Introduction
Gastric cancer is the most common malignant tumor in
the digestive tract. In China, over 160,000 individuals succumb to
gastric cancer annually, accounting for approximately one fifth of
all tumor deaths, posing a serious threat to human health (1,2). It has
been shown that both bacterial and host genetic factors affect the
progression of gastric diseases with individual differences. The
Helicobacter pylori (HP) strain is a main pathogenic factor,
whose toxic areas are fatal pathogenicity island (PAI) and
vacuolatingcytotoxin (VacA) (3,4). In 10–20%
infected patients, HP induces chronic gastritis to develop into
gastroduodenal ulcer, gastric cancer or gastric mucosa-associated
lymphoid tissue lymphoma (5,6). Gastric precancerous lesions refer to
histopathological changes in gastric mucosa, namely the gastric
mucosa dysplasia and intestinal metaplasia, which are prone to
cancerization (7).
Phosphatidylinositol-3-kinase (PI3K) signals are
involved in cell proliferation, differentiation, apoptosis, and
glucose transport (8). After PI3K
activation, a second messenger, phosphatidylinositol triphosphate
(PIP3), is generated on the cytoplasmic membrane, which binds to
the signal proteins, Akt and phosphoinositide-dependent kinase 1
(PDK1), containing the PH structural domain in cells, leading to
Akt activation (9). Akt, also known
as protein kinase B (PKB), is the main downstream effector of PI3K.
The phosphorylation of Ser473 and Thr308 sites is a necessary
condition for Akt activation, and the activated Akt further
phosphorylates or inhibits its downstream target proteins, such as
glycogen synthase kinase-3 (GSK-3), glucose transporter (GLUT),
mammalian target of rapamycin (mTOR), caspase-9 and nuclear
factor-κB (NF-κB), thus regulating the cell proliferation,
differentiation, apoptosis and migration (10,11).
However, there are few reports on the correlation between HP
infection and PI3K/Akt pathway activation in mucosal tissues of
gastric cancer and precancerous lesions.
In this study, the correlation between HP infection
and PI3K/Akt pathway activation in mucosal tissues of gastric
cancer and precancerous lesions was analyzed, providing strong
evidence for the clinical treatment of chronic gastritis and
application of anti-inflammatory drugs in gastric cancer.
Materials and methods
General data
Patients with chronic atrophic gastritis (n=52) and
gastric cancer (n=98) treated at the Department of Gastroenterology
at at The Fifth People's Hospital of Chongqing (Chongqing, China)
from August 2010 to August 2016 were selected. Patients were
diagnosed via gastroscopy and pathological examination. The biopsy
tissue and serum specimens were collected, and tissues were fixed
via 4% neutral formalin, embedded in paraffin, and serially
sectioned (4 µm) for immunohistochemistry (IHC). None of patients
received radio- and/or chemotherapy. There was no history of taking
anti-HP drugs or non-steroidal anti-inflammatory drugs within 2
weeks before gastroscopy, and patients with other systemic
malignancies were excluded. Early gastric cancer was defined as
cancer tissue infiltration in mucosal and submucosal layers.
Moderate-advanced or progressive gastric cancer was defined as
invasion of cancer tissues into the gastric muscular wall and
serosal layer. The study was approved by the Ethics Committee of
The Fifth People's Hospital of Chongqing and written informed
consents were signed by the patients and/or guardians.
Enzyme-linked immunosorbent assay
(ELISA)
Whole blood was collected and centrifuged at 600 × g
for 10 min to separate the serum. The PI3K activity assay kit (Art.
No. K-1000S, Echelon, New York, NY, USA) was used. According to the
instructions provided, the serum was added, and 50 µl enzyme
conjugate was also added into each well, except the control well.
The mixture was mixed evenly and incubated at 37°C for 30 min, and
the supernatant was discarded. The wells were washed with washing
liquid 5 times, and 50 µl color developing agents A and B (1:1)
were added into each well, mixed evenly and incubated in the dark
at 37°C for 15 min. Then, 50 µl stop buffer was added into each
well to terminate the immune reaction, and the results were read at
the wavelength of 450 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). HP immunoglobulin G (IgG)
>20 U/ml indicated a positive result.
