Introduction
Epstein-Barr virus (EBV) is widespread among humans,
with >90% of adults latently infected with EBV throughout their
lifetime (1). EBV infection may cause
mononucleosis and several malignancies including nasopharyngeal
carcinoma (NPC), Hodgkin's lymphoma and Burkitt's lymphoma
(2–4).
NPC is one of the most common malignant types of cancer in people
living in the southern region of China. EBV DNA, RNA and proteins
are expressed in NPC tumor cells (5,6). The
presence of EBV in all NPC tumor cells provides an opportunity for
the development of novel diagnostic and therapeutic approaches to
this type of cancer.
EBV establishes a latent infection (transformation)
or undergoes lytic replication by infecting epithelial cells or B
lymphocytes. Accumulating evidence suggests that carcinogenesis is
principally associated with latent EBV infection (5,6). During
latency, all 11 late viral genes express their protein products,
including 6 Epstein-Barr nuclear antigens (EBNAs 1, 2, 3A, 3B and
3C, and EBNA-LP), 2 latent membrane proteins (LMP1 and LMP2) and 2
small nuclear RNAs (EBERs) (6). Among
these proteins, LMP1 is considered the primary viral oncoprotein,
responsible for the malignant phenotype in NPC, whereas LMP2
maintains EBV latency (7). LMP2
localizes to cell membranes and perinuclear regions in transiently
transfected cells (8). Expression of
LMP2 in malignant cells and B cells suggests that LMP2 may also
serve a role in cell transformation (9). Thus, LMP2 has been selected as a
possible diagnostic target (10).
A number of studies have demonstrated increased
levels of serum antibodies, particularly immunoglobulin (Ig)A
antibody, against lytic and latent proteins in patients with NPC
(11–13). At the time of writing, viral capsid
antigen-IgA (VCA-IgA) and early antibody-IgA (EA-IgA), which are
associated with NPC, are used as markers for serological diagnosis;
however, neither marker is particularly specific (14). Accordingly, the aim of the present
study was to develop a novel ELISA for serological diagnosis of NPC
using multiple B cell epitopes of EBV LMP2.
Materials and methods
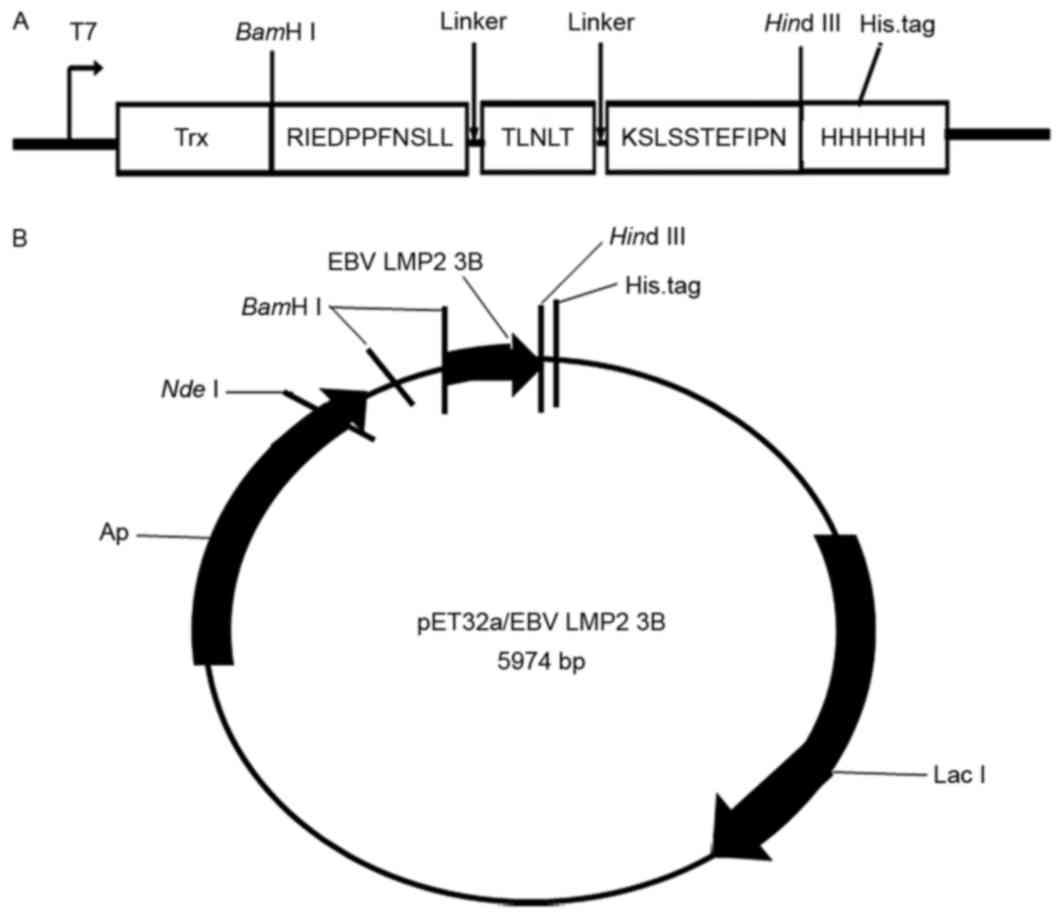
Preparation of the EBV-LMP2-3B
antigen
EBV-LMP2-3B comprises three B cell linear epitopes
[amino acids 199–209 (RIEDPPFNSLL), 318–322 (TLNLT) and 381–391
(KSLSSTEFIPN)], which are located in the extracellular region of
the LMP2 protein, with each epitope connected by a flexible peptide
linker (GS), to construct a novel multi-epitope peptide,
EBV-LMP2-3B. The corresponding DNA sequence was modified based on
prokaryotic codon usage by Java Codon Adaptation Tool (http://www.jcat.de/), synthesized (by Shanghai Sangon
Pharmaceutical Co., Ltd., Shanghai, China), and cloned into the
pET32a(+) vector using BamHI and HindIII to generate
pET32a(+)/EBV-LMP2-3B, which expressed a thioredoxin (Trx) fusion
EBV-LMP2-3B protein with three tags (Trx, His and S) in
Escherichia coli BL21(DE3) induced by 1 mM isopropyl
β-D-1-thiogalactopyranoside (Merck KGaA, Darmstadt, Germany). The
successful creation of the fusion protein was verified using 12%
Tricine SDS-PAGE and western blotting with horseradish peroxidase
(HRP)-conjugated-anti-His monoclonal antibody (cat. no. A00174;
1:5,000; KPL, Inc., Gaithersburg, MD, USA). Following confirmation,
the EBV-LMP2-3B proteins were purified using an
Ni2+-nitrilotriacetate-Sepharose column (Qiagen, Inc.,
Valencia, CA, USA). On the basis of previous methods (15), the native LMP2 was prepared from EBV
B95-8 cells (American Type Culture Collection, Manassas, VA, USA)
using a membrane protein extraction kit (BestBio Biotechnology Co.,
Shanghai, China).
