Introduction
Due to recent advancements in diagnostic techniques,
the incidence of patients with multiple primary cancer (MPC) has
increased (1). Evidence suggests that
the prevalence of MPC is between 0.73 and 17% globally from 1966 to
2015 (2–7). Different values were reported in the
literature on the prevalence of MPC, this is due to the long
evaluation period and the inclusion of autopsy series; therefore,
there were differences between the studies (2–4,7) The risk of developing MPC varies between
different cancer sites and is reported in a range from 1% (primary
liver malignancy) up to 16% (primary bladder cancer) (4). MPC reflects not only the late effects of
therapy but also the influence of shared etiologic factors (in
particular, tobacco and excessive alcohol intake), genetic
susceptibility, environmental exposures, host effects, and
combinations of factors, including gene-environment interactions
(8). Risks for selected MPC are also
modified by age at exposure and attained age (9). For simplicity, Travis et al
(10) categorized MPC into three
major groups according to the predominant etiologic factor:
Treatment-related, syndromic, and those due to shared etiologic
influences; however, they underscored the nonexclusivity of these
groups.
Human papilloma viruses 16 and 18 have been
implicated in the development of synchronous cancer of the anal
canal and cervix (SCACC), although this disease is rare. According
to National Cancer Center data, there were 123 patients with MPC
(2.7%) among 4,480 patients with cervical cancer between 1962 and
1996, but there were no reports of patients with anal canal cancer
(11). The standard treatment for
locally advanced anal canal cancer is chemoradiotherapy (CRT), and
for early-stage cervical cancer the standard treatment is surgery
or radiotherapy (RT); however, to the best of our knowledge, no
previous studies have reported on the treatment of SCACC. The
present study reports a case of SCACC treated with
intensity-modulated RT (IMRT).
Case report
A 64-year-old female presented with inguinal
lymphadenopathy and anal pain. The patient was admitted to Komagome
Hospital (Tokyo, Japan) in January 2017. The patient had undergone
total mastectomy for left breast cancer at the age of 42 years and
cervical conization for cervical cancer (cervical intraepithelial
neoplasia) at the age of 46 years, but they had no family history
of cancer. The patient smoked one pack of cigarettes per day and
drank socially, and they also had a history of frequent sexual
intercourse with multiple sexual partners, but no anal intercourse.
Laboratory tests determined elevated serum squamous cell carcinoma
(SCC) antigen, cytokeratin 19 fragment and cancer antigen 19-9
levels, and the patient tested negative for human immunodeficiency
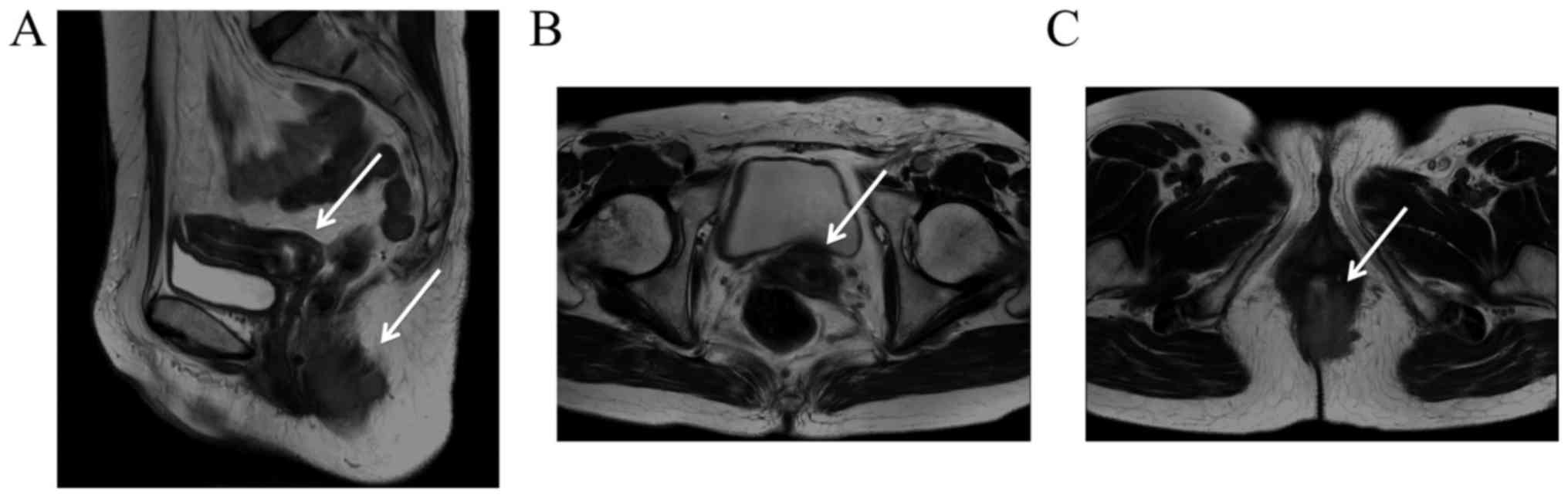
virus. Magnetic resonance imaging of the pelvis depicted a