IHC
Paraffin-embedded tissue sections were taken and
used for IHC. After dewaxing via xylene, dehydration via gradient
ethanol, and antigen retrieval via sodium citrate buffer solution
in a microwave were carried out, 3% H2O2
blocker was added to block the peroxidase. Sections were sealed in
10% donkey serum, added with the primary antibody (total p-Akt,
Abcam, Cambridge, MA, USA, diluted at 1:500), and placed in a wet
box for incubation at 4°C overnight. The following day, the
sections were washed with phosphate-buffered saline (PBS) three
times, and the ready-to-use universal secondary antibody was
incubated, followed by color development via diaminobenzidine (DAB)
and capturing of images under a microscope.
Western blotting
Four cases of HP-positive and 4 cases of HP-negative
gastric cancer tissues were randomly selected and ground to extract
the proteins using IP lysis (with cocktail). The protein
concentration was detected via bicinchoninic acid (BCA); the
protein was separated via 12% polyacrylamide gel electrophoresis,
transferred onto the polyvinylidene fluoride (PVDF) membrane via
semi-dry process, and sealed with 5% skimmed milk powder at room
temperature for 1 h. The bands were incubated with rabbit
anti-human primary monoclonal antibodies, p-Akt Ser473 (1:2,000),
p-Akt Thr308 (1:1,000), total p-Akt (1:2,000) and β-actin (1:5,000)
(cat. nos. 4060, 13038, 4685, 8457, respectively; Cell Signaling
Technology, Danvers, MA, USA) at 4°C overnight. The following day,
the membrane was washed with PBST three times (10 min/time). The
secondary goat anti-rabbit polyclonal antibody (1:2,000; cat. no.
7074; Cell Signaling Technology) was added for incubation at room
temperature for 2 h, and the membrane was washed again with PBST
three times (10 min/time). Then the hypersensitive luminescent
solution was added, and images were captured using a gel imaging
system.
HP infection
HP strains were purchased from the American Type
Culture Collection (Shanghai, China). Gastric cancer cell lines
MGC-803 and AGS were cultured with RPMI-1640 and 10% fetal bovine
serum. Bacterial liquid was added into the medium for co-culture,
and the proteins were extracted at 0, 6 and 12 h. The p-Akt protein
level was detected via western blot analysis.
Statistical analysis
MedCalc software (Marie-Kerque, Belgium) was used
for data statistics and processing. Measurement data were presented
as mean ± standard deviation. ANOVA was used for comparison between
multiple groups and the post hoc test was SNK test. The
χ2 test was used for the comparison of enumeration data.
P<0.05 indicated the difference was statistically
significant.
Results
Comparison of positive rate of HP
infection between patients with precancerous lesions and those with
gastric cancer
The serum HP infections in patients with
precancerous lesions and gastric cancer were detected via ELISA
(Table I). The positive rate of HP
infection in patients with precancerous lesions was 84.6% (44/52),
which was significantly higher than that in patients with gastric
cancer [73.5% (72/98)] (p<0.05). The positive rate of HP
infection in patients with early gastric cancer (86.4%) was
significantly higher than that in patients with moderate-advanced
gastric cancer (69.7%) (p<0.05).
 | Table I.Comparison of positive rate of HP
infection between patients with precancerous lesions and those with
gastric cancer. |
Table I.
Comparison of positive rate of HP
infection between patients with precancerous lesions and those with
gastric cancer.
|
|
| HP infection |
|
|---|
|
|
|
|
|
|---|
| Variables | No. | Negative | Positive | Positive rate
(%) | χ2 | P-value |
|---|
| Chronic atrophic
gastritis | 52 | 8 | 44 | 84.6 | 5.782 | 0.012a |
| Gastric cancer | 98 | 26 | 72 | 73.5 |
|
|
| Early | 22 | 3 | 19 | 86.4 | 4.265 | 0.026b |
| Advanced | 76 | 23 | 53 | 69.7 |
|
|
Detection of p-Akt levels in both
groups via IHC
P-Akt was mainly located in the cytoplasm, and the
p-Akt expression level in HP-positive tissues was obviously higher
than that in HP-negative tissues (p<0.05) (Fig. 1).
Detection of p-Akt protein level via
western blot analysis
Four cases of HP-positive and 4 cases of HP-negative
gastric cancer tissues were randomly selected to detect the Akt
phosphorylation level via western blotting. The p-Akt expression
level was high in HP-positive tissues, but low in HP-negative
tissues (Fig. 2).