Preparation of mice immune sera
Female BALB/c mice (n=27; mean weight, 16.12±0.25 g)
between 6 and 8 weeks of age (Shanghai Laboratory Animal Co., Ltd.,
Shanghai, China) were used for experiments according to approved
protocols and in accordance with recommendations for the proper use
and care of laboratory animals. Mice were maintained at a constant
temperature (22±2°C) with humidity between 40 and 70%. A 12 h
light/dark cycle was maintained and mice had free access to food
and water. The mice were randomly divided into three equal groups
(9 mice per group) and immunized with purified EBV-LMP2-3B fusion
protein (50 µg/100 µl), Trx-His-tag [pET32a(+) basal plasmid
protein] (50 µg/100 µl) or PBS (100 µl) as a negative control. This
was repeated three times at 2-week intervals. Blood was collected
at weeks 0, 2, 4 and 8, and sera were removed and stored at
−80°C.
ELISA detection
Purified EBV-LMP2-3B, native EBV-LMP2, Trx-His-tag,
or synthetic peptides RIEDPPFNSLL, TLNLT and KSLSSTEFIPN (1 µg)
were dissolved in 100 µl PBS and used to coat each well of a plate
(96-well plates; Corning Incorporated, Corning, NY, USA). Plates
were incubated at 4°C overnight. The coated plates were blocked for
1 h at 37°C with blocking buffer [5% non-fat dry milk and 0.05%
Tween-20 in PBS (PBS-T)] and incubated with serum samples (diluted
1:100) for 1 h at 37°C. Following washing with PBS-T, bound
antibodies were detected following incubation for 1 h at 37°C with
HRP-conjugated anti-mouse IgG (1:2,000; cat. no. A0216; Unitech
Co., Ltd., Chiba, Japan) in blocking buffer, followed by washing
with PBS-T, 3,3′,5,5′-tetramethylbenzidine and
H2O2 for 10 min at 37°C. Color development
was determined at 490 nm using an ELISA plate reader (ELx800;
Bio-Tek Instruments, Inc., Winooski, VT, USA). All samples were run
in triplicate.
Western blot analysis
The purified EBV-LMP2-3B, Trx-His-tag and native
EBV-LMP membrane protein samples were analyzed using SDS-PAGE (12%
gel) and western blotting. Rabbit serum against EBV membrane
protein (1:5,000) (16), mouse immune
sera against EBV-LMP2-3B fusion proteins (1:5,000) and sera from
patients with NPC (1:5,000) were used as the primary antibodies.
HRP-conjugated goat anti-rabbit IgG [heavy and light (H+L)] (cat.
no. GAR007; ABR Inc., Fairbanks, AK, USA), HRP-conjugated goat
anti-mouse IgG (H+L) (cat. no. 00001-2; ABR Inc.) or HRP-conjugated
goat anti-human IgG (H+L) (cat. no. ICT-6291; 1:10,000; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) were used as the
corresponding secondary antibodies. Antibodies were diluted in TBS
with Tween-20 containing 5% nonfat milk (w/v). The protein bands
were visualized using 0.005% 4-chloro-1-naphthol and a 0.015%
hydrogen peroxidase color development substrate (cat. no. 2012911;
Branch of the Biological Technology Co., Ltd., Hangzhou China).
Indirect immunofluorescence assay
B95-8 cells, collected and fixed with 4%
paraformaldehyde (PFA) on glass slides, were used as an antigen
substrate. The slides were blocked with blocking buffer [PBS
containing 5% fetal bovine serum (FBS)] at 4°C overnight. The cells
were incubated with either immune sera against anti-EBV-LMP2-3B (50
µg/ml; cat. no. N29018; BestBio Biotechnology Co., Shanghai, China)
or control sera (cat. no. N63728; BestBio Biotechnology Co.)
diluted in PBS with 5% FBS at a 1:100 dilution for 2 h at room
temperature, followed by incubation with goat anti-mouse IgG
fluorescein isothiocyanate (FITC)-conjugated (cat. no. D111035;
Invitrogen; Thermo Fisher Scientific, Inc.) secondary antibody
diluted 1:100 with PBS containing 5% FBS for 1 h at room
temperature. Cells were observed under a fluorescence microscope
(magnification, ×400).