malignant stricture in the anal canal that extended 55 mm
superiorly from the anal verge, and a malignant stricture in the
cervix, with a diameter of up to 25 mm (Fig. 1). Computed tomography (CT) and
positron emission tomography indicated no evidence of distant
metastases, and the pararectal, left inguinal and bilateral iliac
lymph nodes were suspected to exhibit tumor involvement. Pelvic
examination demonstrated a 25-mm ulceroproliferative growth in the
cervix. Rectal examination indicated an ulceroproliferative growth
at the anal verge, extending up to 20 mm. In the two lesions,
micro-photographs showed sheets and nests of tumor cells separated
by fibrous septae. The tumor cells showed squamoid differentiation.
Therefore, biopsy diagnosed SCC, and increased p16 expression was
determined by immunohistochemistry (Fig.
2). Immunohistochemical staining for p16 was performed as
follows (Bond Max Protocol; 3 mm-thick sections were prepared from
10% formalin fixed at ambient temperature for 24 h,
paraffin-embedded tissue blocks. Tissue sections were
deparaffinized with Bond Dewax Solution (Leica Microsystems, Ltd.,
Milton Keynes, UK) according to the manufacturer's protocol at 72°C
for 30 min. Tissue sections were pretreated with the epitope
retrieval (Bond Epitope Retrieval Solution 2 contained an EDTA
based buffer and surfactant, pH 8.8) at 100°C for 10 min. Following
washing, peroxidase blocking with 3% Hydrogen peroxide was
performed at ambient temperature for 10 min. The sections were
again washed with Bond Wash Solution (Leica Microsystems, Ltd.) at
ambient temperature for 6 min and then incubated with the p16
primary antibody (Ventana Medical Systems; cat no. 705-4713;
Tucson, AZ) at ambient temperature for 15 min. The p16 primary
antibody was diluted in 0.05 M Tris-HCl with 1% carrier protein,
and 0.10% ProClin 300, a preservative. The sections were incubated
with Post Primary reagent [Rabbit anti mouse IgG with 10% (v/v)
animal serum in tris-buffered saline/0.09% ProClin™ 950; cat no.
DS9800; Leica Microsystems, Ltd.] for 8 min at ambient temperature,
followed by washing using Bond Wash solution (Leica Microsystems,
Ltd.) for 6 min, and BondTM Polymer Refine Detection (anti-rabbit
Poly-HRP-IgG; cat no. DS9800; Leica Microsystems, Ltd.) according
to the manufacturer's protocol and placed on the slides for 8 min
at ambient temperature, followed by washing using Bond Wash
solution (Leica Microsystems, Ltd.) and distilled water for 4 min.
The sections were developed with 3,39-diaminobenzidine-chromogen at
ambient temperature for 10 min, and counterstained with hematoxylin
at ambient temperature for 5 min. Stained tissues were viewed and
images captured using a light microscope under five fields in each
sample (magnification, ×100). Therefore, cT3N3M0 stage IIIb anal
canal cancer (UICC 2010) and cT1b1N0M0 stage Ib1 (UICC 2010)
cervical cancer were diagnosed (12).
