Introduction
Osteosarcoma is the most common primary bone tumor
and predominantly affects children and adolescents (1–3). The
current treatment of osteosarcoma remains difficult, and
osteosarcoma causes numerous mortalities due to its complex
pathogenesis and resistance to conventional treatments (4).
Photodynamic therapy (PDT) is a disease
site-specific treatment. It involves the local systemic
administration of a photosensitizer followed by irradiation of the
targeted lesion site with non-thermal visible light of an
appropriate wavelength. In the presence of molecular oxygen, the
light irradiation of the photosensitizer and energy transfer may
lead to a number of photochemical reactions and generation of
various cytotoxic species, thereby inducing apoptosis and necrosis
of the targeted lesion (5).
Osteosarcoma has been studied by our group for a number of years
and have investigated the possibility of applying PDT to the
treatment of osteosarcoma (6,7).
Previously, another study indicated that PDT causes
acute inflammation, expression of heat-shock proteins, invasion and
the infiltration of the tumor by leukocytes, and that it may
increase the presentation of tumor-derived antigens to T cells by
regulating dendritic cells (DCs) (8).
DCs are a heterogeneous population of
antigen-presenting cells. Their main function is to process
antigens and present them to T cells to promote immunity toward
foreign antigens and tolerance toward self-antigens (9–15). It has
been demonstrated that the inflammation milieu induced by PDT
promoted the antigen-presenting function of DCs (16).
It has been previously reported that PDT treatment
results in the induction of apoptotic and necrotic cell death, and
that immature DCs co-cultured with PDT-treated tumor cells results
in effective homing to regional and peripheral lymph nodes and
stimulation of the cytotoxic activity of T and natural killer cells
(17). However, the exact mechanism
by which the DCs are initiated remains unclear. In the study, we
firstly hypothesized that the necrosis of osteosarcoma cells
inhibited the function of DCs and treatment with PDT restored the
function of DCs. The present study may provide insight into the
mechanism underling the function of DCs following the treatment of
osteosarcoma via PDT.
Materials and methods
Mice
A total of 20 C57BL/6 mice (6–8 weeks old, 21±2.1 g,
male) were obtained from the Animal Center of the Second Military
Medical University (Shanghai, China). A total of 10 DO11.10
OVA323-339-specific mice (6–8 weeks old, 21±2.1 g, male)
with C57BL/6 background were obtained from the Jackson Laboratory
(C57BL/6 × DO11.10). A total of 10 F1 mice (21±2.1 g, male) were
prepared by crossing C57BL/6 mice with DO11.10 mice. All mice were
maintained under specific pathogen-free conditions and used at 6–8
weeks of age. Mice were provided with ad libitum access to
food and water. Room conditions were controlled for humidity
(40–70%) and temperature (22±3°C) with a 12/12 h light/dark cycle.
The use of the mice was approved by the Ethics Committee of Tongji
University (Shanghai, China).
Photodynamic therapy
The photodynamic treatments were performed at the
Department of Orthopedics of Shanghai Tenth People's Hospital
(Shanghai, China). The entire process was performed as previously
described (18).
Cell culture and treatment
The murine osteosarcoma LM8 cell line was obtained
from the Type Culture Collection of Chinese Academy of Sciences
(Manassas, VA, USA) and was cultured in Dulbecco's modified Eagle's
medium (catalog no. SH30022.01; Hyclone, GE Healthcare Life
Sciences, Logan, UT, USA), containing 10% fetal bovine serum (cat.