Detection of specific IgG antibodies
in sera from patients with NPC
Blood samples were obtained from 198 patients with
NPC and 102 healthy adults at The First Affiliated Hospital of
Wenzhou Medical University (Zhejiang Province, China). Sera were
separated and stored at −80°C until they were retrieved for further
analysis (15). All the NPC cases
were confirmed by clinical and histopathological diagnoses. The
present study was approved by the Human Research Ethics Committee
of The First Affiliated Hospital of Wenzhou Medical University, and
written informed consent was obtained from each patient. Using
EBV-LMP2-3B fusion proteins, native EBV-LMP2 protein or Trx-His-tag
as coated antigens, specific antibodies in the sera of patients
with NPC and healthy individuals were detected by indirect ELISA
performed as aforementioned. HRP-conjugated goat anti-human IgG
(H+L) diluted at 1:5,000 was used as the secondary antibody. The
serum antibody titers of EBV VCA/IgA were also detected using a
commercial ELISA kit (Zeus Scientific, Inc., Branchburg, NJ,
USA).
Statistical analysis
One-way analysis of variance was used to evaluate
the differences in the antibody levels between different groups.
Multiple comparisons between the groups was performed using the
Student-Neumann-Keuls method. P<0.05 was considered to indicate
a statistically significant different. All calculations were
performed with the SPSS software (version 16.0; SPSS, Inc.,
Chicago, IL, USA).
Results
Expression and identification of
EBV-LMP2-3B
The EBV-LMP2-3B fusion protein was expressed in
E. coli BL21(DE3) of EBV-LMP2-3B and three tags, including
Trx, His and S (Fig. 1), with an
approximate molecular mass of 22 kDa, which was greater than that
of the Trx-His-tag protein (21 kDa) produced from the empty
pET32a(+) vector. EBV-LMP2-3B fusion protein may be specifically
probed with 6× his-tag monoclonal antibody in sera from either
Trx-His-tag- or EBV-LMP2-3B-immunized mice or mixed sera from 5
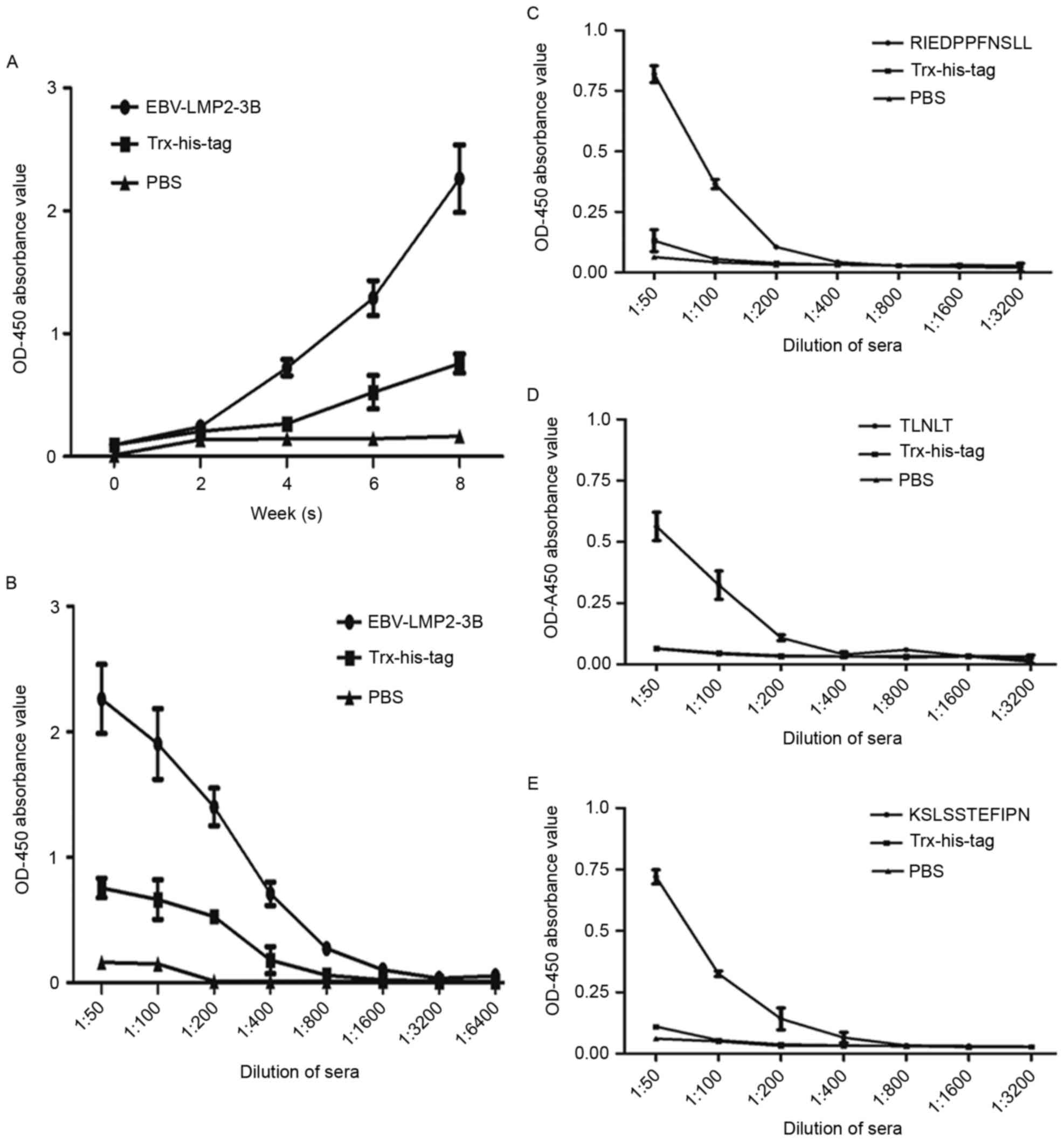
patients with NPC (Fig. 2A-D).