According to the definitive treatment for
advanced-stage anal canal cancer, outpatient treatment with CRT
(59.4 Gy/33 fractions) using S-1 without using high-dose-rate
intracavitary brachytherapy (HDR-ICBT) for SCACC was recommended,
as the patient did not want to undergo resection of the anus.
In order to reduce the dose to the organs at risk
(OARs), such as the small bowel, RT was delivered via a three-step
IMRT technique. Considering the lymph node region affected by SCACC
and the necessary doses, step 1 included the delivery of 36 Gy in
20 fractions to the whole pelvis, the inguinal lymph node region
and the involved primary lesions. Step 2 included the delivery of
an additional 9 Gy in 5 fractions to the whole pelvis and the
involved primary lesions. Finally, step 3 included the delivery of
an additional 14.4 Gy in 8 fractions to the primary lesions
(Table I). The prescribed dose was
defined as 50% of the planning target volume that should receive
100% of the dose. OARs were contoured, including the peritoneal
space (bowel bag), small bowel loops, bladder and bilateral femoral
head. Table II summarizes the dose
constraints for the OARs. Depending on the extent of the tumor, it
was permissible to exceed the dose constraints to the bowel bag and
small bowel loops. Fig. 3 describes
the dose-distribution and dose-volume histogram. The patient was
prescribed S-1 at a dose of 60 mg/m2/day twice daily on
days 1–14 and days 29–42, and treatment was tolerated the well;
however, Common Terminology Criteria for Adverse Events (v4.0),
including grade 2 diarrhea and anal mucositis (13), were demonstrated. CRT was completed
without discontinuation through outpatient treatment.
 | Table I.Target volumes. |
Table I.
Target volumes.
| Steps | Dose, Gy | Targets |
|---|
| Step 1 | 36 | Primary lesions (anal
canal tumor, cervical tumor, metastatic pararectal node, left
inguinal node and bilateral iliac nodes) |
|
|
| Whole pelvis
(mesorectum, parametrium, presacral space, common external and
internal iliac lymph node regions, and obturator lymph node
region) |
|
|
| Inguinal lymph node
region |
| Step 2 | 9 | Primary lesions and
whole pelvis |
| Step 3 | 14.4 | Primary lesions |
 | Table II.Dose constraints for organs at
risk. |
Table II.
Dose constraints for organs at
risk.
| Organs | Desired value |
|---|
| Bowel bag | V15 Gy
<830 ml V25 Gy <650 ml, and V45 Gy
<195 ml |
| Small bowel
loops | V15 Gy
<275 ml, V25 Gy <190 ml, and V45 Gy
<120 ml |
| Bladder | V45 Gy
<70% organ volume |
| Femoral head | V50 Gy
<10% organ volume |
Following treatment, no tumors were detected by
imaging studies or pathological examinations (Fig. 4). At the 15 months total follow-up,
the patient continued to be disease-free, without any
treatment-associated complications.
Discussion
The present report describes a rare case of SCACC
that was successfully treated with IMRT. The current criteria for
the diagnosis of MPC, which were defined by Warren and Gates
(14), are as follows: i) Each lesion
must be malignant; ii) each lesion must exhibit a distinctively
different pathology; and iii) metastases from prior malignancies
must be excluded. Among patients with MPC, double cancer is
primarily observed, with triple cancer occurring in 0.5% of
patients, and quadruple or quintuple cancer occurring in <0.1%
of patients (15). MPC can be divided
into two categories depending on the interval between each
diagnosis: Synchronous cancer is a secondary cancer occurring
simultaneously or within 6 months of the first malignancy; whereas,
metachronous cancer is a secondary cancer that develops >6
months after the first malignancy (16). In the present case, the anal canal and
cervical tumors contained intraepithelial components and the two
tumors were discontinuous. The patient had a medical history of
left-sided breast cancer. Based on these data, the patient was
diagnosed with double primary synchronous and triple primary
metachronous cancer.