no. 10100139; FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 1% penicillin and 1% streptomycin. Cells were placed at
37°C in a humidified 5% CO2 incubator. The remnants of
LM8 cells were produced by Ultrasonic Cell Disruptor
(Fisherbrand™ Model 505 Sonic Dismembratorm; Thermo
Fisher Scientific, Inc.) at 300 watts for 10 sec. Mouse bone
marrow-derived DCs were generated by culturing in
granulocyte-macrophage colony-stimulating factor (GM-CSF; catalog
no. 12-7209-42; Thermo Fisher Scientific, Inc.) and interleukin
(IL)-4 (catalog no. 1-7042-82; Thermo Fisher Scientific, Inc.), as
previously described (19–22). In brief, the back legs above the hip
joint of each mouse were incised, with the knee and ankle joints
intact. Following this, both ends of the bone were incised with
scissors as close to the joints as possible, then a syringe was
filled with ice-cold RPMI complete medium (cat no. 21875091; Gibco;
Thermo Fisher Scientific, Inc.) and the syringe needle was inserted
into the bone in order to flush out the bone marrow into a
centrifuge tube, which was on ice. Following depletion of red
cells, the suspensions were cultured at a density of
2×106 cells/ml in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) with 10% fetal calf serum. rmGM-CSF (10 ng/ml;
cat no. PMC2015; Thermo Fisher Scientific, Inc.) and rmIL-4 (1
ng/ml; cat no. RMIL4; Thermo Fisher Scientific, Inc.) were added at
the beginning of the cell culture. The non-adherent cells were
gently washed at day 3 or day 4. On day 5, the proliferating DC
clusters were collected and purified by anti-CD11c microbeads (cat
no. 130092465; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) as
immature DCs. Immature DCs were stimulated with LPS (1 µg/ml; cat
no. 00-4976-93; Thermo Fisher Scientific, Inc.) for 12 h, and these
cells were collected as mature DCs. The purity of the DCs was
>95%, confirmed anti-CD11c conjugated to fluorescein
isothiocyanate (cata no. 11-0114-85; Thermo Fisher Scientific,
Inc.) by cytometry analysis (BD LSR II; BD Biosciences, Franklin
Lakes, NJ, USA) as previously described (23). The bone marrow-derived DCs were
co-cultured with the remnants of osteosarcoma LM8 cells. DCs
co-cultured with RPMI complete medium (cat no. 21875091; Gibco;
Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum (cat.
no. 10100139; FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) were used as the control. The cells were placed at 37°C in
a humidified 5% CO2 incubator for 24 h.
Phenotype analysis
For assessing the DC phenotypes, cells were
incubated with blocking buffer (0.5% BSA and 0.05% Sodium Azide in
1 × PBS) at room temperature for 30 min. These cells
(5×105) were subsequently stained independently with
fluorescein-conjugated monoclonal antibodies against cluster of
differentiation CD80 (catalog no. 11-0809-42; 1:500), CD86 (catalog
no. 12-0869-42; 1:1,000), Ia (catalog no. 11-0432-42; 1:1,000) or
CD40 (catalog no. 11-1548-42; 1:1,000) (all Thermo Fisher
Scientific, Inc.) in staining buffer (1% FBS with 0.09% NaN3) at
room temperature for 20 min. Following washed by PBS three times
and centrifugation at 500 × g for 3 min at 4°C, cells were
subsequently analyzed by FACS (BD LSR II) for expression of CD80,
CD86, Ia and CD40. For assessing the myeloid-derived suppressor
cell phenotypes, cells (5×105) were double-stained with
fluorescein-conjugated monoclonal antibodies against Gr-1 and
CD11b, and then these cells were analyzed by FACS using a BD LSR II
flow cytometer (LSR II, 1.1.0) with BD FACSDiva™ software version
6.0 (BD LSR II; BD Biosciences, Franklin Lakes, NJ, USA) for Gr-1
and CD11b expression.