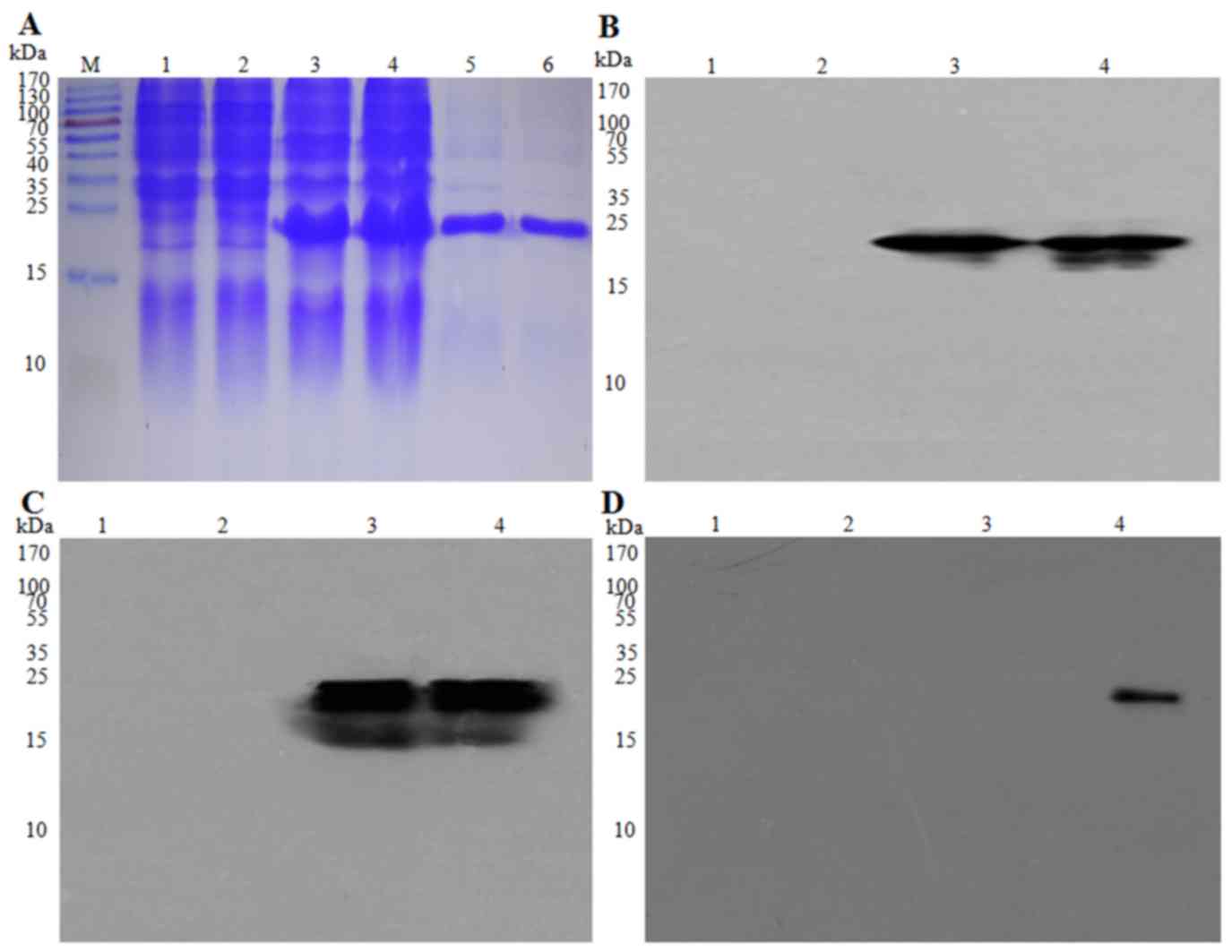 | Figure 2.Expression and identification of the
EBV-LMP2-3B fusion protein. EBV-LMP2-3B and Trx-His-tag protein,
expressed in Escherichia coli BL21(DE3) and purified, were
detected using (A) SDS-PAGE analysis and (B) western blot assay
using anti-His-tag monoclonal antibody (1:5,000 dilution), (C)
mouse immune serum against EBV-LMP2-3B (1:5,000 dilution), and (D)
serum from patients with NPC (1:10,000 dilution). M, pre-stained
protein marker, lanes 1 and 2; E. coli BL21(DE3) lysate;
lane 3; pET32a(+)/EBV-LMP2-3B (Trx-LMP2-3B-His-tag; 22 kDa); lane
4, Trx-His-tag (21 kDa); lanes 5 and 6, purified EBV-LMP2-3B fusion
and Trx-His-tag proteins. Epstein-Barr virus; LMP, latent membrane
protein; Trx, thioredoxin. |
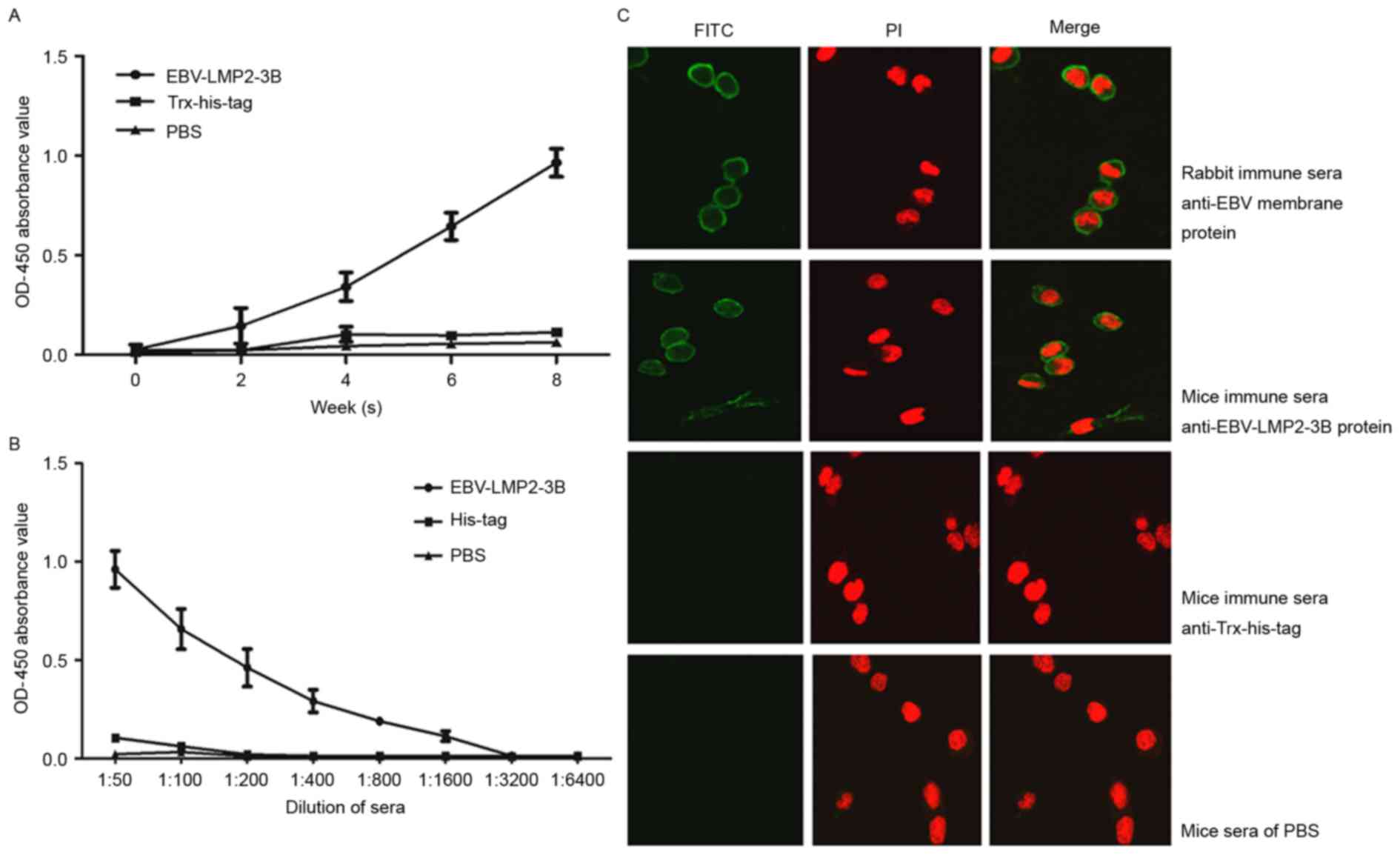
EBV-LMP2-3B-induced specific antibody
responses in mice
Results revealed that EBV-LMP2-3B-specific IgG
antibody levels were significantly increased compared with those in
sera from either the Trx-His-tag-immunized or PBS control groups
(P<0.01). The highest level of antibodies from
EBV-LMP2-3B-immunized mice was obtained at 8 weeks
post-immunization (Fig. 3A and B).
The levels of antibodies against all the synthesized peptides
(RIEDPPFNSLL, TLNLT and KSLSSTEFIPN) detected in the sera of
EBV-LMP2-3B-immunized mice (Fig.
3C-E) were significantly increased compared with those from
either Trx-His-tag-immunized mice or the PBS control group
(P<0.05).
Immune sera against EBV-LMP2-3B
recognizes native LMP2 antigen
Native LMP2 antigen was detected in sera from all
EBV-LMP2-3B-, Trx-His-tag- and PBS-immunized mice. The levels of
specific IgG antibodies in EBV-LMP2-3B-immunized mice were
significantly increased compared with those in either
Trx-His-tag-immunized mice or the PBS control group at 6 and 8
weeks post-immunization (P<0.05; Fig.
4A). Antibody titers were significantly increased in
EBV-LMP2-3B-immunized mice compared with the control mice at week 8
(Fig. 4B). Immunofluorescence
labeling confirmed that three polytopes of LMP, predominantly
located on the surface of EBV B95-8 cells, were recognized by
specific sera from the EBV-LMP2-3B group, as well as by the sera of
B95-8 cell lysate-immunized rabbits (Fig.
4C).