Analysis of correlation between HP
infection and PI3K activity
Total proteins were extracted from cells at 0, 6 and
12 h after HP infection, and the PI3K activity was detected using
the PI3K activity assay kit. PI3K activity was significantly
enhanced after HP infection (p<0.05) (Fig. 3).
Analysis of correlation between HP
infection and cell proliferation
At 0, 6 and 12 h after HP infection of cells, the
infection medium was discarded. The cells were digested and
inoculated in a 96-well plate. The number of cells at 4, 8, 12, 16,
20 and 24 h of culture after HP infection was counted using the
cell counting method. Compared with that at 0 h, the proliferation
rates of cells at 6 h and 12 h after HP infection were
significantly increased in a time-dependent manner (p<0.05)
(Fig. 4).
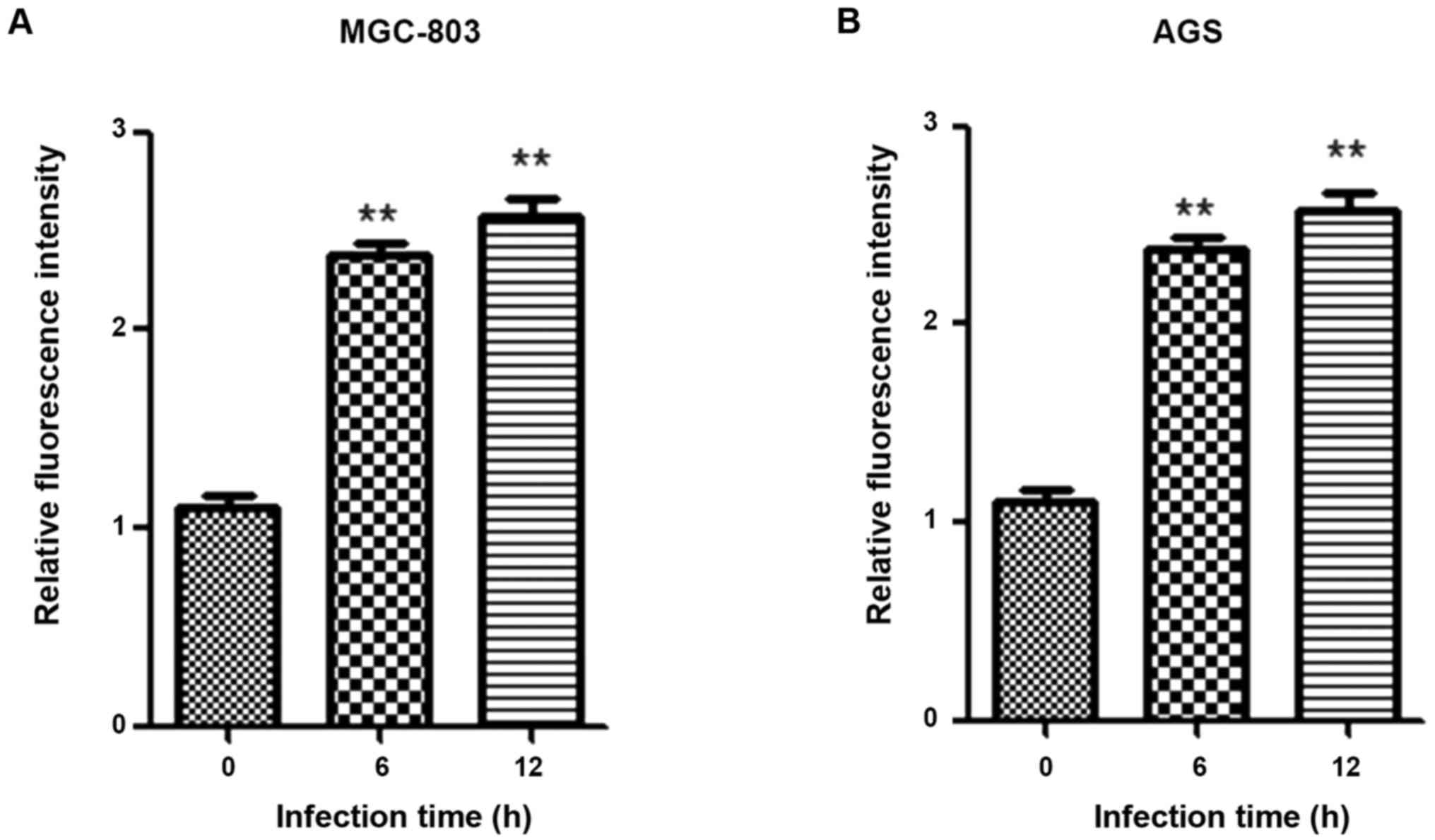
Detection of correlation between HP
infection and p-Akt via in vitro experiments
The gastric cancer MGC-803 and AGS cells were
co-cultured with HP. At 0, 6 and 12 h after HP infection of cells,
the protein was extracted from cells to detect the p-Akt expression
via western blotting. The results showed that the p-Akt expressions
(Ser473 and Thr308) in cells after HP infection were significantly
increased (p<0.05); with the passage of time, the Akt
phosphorylation level was gradually elevated in a time-dependent
manner (Fig. 5).