T-cell proliferation analysis
Ability of DCs in stimulating T cell proliferation
were tested by DC-T cell cocultured experiment. In detail,
OVA323-339-specific T-cell receptor (TCR)-transgenic
mice (6–8 weeks) were euthanized for isolation of the spleen. The
single-cell suspensions of spleen were produced by gentle grinding
and filtration, and then the red blood cells were removed via red
blood cell Lysis Buffer (cat. 00-433-57; Thermo Fisher Scientific,
Inc.). The CD4+ T cells were purified using microbeads
(CD4+ antibody-coated; Miltenyi Biotec GmbH). The
CD4+ T cells (1×105) were co-cultured with
DCs (1×104) in 96-well plates for 5 days. The viable T
cells (CD4+ and 7-AAD−) were then counted by
using a BD LSR II flow cytometer (LSR II, 1.1.0) with BD FACSDiva™
software version 6.0 (BD LSR II; BD Biosciences).
RNA sequencing (RNA-SEQ) and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA were extracted from LM8 cells by
TRIzol® Reagent (catalog no. 15596018, Thermo Fisher
Scientific, Inc.). RNA-SEQ was performed by Shanghai Shengong
Engineering Technology Service, Ltd. (Shanghai, China). The
expression levels of heat shock protein 70 (HSP70), activating
transcription factor 3 (ATF3), B-cell lymphoma 2 (Bcl-2), tumor
protein (P)53, P21, P16 and P27 levels were analyzed by RT-qPCR.
RNA were reversed-transcrpted by SuperScript™ First-Strand
Synthesis System for RT-PCR (catalog no. 11904018, Thermo Fisher
Scientific, Inc). The thermocycling conditions were 42°C for 50
min, 70°C for 15 min. PCR primers were constructed by Shanghai
Shengong Engineering Technology Service, Ltd. qPCR reactions were
performed in 96-well optical reaction plates using the cDNA
equivalent of 20 ng total RNA for each sample in a total volume of
20 µl containing 1X SYBR® Green PCR master mix (24) (cat. no. A25741; Thermo Fisher
Scientific) and forward and reverse primers were listed as
following: HSP70, forward, 5′-GATGCCAGCGTACAGTCC-3′, reverse,
5′-ACAGCATTTGTCACTGTCTTT-3′; ATF3, forward,
5′-TGCAAAAGGAAACTGACCAAG-3′, and reverse,
5′-CTGGCCTGGATGTTGAAGCAT-3′; p53, forward,
5′-CCCCTCCTGGCCCCTGTCATCTTC-3′, and reverse,
5′-GCAGCGCCTCACAACCTCCGTCAT-3′; p21, forward,
5′-GAGGCCGGGATGAGTTGGGAGGAG-3′, and reverse
5′-CAGCCGGCGTTTGGAGTGGTAGAA-3′; p16, forward,
5′-GAGCAGCATGGAGCCTTCGG, and reverse, 5′-CATGGTTACTGCCTCTGGTG-3′;
p27, forward, 5′-GGTTAGCGGAGCAATGCG-3′, and reverse,
5′-TCCACAGAACCGGCATTTG-3′; GAPDH, forward,
5′-CGACCACTTTGTCAAGCTCA-3′, and reverse,
5′-AGGGGTCTACATGGCAACTG-3′. The thermocycling conditions were as
follows: 95°C for 3 min and 40 cycles of amplification comprising
95°C for 12 sec, appropriate annealing temperature (60°C) for 30
sec, and 72°C for 30 sec (25–27).
Statistical analysis
A two-tailed unpaired Student's t-test was used to
analyze the difference between two groups. Analysis of variance,
followed by the least significant difference test, was used to
analyze the difference among three groups. SPSS for Windows, v.16.0
(SPSS Inc., Chicago, IL, USA), was used to perform all statistical
analyses. Values are expressed as the mean ± standard deviation of
three independent tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
The remnants of LM8 cells inhibit the
function of DCs
To begin with, the effects of the lysis remnants of
osteosarcoma cells on the function of DCs were determined. The bone
marrow-derived DCs were co-cultured with the remnants of
osteosarcoma LM8 cells. DCs co-cultured with medium were used as
control. After a 48-h co-culture, the DCs were isolated for
phenotype and cytokine analysis, and their ability to stimulate the
proliferation of T-cells was also investigated. It was revealed
that the remnants of LM8 cells significantly inhibited the
expression of major histocompatibility complex 2 (MHC-2), CD40,
CD86, CD80 and C-C chemokine receptor type 7 (Fig. 1A). The treatment of LM8 cells
decreased IL-12 levels and increased IL-10 levels (Fig. 1B). The mixed lymphocyte reaction
analysis revealed that the treatment of LM8 cells decreased the
ability of DCs to stimulate T-cell proliferation (Fig. 1C).