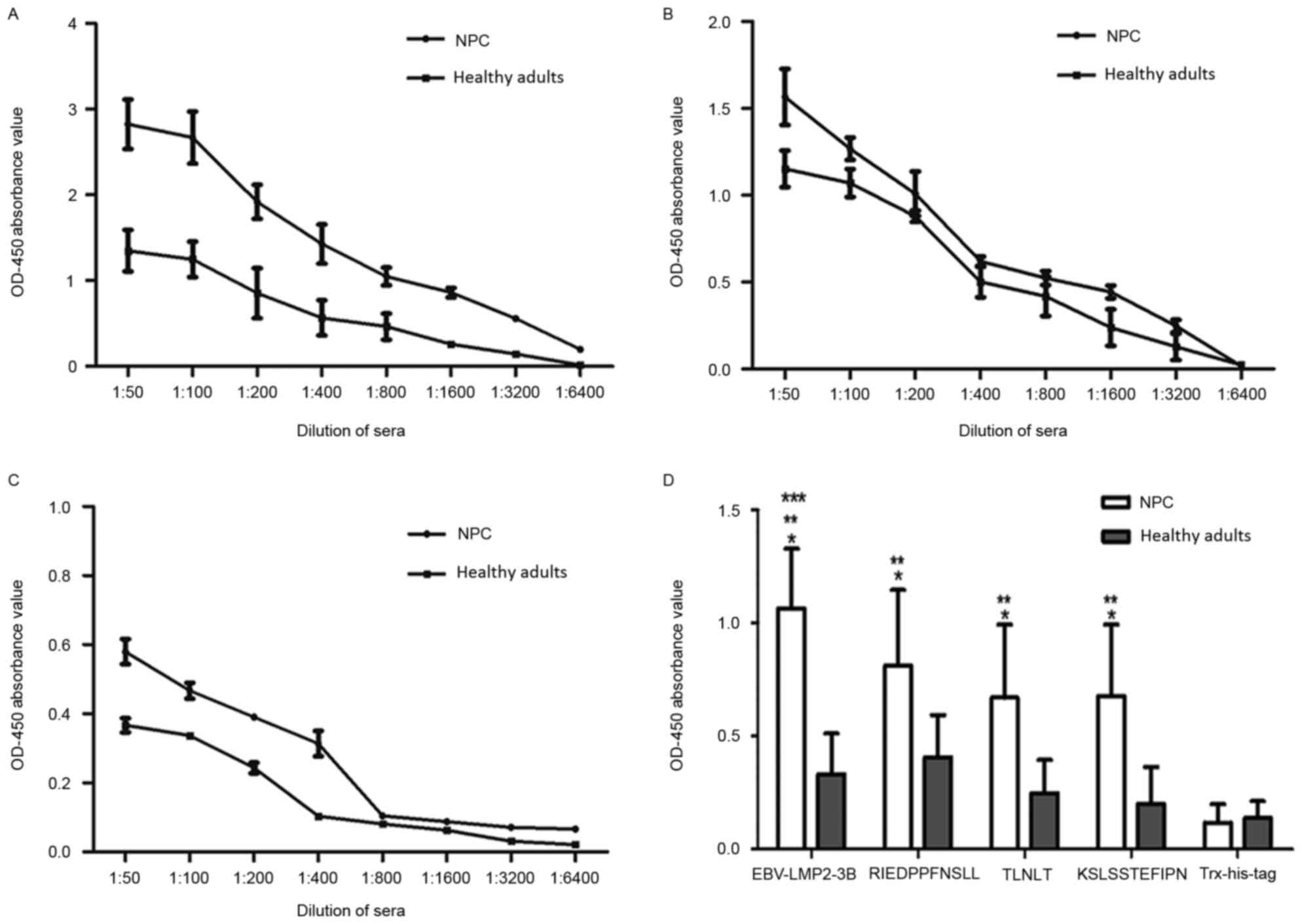
Sensitivity and specificity of the
EBV-LMP2-3B-based ELISA
The reactivity of sera from patients with NPC and
healthy individuals was analyzed using the EBV-LMP2-3B-based ELISA
assay (Fig. 5). Compared with titers
in healthy individuals, the titers of EBV-LMP2-3B-specific serum
IgG antibodies detected in the NPC group were significantly
increased (P<0.05). The rates of serum antibody positivity
against EBV-LMP2-3B and synthesized peptides were further evaluated
in 198 patients with NPC and 102 healthy subjects. Samples with
optical density at 450 nm (OD450) values above the
threshold (i.e. the mean ± 2 standard deviations of the
OD450 value of sera from healthy individuals) were
determined to be antigen-positive. The results revealed that
antibody-positivity rates against EBV-LMP2-3B and the three
synthesized peptides (RIEDPPFNSLL, TLNLT and KSLSSTEFIPN) in the
NPC group were 91.91 (182/198), 63.63 (126/198), 57.57 (114/198)
and 61.11% (121/198), respectively. The antibody-positivity rate
against EBV-LMP2-3B was significantly increased compared with those
against the three synthesized peptides (P<0.05). When the same
patients' sera were tested with an EBV VCA-IgA ELISA kit (the
standard assay used for diagnosing EBV infection), the
antibody-positivity rates in the NPC and healthy control groups
were 59.59 (118/198) and 24.50% (25/102), respectively. This result
indicates that the EBV-LMP2-3B-based ELISA was sensitive and
specific for the detection of NPC (Table
I).
 | Table I.Sensitivity and specificity of the
EBV-LMP2-based ELISA for NPC. |
Table I.
Sensitivity and specificity of the
EBV-LMP2-based ELISA for NPC.
|
| NPC | Healthy adults |
|
|
|---|
|
|
|
|
|
|
|---|
| Immunization
group | Mean ± SD | Ratio, % | Mean ± SD | Ratio, % | Sensitivity, % | Specificity, % |
|---|
| VCA-IgA | 0.325±0.304 | 59.59 (118/198) | 0.033±0.024 | 24.50 (25/102) | 59.59 | 75.49 |
| EBV-LMP2-3B-IgG | 1.064±0.264 | 91.91 (182/198) | 0.332±0.180 | 8.82 (9/102) | 91.91 | 93.14 |
| Synthesized peptide
(RIEDPPFNSLL)-IgG | 0.811±0.335 | 63.63 (126/198) | 0.407±0.187 | 6.86 (7/102) | 63.63 | 93.13 |
| Synthesized peptide
(TLNLT)-IgG | 0.670±0.323 | 57.57 (114/198) | 0.249±0.146 | 5.88 (6/102) | 57.57 | 94.11 |
| Synthesized peptide
(KSLSSTEFIPN)-IgG | 0.677±0.316 | 61.11 (121/198) | 0.201±0.164 | 3.92 (4/102) | 61.11 | 96.08 |
Discussion
Serological detection of EBV has been commonly used
to diagnose suspected cases of NPC and has been proposed for
large-scale screenings and epidemiological surveys, as well as to
monitor the recurrence and progression of EBV-associated tumors
(17–19). Traditionally, serum antibody detection
has been performed by either immunofluorescence labeling or
immunoenzymatic assay using EBV-infected lymphocytes as the binding
substrate (20). Traditional
serological studies are difficult to standardize since they are
semi-quantitative (12,21) and are not suitable for large-scale
testing or automated handling (22).