Generally, the treatments for synchronous cancer
remain unclear. It is vital to determine the treatment strategy for
synchronous cancer, considering the tumor stage, tumor location and
patient situation (17). Based on the
definitive treatment for advanced-stage anal canal cancer, the
present treatment policy involved concurrent therapy for SCACC, as
the tumors were in close proximity. Table III depicts the comparisons of
treatments for anal canal and cervical cancer.
 | Table III.Treatments for anal canal cancer and
uterine cervical cancer. |
Table III.
Treatments for anal canal cancer and
uterine cervical cancer.
| Evaluation item | Anal canal cancer
cT3N3M0c stage IIIB | Cervical cancer
cT1b1N0M0c stage IB1 |
|---|
| Standard
treatment | CRT | RT or surgery |
| Dose to primary
lesions | 54–59.4 Gy/30–33 fr.
(BED = 70.1 Gy10) | WP 20 Gy/10 fr. |
|
|
| MB 30 Gy/15 fr. |
|
|
| HDR-ICBT 24 Gy/4
fr. |
|
|
| (BED = 62.4
Gy10 at point A) |
| Dose to sub
clinical area | 36–45 Gy/20–25
fr. | 45–50.4 Gy/25–28
fr. |
| Radiation
field | Primary lesions,
whole pelvis and inguinal lymph node region | Primary lesions and
whole pelvis (including common iliac lymph node region) |
| RT technique | IMRT or 3D-CRT | 3D-CRT |
| Combined
chemotherapy | 5-FU + MMC | – |
| CRT with S-1 | Phase I/II trial of
CRT with S-1 plus MMC is ongoing | Pilot RCT
demonstrated efficacy for CRT with S-1 plus CDDP in advanced
cervical cancer |
In previous studies, in patients with locally
advanced anal canal cancer, the combination of RT and infused
5-fluorouracil (5-FU) and mitomycin resulted in a significantly
improved locoregional control rate and a reduction in the
requirement for colostomy, without a significant increase in late
side effects, compared with RT alone (18). In patients with T3-, T4- or lymph
node-positive anal canal cancer, RT doses of ≥54 Gy administered
with limited treatment breaks (<60 days) were associated with
increased locoregional control (19).
The effect of further escalation of radiation doses was assessed in
the ACCORD 03 trial, which indicated that doses of >60 Gy
provided no additional benefit to patients with anal canal cancer
(20). In patients with early-stage
cervical cancer, a randomized clinical trial (RCT) demonstrated no
significant difference in the overall survival between patients
treated with surgery and those treated with definitive RT (21); therefore, definitive RT without
chemotherapy has been accepted as a treatment option for
early-stage cervical cancer. In Japan, definitive RT comprising of
whole pelvis external beam RT (EBRT) of 20 Gy/10 fractions, pelvic
EBRT with a midline block of 30 Gy/15 fractions and HDR-ICBT of 24
Gy/4 fractions at point A [biologically effective dose (BED), 62.4
Gy10] was a safe and effective treatment for patients
with stage I and II cervical cancer with a small (<4 cm) tumor
diameter (22). Based on these
reports, it was considered that EBRT doses of 59.4 Gy/33 fractions
for primary lesions (BED, 70.1 Gy10) could be applied
for the two primary tumors without HDR-ICBT. Therefore, in the
present study EBRT doses of 59.4 Gy/33 fractions without HDR-ICBT
were used.
The lymph node region involved in anal canal cancer
is similar to that in cervical cancer, but anal canal cancer
involves the inguinal lymph node region and cervical cancer
involves the common iliac lymph node region (23). Considering comprehensive regional
nodal irradiation to each area, IMRT was adopted in the present
study in order to reduce the dose to the OARs. With regard to the
effectiveness of IMRT, the results of a phase II trial for anal
canal cancer treated with IMRT demonstrated that the incidence of
acute gastrointestinal morbidity of at least grade 3 was
significantly lower, compared with previous three-dimensional
conformal RT (21 vs. 37%; P=0.005) (24,25).