 | Figure 1.The remnants of LM8 decreased the
co-stimulatory molecules, and inhibited IL-12 and IL-6 levels,
increased IL-10 levels, and inhibited the ability of DCs to
stimulate T-cell proliferation. (A) The DCs were isolated following
a 48-h co-culture with the remnants of LM8 cells. The cells were
then labeled with antibodies against CD11c, MHC-2, CD40, CD86, CD80
and CCR7 for phenotypic analysis by flow cytometry. The numbers in
the histograms indicate the geometric mean fluorescence intensity.
(B) Following isolation from the co-culture system, the DCs were
cultured for 12 h. Subsequently, the expression levels of IL-12,
IL-6 and IL-10 in the supernatant were analyzed by ELISA. (C)
CD4+ T cells from DO11.10 OVA323-339-specific
(TCR-transgenic × C57BL/6) F1 hybrid mice were co-cultured with DCs
or mDCs (control) in the presence of OVA peptides. Five days later,
the total number of viable CD4+ T (CD4+
7-AAD−) cells in each well was measured by flow
cytometry. Data represent one of at least three experiments with
similar results. *P<0.05 LM8 DC/T vs. Control DC/T. IL,
interleukin; DCs, dendritic cells; CD, cluster of differentiation;
MHC-2, major histocompatibility complex 2; CCR7, C-C chemokine
receptor 7; DC/T, DCs and T cell co-culture; mDCs, mature DCs. |
PDT treatment reduces the inhibitory
function of the LM8 remnants
To investigate the role of PDT in the inflammation,
we first treated LM8 cells with PDT and then collected the remnants
of LM8 treated cells (PDT-LM8) for co-cultured with the bone
marrow-derived DCs. We found that PDT-LM8 upregulated MHC-2
expression (Fig. 2A). We then
analyzed the co-expression molecules and found that PDT-LM8 treated
by PDT up-regulated the CD80, CD86, Ia, CD11c, CD40, and CCR7
(Fig. 2B). The mixed lymphocyte
reaction analysis revealed that the PDT treatment may increase the
ability of DCs in stimulating T cell proliferation (Fig. 2C).
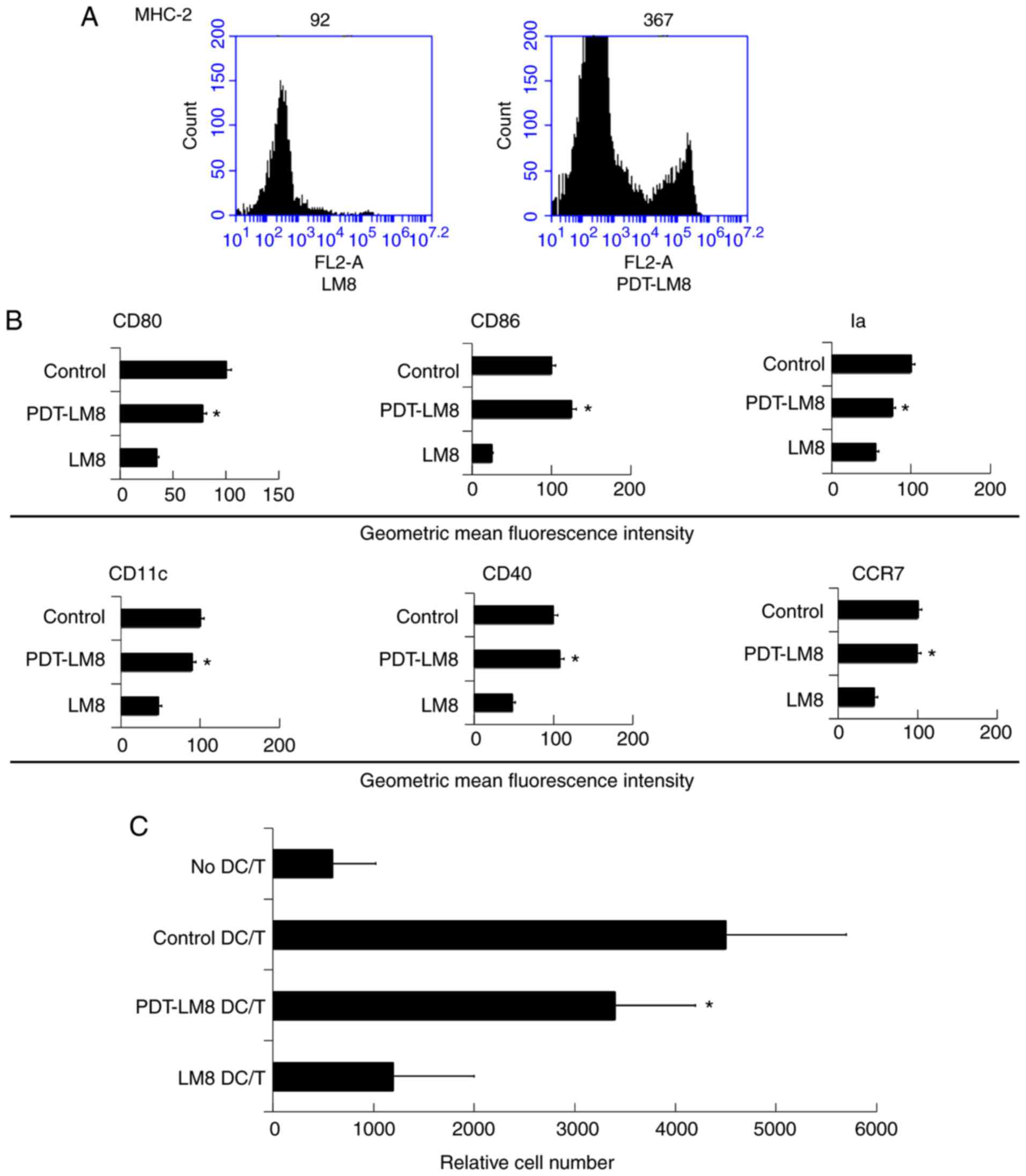 | Figure 2.PDT treatment partly reversed the