Detection of correlation between HP
infection and p-Akt
After LY294002, a PI3K inhibitor, was added, the
PI3K activity was suppressed, thus inhibiting the Akt activation,
namely the Akt phosphorylation. Therefore, LY294002 was added into
the cell culture solution for co-culture with HP. The protein was
extracted to detect the p-Akt expression level. Akt was not
significantly activated by HP infection after inhibition of PI3K
activity (Fig. 6).
Discussion
At present, the pathogenesis of gastric cancer is
not yet fully clear, and the implementation of etiological primary
prevention is more difficult; thus, research on gastric
precancerous lesions is crucial in the secondary prevention of
gastric cancer. Early identification, prevention and treatment of
precancerous diseases and lesions have become effective methods
used to reduce the incidence and mortality rates of gastric cancer
(12).
In tumor cells, the Akt/PI3K pathway is abnormally
activated. Activated Akt phosphorylates a large number of
downstream substrates and promotes the upregulation of cell
proliferation. Moreover, Akt directly promotes cell survival
through phosphorylation and the inactivation of several
pro-apoptotic targets (including Bad, Bim, Bax and FoxO1/3a
transcription factors) (13). In
addition, Akt also plays an important role in metabolism, which
regulates glycolysis through phosphorylation of phosphofructokinase
(PFK) and hexokinase, and exerts important functions in the
anaerobic glycolysis (also known as the Warburg effect) of cancer
cells (14). The activation of lipid
kinase PI3K can activate Akt, thus generating PIP3 in the
cytoplasmic membrane. Akt binds to PIP3 through its substrate
protein homology (PH) domain, resulting in the translocation of Akt
to the cell membrane. Akt can be activated via dual phosphorylation
mechanism. PDK1 is also translocated to the cytoplasmic membrane
due to its own PH domain, which can phosphorylate Akt at the Thr308
site in the activation loop (15,16). The
secondary phosphorylation of carboxy-terminal Ser473 site of Akt is
completed by the mTOR-Rictor complex, mTORC2, which is also
necessary for Akt activation (17).
A large number of studies have shown that HP
infection is closely related to the occurrence and development of
gastric cancer. Currently, HP infection can be clinically diagnosed
via endoscopy and breath test (18).
Tabassam et al found that HP virulence factors, cag PAI and
OipA, regulate the phosphorylation of Akt sites, Thr308 and Ser473,
respectively. OipA mutation reduces the phosphorylation level of
Akt Ser473, whereas HP infection-induced cag PAI mutation reduces
the activation of Akt Thr308. The specific function of cag PAI or
OipA in activating the signal transduction in Akt Ser473 or Thr308
may lead to the imbalance of downstream proliferation and apoptosis
signaling (11). Importantly,
infection with the cag PAI/OipA double mutants completely blocks
the activation of two HP-mediated Akt sites, suggesting that both
OipA and cag PAI are necessary for the complete activation of Akt
(19). Activated Akt signaling
pathway is a key regulator in many cellular biological effects,
such as cell survival, proliferation and motility (20). The upregulation of Akt activation is
also observed in tissues adjacent to gastric tumors, and the
activation of Akt affects the chemoresistance of gastric cancer
(21). In summary, the
phosphorylation of Akt mediated jointly by cag PAI and OipA is
thought to be an intracellular signaling regulator of occurrence of
gastric cancer, as well as a key regulator of many cellular
functions.