At present, ELISAs are used to detect EBV-specific serum antibodies
in the majority of laboratories; however, this method may overcome
the shortcomings of the traditional method, and the data generated
are more accurate and objective than those of earlier assays.
Although VCA-IgA and EA-IgA have been widely used as serological
markers of NPC in clinical screening, early diagnosis and
determining the prognosis of NPC (18,23,24), the
VCA-IgA ELISA method has a high false positive rate (8).
A variety of EBV antigens, including those from
native or recombinant EBV proteins VCA, EA, BamHI Z EBV
replication activator, EBNA1 and thymidine kinase or synthetic
peptides have been used in ELISAs to screen for and diagnose NPC
(25–28). We previously focused on a recombinant
protein with the B cell linear epitopes of LMP2 to detect the
specific antibodies in sera from patients with NPC to develop
ELISAs with higher sensitivity (15),
and B cell epitopes of LMP2 are optimal for use as a serological
marker to diagnose NPC. In the present study, EBV-LMP2-3B was
designed using three B cell linear epitopes (RIEDPPFNSLL, TLNLT and
KSLSSTEFIPN). This EBV-LMP2-3B fusion protein expressed in a
prokaryotic expression system is easily prepared and purified and
demonstrates high immunogenicity. The EBV-LMP2-3B-specific
antibodies in the sera of immunized mice recognize not only the
native LMP2, but also the three synthesized peptides. Furthermore,
immunofluorescence labeling confirmed that the antigen recognition
site was located in the membrane region of B95-8 cells. The
EBV-LMP2-3B protein was also recognized by the sera of patients
with NPC. Taken together, these results indicate that the
EBV-LMP2-3B fusion protein is highly immunogenic and that sera from
immunized mice may specifically recognize and bind to native LMP2
protein. The results suggest that the EBV-LMP2-3B fusion protein
may be useful as a target antigen in ELISAs to screen for
antibodies against EBV, particularly in patients with NPC.
EBV-LMP2-3B-specific serum antibodies may be
detected in patients with NPC, and the antibody level and
positivity rate in the NPC group were significantly increased
compared with those in the healthy group (P<0.05). Although
serum antibodies specific for the synthesized peptides were also
detected in patients with NPC, the antibody levels and positivity
rates against EBV-LMP2-3B protein were significantly increased
compared with those against each of the three synthetic peptides.
Furthermore, IgG antibodies specific for EBV-LMP2-3B protein in
serum specimens from 198 patients with NPC were effectively
detected, representing an improvement over the traditional
VCA-IgA-based assay. In addition, the antibody level and positive
rate were increased compared with those against VCA-IgA. These data
provide evidence that EBV-LMP2-3B is immunodominant and may be used
as a target antigen in ELISAs to diagnose NPC serologically.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372447).
Competing interests
All authors declare that there are no conflicts of
interest.
References
|
1
|
Niedobitek G, Meru N and Delecluse HJ:
Epstein-Barr virus infection and human malignancies. Int J Exp
Path. 82:149–170. 2001. View Article : Google Scholar
|
|
2
|
Hutajulu SH, Kurnianda J, Tan IB and
Middeldorp JM: Therapeutic implications of Epstein-Barr virus
infection for the treatment of nasopharyngeal carcinoma. Ther Clin
Risk Manag. 10:721–736. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niedobitek G: Epstein-Barr virus infection
in the pathogenesis of nasopharyngeal carcinoma. Mol Pathol.
53:248–254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shah KM and Young LS: Epstein-Barr virus
and carcinogenesis: Beyond Burkitt's lymphoma. Clin Microbiol
Infect. 15:982–988. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sung NS, Edwards RH, Seillier-Moiseiwitsch
F, Perkins AG, Zeng Y and Raab-Traub N: Epstein-Barr virus strain
variation in nasopharyngeal carcinoma from the endemic and
non-endemic regions of China. Int J Cancer. 76:207–215. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Middeldorp JM, Brink AA, van den Brule AJ
and Meijer CJ: Pathogenic roles for Epstein-Barr virus (EBV) gene
products in EBV-associated proliferative disorders. Crit Rev Oncol
Hematol. 45:1–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dawson CW, Port RJ and Young LS: The role
of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the
pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol.
22:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cai WM, Li YW, Wu B, Liu YY, Hu YH, Gu XZ,
Liu HY and Wang GD: Serologic diagnosis of nasopharyngeal
carcinoma. A double-blind study of four EB virus antibodies with
evaluation by sequential discrimination. Int J Radiat Oncol Biol
Phys. 9:1763–1768. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pang MF, Lin KW and Peh SC: The signaling
pathways of Epstein-Barr virus-encoded latent membrane protein 2A
(LMP2A) in latency and cancer. Cell Mol Biol Lett. 14:222–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Straathof KC, Leen AM, Buza EL, Taylor G,
Huls MH, Heslop HE, Rooney CM and Bollard CM: Characterization of
latent membrane protein 2 specificity in CTL lines from patients
with EBV-positive nasopharyngeal carcinoma and lymphoma. J Immunol.