effect of LM8 remnants on the phenotype of DCs and their ability to
stimulate T cells proliferation. (A) The LM8 cells were pre-treated
with PDT, and then labeled with antibodies against MHC-2, CD11c,
CD40, CD86, CD80 and CCR7, for phenotypic analysis by flow
cytometry. (B) The numbers in the histograms indicate the geometric
mean fluorescence intensity. *P<0.05 PDT-LM8 vs LM8. (C)
CD4+ T cells from DO11.10 OVA323-339-specific
(TCR-transgenic × C57BL/6) F1 hybrid mice were co-cultured with DCs
or mDCs (control) in the presence of OVA peptides. Five days later,
the total number of viable CD4+ T (CD4+
7-AAD−) cells in each well was measured by flow
cytometry. Data represent one of at least three experiments with
similar results. *P<0.05 PDT-LM8 DC/T vs LM8 DC/T. PDT,
photodynamic therapy; DCs, dendritic cells; MHC-2, major
histocompatibility complex 2; CD, cluster of differentiation; CCR7,
C-C chemokine receptor 7; DC/T, DCs and T cell co-culture. |
HSP70 is upregulated by PDT
To identify the key molecular elements altered in
osteosarcoma cells treated with PDT, the differential gene
expression between LM8 cells treated with PDT and the control was
determined by RNA-SEQ analysis. The data indicated that HSP70 was
upregulated in the PDT-treated LM8 cells (Fig. 3A). As HSP70 serves an important role
in the activation of DCs (28–30), the
expression of HSP70 and related genes, including ATF3, BCL2, P53,
P21, P16 and P27, was subsequently assessed by RT-qPCR. It was
revealed that HSP70 was upregulated following PDT treatment
(Fig. 3B). Next, the HSP70 levels
were inhibited in LM8 cells via siRNA and then the remnants of
these transfected LM8 cells were cultured with DCs. It was observed
that the increased downregulation of HSP70 cells at least partly
reduced the effect of PDT treatment on the phenotype of DCs (CD86
expression). Therefore, it was concluded that the HSP70 expression
induced by PDT may promote the function of DCs (Fig. 3C).