Conversely, the effectiveness of IMRT for cervical cancer remains
controversial due to uterine motion (26). To account for internal uterine motion,
the following steps were taken in the present study: Firstly, to
ensure reproducibility, the patient was instructed to empty their
bladder and consume 500 cc of water 30 min prior to the scan and
treatment; secondly, to ensure reproducibility, magnesium oxide for
controlling intestinal function and a laxative to maintain regular
bowel function and an empty rectum was administered; thirdly, the
internal target volume was defined as the uterine clinical target
volume with 10-mm anteroposterior, 10-mm superoinferior and 5-mm
lateral margins, according to a previous report (27) and finally, the treatment position was
verified using daily megavoltage CT imaging. As a result, the
patient achieved a complete response without diarrhea or anal
mucositis of grade 3 or higher.
With regard to chemotherapy, S-1 is an orally
administered antitumor drug composed of tegafur,
5-chloro-2,4-dihydroxypyridine and oteracil potassium. S-1 results
in an increase in radiosensitivity of the tumor cells, as reported
in a previous study (28). Clinical
trials of S-1 have been performed for various cancer types. S-1 is
not inferior to 5-FU in a superiority style, and in view of the
convenience of oral administration, S-1 could replace intravenous
5-FU for the treatment of unresectable or recurrent gastric cancer
(29). According to these data, a
phase I/II trial of CRT with S-1 plus mitomycin C is ongoing in
patients with stage II/III squamous cell carcinoma of the anal
canal (30). In advanced cervical
cancer, the results of a pilot RCT demonstrated promising efficacy
and an acceptable toxicity of CRT with S-1 plus cisplatin, compared
with CRT with cisplatin alone (31).
Based on these reports, it was considered that S-1 may be an
effective treatment for SCACC and recommended for outpatient
treatment.
Increased p16 expression has been demonstrated to be
an important prognostic determinant for patients with anal canal
and cervical cancer treated with RT (32,33). In
the present case, although the definitive treatment was lacking in
terms of combination chemotherapy without 5-FU and mitomycin for
anal canal cancer and an irradiation method without ICBT for
cervical cancer, the promising treatment results may have been
reflected by the increased p16 expression simultaneously observed
in each tumor.
In conclusion, a rare case of SCACC that was
successfully treated with IMRT is reported in the present study. It
is important to determine the treatment strategy for synchronous
cancer, taking into consideration the tumor stage, tumor location
and patient situation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TK prepared the manuscript and the literature
search. TK, KI and TS contributed to analysis and interpretation of
data, and assisted in the preparation of the manuscript. SK, KN, KS
and KK have contributed to data collection and interpretation, and
critically reviewed the manuscript. All authors approved the final
version of the manuscript, and agree to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication of this case report, and the privacy policy was fully
explained.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MPC
|
multiple primary cancer
|
|
SCACC
|
synchronous cancer of the anal canal
and cervix
|
|
CRT
|
chemoradiotherapy
|
|
RT
|
radiotherapy
|
|
IMRT
|
intensity-modulated radiotherapy
|
|
HDR-ICBT
|
high-dose-rate intracavitary
brachytherapy
|
|
EBRT
|
external beam radiotherapy
|
|
RCT
|
randomized clinical trial
|
References
|
1
|
Kanguru L, Bikker A, Cavers D, Barnett K,
Brewster DH, Weller D and Campbell C: Pathways to diagnosis of a
second primary cancer: Protocol for a mixed-methods systematic
review. BMJ Open. 7:e0179292017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spratt JS Jr and Hoag MG: Incidence of
multiple primary cancers per man-year of follow up: 20-year review
from the Ellis Fischel State Cancer Hospital. Ann Surg.