175:4137–4147. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chien YC, Chen JY, Liu MY, Yang HI, Hsu
MM, Chen CJ and Yang CS: Serologic markers of Epstein-Barr virus
infection and nasopharyngeal carcinoma in Taiwanese men. N Engl J
Med. 345:1877–1882. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ji MF, Wang DK, Yu YL, Guo YQ, Liang JS,
Cheng WM, Zong YS, Chan KH, Ng SP, Wei WI, et al: Sustained
elevation of Epstein-Barr virus antibody levels preceding clinical
onset of nasopharyngeal carcinoma. Br J Cancer. 96:623–630. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao SM, Liu Z, Jia WH, Huang QH, Liu Q,
Guo X, Huang TB, Ye W and Hong MH: Fluctuations of epstein-barr
virus serological antibodies and risk for nasopharyngeal carcinoma:
A prospective screening study with a 20-year follow-up. PLoS One.
6:e191002011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu KJ, Hsu WL, Pfeiffer RM, Chiang CJ,
Wang CP, Lou PJ, Cheng YJ, Gravitt P, Diehl SR, Goldstein AM, et
al: Prognostic utility of anti-EBV antibody testing for defining
NPC risk among individuals from high-risk NPC families. Clin Cancer
Res. 17:1906–1914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue X, Zhu S, Li W, Chen J, Ou Q, Zheng M,
Gong W and Zhang L: Identification and characterization of novel
B-cell epitopes within EBV latent membrane protein 2 (LMP2). Viral
Immunol. 24:227–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin X, Chen S, Xue X, Lu L, Zhu S, Li W,
Chen X, Zhong X, Jiang P, Sename TS, et al: Chimerically fused
antigen rich of overlapped epitopes from latent membrane protein 2
(LMP2) of Epstein-Barr virus as a potential vaccine and diagnostic
agent. Cell Mol Immunol. 13:492–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Han BL, Xu XY, Zhang CZ, Wu JJ, Han CF,
Wang H, Wang X, Wang GS, Yang SJ and Xie Y: Systematic review on
Epstein-Barr virus (EBV) DNA in diagnosis of nasopharyngeal
carcinoma in Asian populations. Asian Pac J Cancer Prev.
13:2577–2581. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Deng Y, Li X, Chen QP, Liao XC and
Qin X: Diagnostic value of Epstein-Barr virus capsid antigen-IgA in
nasopharyngeal carcinoma: A meta-analysis. Chin Med J (Engl).
123:1201–1205. 2010.PubMed/NCBI
|
|
19
|
Ng WT, Yau TK, Yung RW, Sze WM, Tsang AH,
Law AL and Lee AW: Screening for family members of patients with
nasopharyngeal carcinoma. Int J Cancer. 113:998–1001. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rea TD, Ashley RL, Russo JE and Buchwald
DS: A systematic study of Epstein-Barr virus serologic assays
following acute infection. Am J Clin Pathol. 117:156–161. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi Z, Yuxi L, Chunren L, Sanwen C, Jihneng
W, Jisong Z and Huijong Z: Application of an immunoenzymatic method
and an immunoautoradiographic method for a mass survey of
nasopharyngeal carcinoma. Intervirology. 13:162–168. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stevens SJ, Zwaan CM, Verkuijlen SA and
Middeldorp JM: Epstein-Barr virus (EBV) serology for predicting
distant metastases in a white juvenile patient with nasopharyngeal
carcinoma and no clinical response to EBV lytic induction therapy.
Head Neck. 28:1040–1045. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai YL, Zheng YM, Wang W, Wei Y, Shen XX,
Cheng JR, Wu YS, Gao JQ, Zhong WM and Li J: Combined detection of
Epstein-Barr virus antibodies for serodiagnosis of nasopharyngeal
carcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 30:2746–2748. 2010.(In
Chinese). PubMed/NCBI
|
|
24
|
Sun P, Chen C, Cheng YK, Zeng ZJ, Chen XL,
Liu LZ and Gu MF: Serologic biomarkers of Epstein-Barr virus
correlate with TNM classification according to the seventh edition
of the UICC/AJCC staging system for nasopharyngeal carcinoma. Eur
Arch Otorhinolaryngol. 271:2545–2554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Connolly Y, Littler E, Sun N, Chen X,
Huang PC, Stacey SN and Arrand JR: Antibodies to Epstein-Barr virus
thymidine kinase: A characteristic marker for the serological
detection of nasopharyngeal carcinoma. Int J Cancer. 91:692–697.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dardari R, Hinderer W, Lang D, Benider A,
El Gueddari B, Joab I, Benslimani A and Khyatti M: Antibody
responses to recombinant Epstein-Barr virus antigens in
nasopharyngeal carcinoma patients: Complementary test of ZEBRA
protein and early antigens p54 and p138. J Clin Microbiol.
39:3164–3170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Karray H, Ayadi W, Fki L, Hammami A, Daoud
J, Drira MM, Frikha M, Jlidi R and Middeldorp JM: Comparison of
three different serological techniques for primary diagnosis and
monitoring of nasopharyngeal carcinoma in two age groups from
Tunisia. J Med Virol. 75:593–602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Grunsven WM, Spaan WJ and Middeldorp
JM: Localization and diagnostic application of immunodominant
domains of the BFRF3-encoded Epstein-Barr virus capsid protein. J
Infect Dis. 170:13–19. 1994. View Article : Google Scholar : PubMed/NCBI
|