 | Figure 3.PDT treatment upregulated the HSP70
expression in LM8 cells and promoted upregulation of
HSP70-activated DCs. (A) The LM8 cells with and without PDT
pre-treatment were collected for RNA sequencing analysis. (B) The
expression of HSP70, ATF3, Bcl-2, P53, P21, P16 and P27 was
analyzed by reverse transcription-quantitative PCR. (C) LM8 cells
were transfected with HSP70 small interfering RNA, and the DCs were
then co-cultured with LM8 for 48 h, prior to being labeled with an
antibody against CD86 for phenotypic analysis by flow cytometry.
The numbers in the histograms indicate the geometric mean
fluorescence intensity. Data represent one of at least three
experiments with similar results. *P<0.05 HSP70-si-PDT-LM8 vs
PDT-LM8. PDT, photodynamic therapy; HSP70, heat shock protein 70;
DCs, dendritic cells; ATF3, activating transcription factor 3;
Bcl-2, B-cell lymphoma 2; PCR, polymerase chain reaction; CD,
cluster of differentiation. |
Discussion
PDT is a novel approach for the treatment of
osteosarcoma. However, the underlying mechanisms affecting
subsequent immune reaction remains unclear. The present study
provided insights into these molecular mechanisms and revealed that
the remnants of osteosarcoma cells inhibited the functions of DCs
and that treatment with PDT reduced this inhibitory function.
Notably, the upregulation of HSP70 was involved in the underlying
mechanism regarding this phenomenon.
It has been established that the function of DCs in
tumors is inhibited. In a previous study, authors revealed that the
tumor microenvironment was able to induce DCs to differentiate into
regulatory DCs with a CD11c(low) CD11b(high) Ia(low) phenotype and
a high expression of IL-10, nitric oxide, vascular endothelial
growth factor and arginase I. These tumor-educated regulatory DCs
suppress T cell response (31).
Notably, PDT treatment changed the gene expression of osteosarcoma
cells and induced upregulation of HSP70. HSP70 has been
demonstrated to activate the function of DCs (28–30). As it
has been established that tumor-educated DCs develop into DCs with
immune suppressive functions. The present study revealed that PDT
treatment promoted the normal function of DCs.
However, for CD11c(low) CD11b(high) Ia(low)
regulatory DCs, tumor-derived transforming growth factor β (TGF-β)
and prostaglandin E2 (PGE2) are responsible for the generation of
regulatory DCs. The present study revealed that upregulation of
HSP70 reversed the inhibitory function of the tumor on DCs.
Therefore, we concluded that there were two possible reasons for
this: i) PDT treatment may prevent the process of tumor-educated
DCs via HSP70, or ii) PDT treatment may re-educate the
tumor-educated DCs in a HSP70-dependent manner.
In the process of inducing DCs with immune
suppressive functions, the soluble factors TGF-β and PGE2 served
central roles. Following PDT treatment, HSP70, an insoluble factor,
was able to reverse the function of DCs. Therefore, the results of
the present study indicated that soluble factors and other
molecules expressed by tumors may affect the function of DCs.