164:775–784. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demandante CG, Troyer DA and Miles TP:
Multiple primary malignant neoplasms: Case report and a
comprehensive review of the literature. Am J Clin Oncol. 26:79–83.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayat MJ, Howlader N, Reichman ME and
Edwards BK: Cancer statistics, trends, and multiple primary cancer
analyses from the surveillance, epidemiology, and end results
(SEER) program. Oncologist. 12:20–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coyte A, Morrison DS and McLoone P: Second
primary cancer risk-the impact of applying different definitions of
multiple primaries: Results from a retrospective population-based
cancer registry study. BMC cancer. 14:2722014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Utada M, Ohno Y, Hori M and Soda M:
Incidence of multiple primary cancers and interval between first
and second primary cancers. Cancer Sci. 105:890–896. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Molina-Montes E, Requena M,
Sánchez-Cantalejo E, Fernández MF, Arroyo-Morales M, Espín J,
Arrebola JP and Sánchez MJ: Risk of second cancers cancer after a
first primary breast cancer: A systematic review and meta-analysis.
Gynecol Oncol. 136:158–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Travis LB: Therapy-associated solid
tumors. Acta Oncol. 41:323–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Travis LB, Fosså SD, Schonfeld SJ,
McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A,
Pukkala E, et al: Second cancers among 40,576 testicular cancer
patients: Focus on long-term survivors. J Natl Cancer Inst.
97:1354–1365. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis LB, Rabkin CS, Brown LM, Allan JM,
Alter BP, Ambrosone CB, Begg CB, Caporaso N, Chanock S, DeMichele
A, et al: Cancer survivorship-genetic susceptibility and second
primary cancers: Research strategies and recommendations. J Natl
Cancer Inst. 98:15–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaneko S and Yamaguchi N: Epidemiological
analysis of site relationships of synchronous and metachronous
multiple primary cancers in the National Cancer Center, Japan,
1962–1996. Jpn J Clin Oncol. 29:96–105. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sobin LH, Gospodarowicz MK and Wittekind
C; and International Union against Cancer, : TNM classification of
malignant tumours. Wiley-Blackwell; Chichester, West Sussex, UK;
Hoboken, NJ: 2010
|
|
13
|
National Cancer Institute (U.S.): Common
terminology criteria for adverse events (CTCAE), . U.S. Dept. of
Health and Human Services. National Institutes of Health, National
Cancer Institute; Bethesda, Md.: 2009
|
|
14
|
Warren S and Gates O: Multiple primary
malignant tumors: A survey of the literature and statistical study.
Am J Cancer. 16:1358–1414. 1932.
|
|
15
|
Németh Z, Czigner J, Iván L, Ujpál M,
Barabás J and Szabó G: Quadruple cancer, including triple cancers
in the head and neck region. Neoplasma. 49:412–414. 2002.PubMed/NCBI
|
|
16
|
Moertel CG, Dockerty MB and Baggenstoss
AH: Multiple primary malignant neoplasms. I. Introduction and
presentation of data. Cancer. 14:221–230. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vogt A, Schmid S, Heinimann K, Frick H,
Herrmann C, Cerny T and Omlin A: Multiple primary tumours:
Challenges and approaches, a review. ESMO Open. 2:e0001722017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bartelink H, Roelofsen F, Eschwege F,
Rougier P, Bosset JF, Gonzalez DG, Peiffert D, van Glabbeke M and
Pierart M: Concomitant radiotherapy and chemotherapy is superior to
radiotherapy alone in the treatment of locally advanced anal
cancer: Results of a phase III randomized trial of the European
Organization for Research and Treatment of Cancer Radiotherapy and
Gastrointestinal Cooperative Groups. J Clin Oncol. 15:2040–2049.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang K, Haas-Kogan D, Weinberg V and
Krieg R: Higher radiation dose with a shorter treatment duration
improves outcome for locally advanced carcinoma of anal canal.
