ‘how-to’ technique
using helical tomotherapy and linear accelerator-based
intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys.
78:1244–1252. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolff D, Abo-Madyan Y, Dobler B, Lohr F,
Mai S, Polednik M and Wenz F: Serial tomotherapy vs. MLC-IMRT
(multileaf collimator intensity modulated radiotherapy) for
simultaneous boost treatment large intracerebral lesions. Z Med
Phys. 19:58–66. 2009.(In German).
|
|
8
|
Langer CJ and Mehta MP: Current management
of brain metastases, with a focus on systemic options. J Clin
Oncol. 23:6207–6219. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mehta MP, Paleologos NA, Mikkelsen T,
Robinson PD, Ammirati M, Andrews DW, Asher AL, Burri SH, Cobbs CS,
Gaspar LE, et al: The role of chemotherapy in the management of
newly diagnosed brain metastases: A systematic review and
evidence-based clinical practice guideline. J Neurooncol. 96:71–83.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tatar Z, Thivat E, Planchat E, Gimbergues
P, Gadea E, Abrial C and Durando X: Temozolomide and unusual
indications: Review of literature. Cancer Treat Rev. 39:125–135.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Addeo R, Caraglia M, Faiola V, Capasso E,
Vincenzi B, Montella L, Guarrasi R, Caserta L and Del Prete S:
Concomitant treatment of brain metastasis with whole brain
radiotherapy [WBRT] and temozolomide [TMZ] is active and improves
quality of life. BMC Cancer. 7:182007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Wang X, Ma B, Yang K, Zhang Q and
Tian J: Concomitant or adjuvant temozolomide with whole-brain
irradiation for brain metastases: A meta-analysis. Anticancer
Drugs. 21:120–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
EuroQol Group, : EuroQol - a new facility
for the measurement of health-related quality of life. Health
Policy. 16:199–208. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferro M, Cilla S, Macchia G, Deodato F,
Pierro A, Digesu C, Ferrandina G, Ciuffreda M, Sallustio G and
Morganti AG: On the cutting edge of intensity modulated
radiotherapy and simultaneous integrated boost (IMRT-SIB): The case
of a patient with 8 brain metastases. Rep Pract Oncol Radiother.
20:316–319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edwards AA, Keggin E and Plowman PN: The
developing role for intensity-modulated radiation therapy (IMRT) in
the non-surgical treatment of brain metastases. Br J Radiol.
83:133–136. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Slade AN and Stanic S: The impact of RTOG
0614 and RTOG 0933 trials in routine clinical practice: The US
Survey of Utilization of Memantine and IMRT planning for
hippocampus sparing in patients receiving whole brain radiotherapy
for brain metastases. Contemp Clin Trials. 47:74–77. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gondi V, Pugh SL, Tome WA, Caine C, Corn
B, Kanner A, Rowley H, Kundapur V, DeNittis A, Greenspoon JN, et
al: Preservation of memory with conformal avoidance of the
hippocampal neural stem-cell compartment during whole-brain
radiotherapy for brain metastases (RTOG 0933): A phase II
multi-institutional trial. J Clin Oncol. 32:3810–3816. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guerrieri M, Wong K, Ryan G, Millward M,
Quong G and Ball DL: A randomised phase III study of palliative
radiation with concomitant carboplatin for brain metastases from
non-small cell carcinoma of the lung. Lung Cancer. 46:107–111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robinet G, Thomas P, Breton JL, Léna H,
Gouva S, Dabouis G, Bennouna J, Souquet PJ, Balmes P, Thiberville
L, et al: Results of a phase III study of early versus delayed
whole brain radiotherapy with concurrent cisplatin and vinorelbine
combination in inoperable brain metastasis of non-small-cell lung
cancer: Groupe Français de Pneumo-Cancérologie (GFPC) Protocol
95-1. Ann Oncol. 12:59–67. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee DH, Han JY, Kim HT, Yoon SJ, Pyo HR,
Cho KH, Shin SH, Yoo H, Lee SH and Lee JS: Primary chemotherapy for
newly diagnosed nonsmall cell lung cancer patients with synchronous
brain metastases compared with whole-brain radiotherapy
administered first: Result of a randomized pilot study. Cancer.
113:143–149. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim DY, Lee KW, Yun T, Kim DW, Kim TY, Heo
DS, Bang YJ and Kim NK: Efficacy of platinum-based chemotherapy
after cranial radiation in patients with brain metastasis from
non-small cell lung cancer. Oncol Rep. 14:207–211. 2005.PubMed/NCBI
|
|
22
|
Neuhaus T, Ko Y, Muller RP, Grabenbauer
GG, Hedde JP, Schueller H, Kocher M, Stier S and Fietkau R: A phase
III trial of topotecan and whole brain radiation therapy for
patients with CNS-metastases due to lung cancer. Br J Cancer.
100:291–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Stevens MF and Bradshaw TD:
Temozolomide: Mechanisms of action, repair and resistance. Curr Mol
Pharmacol. 5:102–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Q, Jiang Z, Qi X, Lu S, Wang S, Leng
C, Lu F, Liu H, Liang S and Shi J: Whole brain radiation therapy
followed by intensity-modulated boosting treatment combined with
concomitant temozolomide for brain metastases from non-small-cell
lung cancer. Clin Transl Oncol. 16:1000–1005. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Addeo R, De Rosa C, Faiola V, Leo L,
Cennamo G, Montella L, Guarrasi R, Vincenzi B, Caraglia M and Del
Prete S: Phase 2 trial of temozolomide using protracted low-dose
and whole-brain radiotherapy for nonsmall cell lung cancer and
breast cancer patients with brain metastases. Cancer.
113:2524–2531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gamboa-Vignolle C, Ferrari-Carballo T,
Arrieta Ó and Mohar A: Whole-brain irradiation with concomitant
daily fixed-dose temozolomide for brain metastases treatment: A
randomised phase II trial. Radiother Oncol. 102:187–191. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Verger E, Gil M, Yaya R, Viñolas N, Villà
S, Pujol T, Quintó L and Graus F: Temozolomide and concomitant
whole brain radiotherapy in patients with brain metastases: A phase
II randomized trial. Int J Radiat Oncol Biol Phys. 61:185–191.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Antonadou D, Paraskevaidis M, Sarris G,
Coliarakis N, Economou I, Karageorgis P and Throuvalas N: Phase II
randomized trial of temozolomide and concurrent radiotherapy in
patients with brain metastases. J Clin Oncol. 20:3644–3650. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hassler MR, Pfeifer W, Knocke-Abulesz TH,
Geissler K, Altorjai G, Dieckmann K and Marosi C: Temozolomide
added to whole brain radiotherapy in patients with multiple brain
metastases of non-small-cell lung cancer: A multicentric Austrian
phase II study. Wien Klin Wochenschr. 125:481–486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chua D, Krzakowski M, Chouaid C, Pallotta
MG, Martinez JI, Gottfried M, Curran W and Throuvalas N:
Whole-brain radiation therapy plus concomitant temozolomide for the
treatment of brain metastases from non-small-cell lung cancer: A
randomized, open-label phase II study. Clin Lung Cancer.
11:176–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hamilton E and Infante JR: Targeting
CDK4/6 in patients with cancer. Cancer Treat Rev. 45:129–138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cardama GA, Gonzalez N, Maggio J, Menna PL
and Gomez DE: Rho GTPases as therapeutic targets in cancer
(Review). Int J Oncol. 51:1025–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|