Furthermore, these data also indicated that that p53
was upregulated following treatment of LM8 cells with PDT (Fig. 2B). However, it was previously
demonstrated that the p53 gene was most often mutated/deleted in
human osteosarcoma (32,33), and it is possible that the OS cell
lines used in those studies lacked p53 expression. Due to the fact
that the results of the present study cannot confirm that p53 was
upregulated by PDT treatment, this will be investigated in future
studies.
In conclusion, the results of the present study
revealed that the remnants of osteosarcoma treated with PDT induced
the activation of DCs, and that the molecular mechanism involved
upregulation of HSP70 expression induced by PDT. Therefore, the
present study may provide novel insight into the treatment of
osteosarcoma via PDT and its effects on the function of DCs.
Acknowledgements
The authors would like to thank Dr Chaoxiong Zhang
(Research Center for Public Health and Preventive Medicine, West
China School of Public Health, Sichuan Univeristy, Chengdu, China)
for their assistance.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FZ and YZ collected patient data and performed cell
experiments. GF performed the PCR molecular experiment and FACS. SH
contributed to the study design and manuscript writing.
Ethics statement and consent to
participate
The present study was approved by Ethics Committee
of Tongji University (Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miao J, Wu S, Peng Z, Tania M and Zhang C:
MicroRNAs in osteosarcoma: Diagnostic and therapeutic aspects.
Tumor Biol. 34:2093–2098. 2013. View Article : Google Scholar
|
|
5
|
Huang Z: A review of progress in clinical
photodynamic therapy. Technol Cancer Res Treat. 4:283–293. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gong HY, Sun MX, Hu S, Tao YY, Gao B, Li
GD, Cai ZD and Yao JZ: Benzochloroporphyrin derivative induced
cytotoxicity and inhibition of tumor recurrence during photodynamic
therapy for osteosarcoma. Asian Pac J Cancer Prev. 14:3351–3355.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng H, Sun M, Zhou C, Yin F, Wang Z, Hua
Y and Cai Z: Hematoporphyrin monomethyl ether-mediated photodynamic
therapy selectively kills sarcomas by inducing apoptosis. PloS One.
8:e777272013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castano AP, Mroz P and Hamblin MR:
Photodynamic therapy and anti-tumour immunity. Nat Rev Cancer.
6:535–545. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Merad M: Dendritic cells: Controllers of
adaptive immunity. Nat Rev Immunol. 11:1–2. 2011.
|
|
10
|
Schuler G, Schuler-Thurner B and Steinman
RM: The use of dendritic cells in cancer immunotherapy. Curr Opin
Immunol. 15:138–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merad M, Sathe P, Helft J, Miller J and
Mortha A: The dendritic cell lineage: Ontogeny and function of
dendritic cells and their subsets in the steady state and the
inflamed setting. Annu Rev Immunol. 31:563–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen R, Deng X, Wu H, Peng P, Wen B and Li
F and Li F: Combined immunotherapy with dendritic cells and
cytokine-induced killer cells for malignant tumors: A systematic
review and meta-analysis. Int Immunopharmacol. 22:451–464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guilliams M, Ginhoux F, Jakubzick C, Naik
SH, Onai N, Schraml BU, Segura E, Tussiwand R and Yona S: Dendritic
cells, monocytes and macrophages: A unified nomenclature based on
ontogeny. Nat Rev Immunol. 14:571–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anguille S, Smits EL, Lion E, van Tendeloo
VF and Berneman ZN: Clinical use of dendritic cells for cancer
therapy. Lancet Oncol. 15:e257–e267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pizova K, Tomankova K, Daskova A, Binder
S, Bajgar R and Kolarova H: Photodynamic therapy for enhancing
antitumour immunity. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 156:93–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jalili A, Makowski M, Switaj T, Nowis D,
Wilczynski GM, Wilczek E, Chorazy-Massalska M, Radzikowska A,
Maslinski W, Biały L, et al: Effective photoimmunotherapy of murine
colon carcinoma induced by the combination of photodynamic therapy
and dendritic cells. Clin Cancer Res. 10:4498–4508. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saczko J, Nowak M, Skolucka N, Kulbacka J
and Kotulska M: The effects of the electro-photodynamic in vitro
treatment on human lung adenocarcinoma cells. Bioelectrochemistry.