World J Gastroenterol. 13:895–900. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peiffert D, Tournier-Rangeard L, Gérard
JP, Lemanski C, François E, Giovannini M, Cvitkovic F, Mirabel X,
Bouché O, Luporsi E, et al: Induction chemotherapy and dose
intensification of the radiation boost in locally advanced anal
canal carcinoma: Final analysis of the randomized UNICANCER ACCORD
03 trial. J Clin Oncol. 30:1941–1948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Landoni F, Maneo A, Colombo A, Placa F,
Milani R, Perego P, Favini G, Ferri L and Mangioni C: Randomised
study of radical surgery versus radiotherapy for stage Ib-IIa
cervical cancer. Lancet. 350:535–540. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toita T, Kato S, Niibe Y, Ohno T, Kazumoto
T, Kodaira T, Kataoka M, Shikama N, Kenjo M, Tokumaru S, et al:
Prospective multi-institutional study of definitive radiotherapy
with high-dose-rate intracavitary brachytherapy in patients with
nonbulky (<4-cm) stage I and II uterine cervical cancer
(JAROG0401/JROSG04-2). Int J Radiat Oncol Biol Phys. 82:e49–e56.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. Wiley Blackwell;
Chichester, West Sussex, UK; Hoboken, NJ: 2017
|
|
24
|
Ajani JA, Winter KA, Gunderson LL,
Pedersen J, Benson AB III, Thomas CR Jr, Mayer RJ, Haddock MG, Rich
TA and Willett C: Fluorouracil, mitomycin, and radiotherapy vs
fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal
canal: A randomized controlled trial. JAMA. 299:1914–1921. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kachnic LA, Winter K, Myerson RJ, Goodyear
MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran
H and Willett CG: RTOG 0529: A phase 2 evaluation of dose-painted
intensity modulated radiation therapy in combination with
5-fluorouracil and mitomycin-C for the reduction of acute morbidity
in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys.
86:27–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jadon R, Pembroke CA, Hanna CL,
Palaniappan N, Evans M, Cleves AE and Staffurth J: A systematic
review of organ motion and image-guided strategies in external beam
radiotherapy for cervical cancer. Clin Oncol (R Coll Radiol).
26:185–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taylor A and Powell ME: An assessment of
interfractional uterine and cervical motion: Implications for
radiotherapy target volume definition in gynaecological cancer.
Radiother Oncol. 88:250–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng L, Ou G, Itasaka S, Harada H, Xie X,
Shibuya K, Kizaka-Kondoh S, Morinibu A, Shinomiya K and Hiraoka M:
TS-1 enhances the effect of radiotherapy by suppressing
radiation-induced hypoxia-inducible factor-1 activation and
inducing endothelial cell apoptosis. Cancer Sci. 99:2327–2335.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boku N, Yamamoto S, Fukuda H, Shirao K,
Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, et
al: Fluorouracil versus combination of irinotecan plus cisplatin
versus S-1 in metastatic gastric cancer: A randomised phase 3
study. Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takashima A, Shimada Y, Hamaguchi T, Ito
Y, Nakano A, Nakamura K, Shibata T, Fukuda H and Moriya Y:
Colorectal Cancer Study Group of the Japan Clinical Oncology Group:
A Phase I/II trial of chemoradiotherapy concurrent with S-1 plus
mitomycin C in patients with clinical Stage II/III squamous cell
carcinoma of anal canal (JCOG0903: SMART-AC). Jpn J Clin Oncol.
41:713–717. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Z, Mao W, Lin N and Han S: Concurrent
radiotherapy with S-1 plus cisplatin versus concurrent radiotherapy
with cisplatin alone for the treatment of locally advanced cervical
carcinoma: A pilot randomised controlled trial. Clin Transl Oncol.
18:413–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schwarz JK, Lewis JS Jr, Pfeifer J,
Huettner P and Grigsby P: Prognostic significance of p16 expression
in advanced cervical cancer treated with definitive radiotherapy.
Int J Radiat Oncol Biol Physics. 84:153–157. 2012. View Article : Google Scholar
|
|
33
|
Serup-Hansen E, Linnemann D,
Skovrider-Ruminski W, Høgdall E, Geertsen PF and Havsteen H: Human
papillomavirus genotyping and p16 expression as prognostic factors
for patients with American Joint Committee on Cancer stages I to
III carcinoma of the anal canal. J Clin Oncol. 32:1812–1817. 2014.
View Article : Google Scholar : PubMed/NCBI
|