79:90–94. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang M, Tang H, Guo Z, An H, Zhu X, Song
W, Guo J, Huang X, Chen T, Wang J and Cao X: Splenic stroma drives
mature dendritic cells to differentiate into regulatory dendritic
cells. Nat Immunol. 5:1124–1133. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang H, Guo Z, Zhang M, Wang J, Chen G and
Cao X: Endothelial stroma programs hematopoietic stem cells to
differentiate into regulatory dendritic cells through IL-10. Blood.
108:1189–1197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia S, Guo Z, Xu X, Yi H, Wang Q and Cao
X: Hepatic microenvironment programs hematopoietic progenitor
differentiation into regulatory dendritic cells, maintaining liver
tolerance. Blood. 112:3175–3185. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Q, Guo Z, Xu X, Xia S and Cao X:
Pulmonary stromal cells induce the generation of regulatory DC
attenuating T-cell-mediated lung inflammation. Eur J Immunol.
38:2751–2761. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lutz MB, Kukutsch N, Ogilvie AL, Rössner
S, Koch F, Romani N and Schuler G: An advanced culture method for
generating large quantities of highly pure dendritic cells from
mouse bone marrow. J Immunol Methods. 223:77–92. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morrison TB, Weis JJ and Wittwer CT:
Quantification of low-copy transcripts by continuous SYBR Green I
monitoring during amplification. Biotechniques. 24:954–958, 960,
962. 1998.PubMed/NCBI
|
|
25
|
Chidlow G, Wood JP and Casson RJ:
Expression of inducible heat shock proteins Hsp27 and Hsp70 in the
visual pathway of rats subjected to various models of retinal
ganglion cell injury. PloS One. 9:e1148382014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dorak MT: Real-time PCR. Taylor &
Francis; 2007, View Article : Google Scholar
|
|
27
|
Fraga D, Meulia T and Fenster S: Real-time
PCR. Current protocols essential laboratory techniques: 10.13.
11-10.13. 40. 2008. View Article : Google Scholar
|
|
28
|
Floto RA, MacAry PA, Boname JM, Mien TS,
Kampmann B, Hair JR, Huey OS, Houben EN, Pieters J, Day C, et al:
Dendritic cell stimulation by mycobacterial Hsp70 is mediated
through CCR5. Science. 314:454–458. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mac Ary PA, Javid B, Floto RA, Smith KG,
Oehlmann W, Singh M and Lehner PJ: HSP70 peptide binding mutants
separate antigen delivery from dendritic cell stimulation.
Immunity. 20:95–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu Y, Wan T, Zhou X, Wang B, Yang F, Li N,
Chen G, Dai S, Liu S, Zhang M and Cao X: Hsp70-like protein 1
fusion protein enhances induction of carcinoembryonic
antigen-specific CD8+ CTL response by dendritic cell vaccine.
Cancer Res. 65:4947–4954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu Q, Zhang C, Sun A, Zheng Y, Wang L and
Cao X: Tumor-educated CD11bhighIalow regulatory dendritic cells
suppress T cell response through arginase I. J Immunol.
182:6207–6216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen X, Bahrami A, Pappo A, Easton J,
Dalton J, Hedlund E, Ellison D, Shurtleff S, Wu G, Wei L, et al:
Recurrent somatic structural variations contribute to tumorigenesis
in pediatric osteosarcoma. Cell Rep. 7:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|

