In the present study, serum specimens of patients
with chronic gastritis and gastric cancer were collected. The serum
HP level was detected via ELISA to further confirm whether the
phosphorylation level of Akt could be significantly upregulated
after HP infection in patients with gastritis or gastric cancer,
namely the PI3K/Akt pathway activation, thereby promoting the tumor
occurrence and development. At the same time, biopsy tissue
specimens of patients were collected. The p-Akt level, namely the
PI3K/Akt pathway activation, in tissues was detected via IHC. In
in vitro experiments, gastric cancer MGC-803 and AGS cells
were co-cultured with HP, and it was found that both PI3K activity
and p-Akt protein level were significantly increased after cell
infection. Moreover, the cell count experiments showed that HP
infection could significantly increase the proliferative activity
of gastric cancer cells. In addition, the co-culture of LY294002
and HP revealed that there was no significant change in the p-Akt
protein level. In conclusion, it is proved in this study through
clinical cases combined with in vitro experiments that the
PI3K/Akt pathway can be activated after HP infection, thereby
promoting the occurrence and development of tumors.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
YX collected the general data of patients. YX and LL
were responsible for IHC and western blot analysis. Both authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Fifth People's Hospital of Chongqing (Chongqing, China) and
written informed consents were signed by the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Smith MG, Hold GL, Tahara E and El-Omar
EM: Cellular and molecular aspects of gastric cancer. World J
Gastroenterol. 12:2979–2990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gotoda T, Yanagisawa A, Sasako M, Ono H,
Nakanishi Y, Shimoda T and Kato Y: Incidence of lymph node
metastasis from early gastric cancer: Estimation with a large
number of cases at two large centers. Gastric Cancer. 3:219–225.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SS, Ruiz VE, Carroll JD and Moss SF:
Helicobacter pylori in the pathogenesis of gastric cancer and
gastric lymphoma. Cancer Lett. 305:228–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moss SF and Sood S: Helicobacter pylori.
Curr Opin Infect Dis. 16:445–451. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tammer I, Brandt S, Hartig R, König W and
Backert S: Activation of Abl by Helicobacter pylori: A novel kinase
for CagA and crucial mediator of host cell scattering.
Gastroenterology. 132:1309–1319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murata-Kamiya N, Kurashima Y, Teishikata
Y, Yamahashi Y, Saito Y, Higashi H, Aburatani H, Akiyama T, Peek RM
Jr, Azuma T, et al: Helicobacter pylori CagA interacts with
E-cadherin and deregulates the β-catenin signal that promotes
intestinal transdifferentiation in gastric epithelial cells.
Oncogene. 26:4617–4626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Vries AC, van Grieken NCT, Looman CWN,
Casparie MK, de Vries E, Meijer GA and Kuipers EJ: Gastric cancer
risk in patients with premalignant gastric lesions: A nationwide
cohort study in the Netherlands. Gastroenterology. 134:945–952.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thorpe LM, Yuzugullu H and Zhao JJ: PI3K
in cancer: Divergent roles of isoforms, modes of activation and
therapeutic targeting. Nat Rev Cancer. 15:7–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang J and Slingerland JM: Multiple roles
of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell
Cycle. 2:339–345. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hers I, Vincent EE and Tavaré JM: Akt
signalling in health and disease. Cell Signal. 23:1515–1527. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabassam FH, Graham DY and Yamaoka Y:
Helicobacter pylori activate epidermal growth factor receptor- and
phosphatidylinositol 3-OH kinase-dependent Akt and glycogen
synthase kinase 3β phosphorylation. Cell Microbiol. 11:70–82. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oda I, Saito D, Tada M, Iishi H, Tanabe S,
Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y, et al: A multicenter
retrospective study of endoscopic resection for early gastric
cancer. Gastric Cancer. 9:262–270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Wang H, Kuang CY, Zhu JK, Yu Y, Qin
ZX, Liu J and Huang L: An essential role for the
Id1/PI3K/Akt/NFkB/survivin signalling pathway in promoting the
proliferation of endothelial progenitor cells in vitro. Mol Cell
Biochem. 363:135–145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurup PA: Endosymbioticactinidicarchaeal
mediated warburg phenotype mediates human disease state. Adv Nat
Sci. 5:81–84. 2012.
|
|
15
|
Chappell WH, Steelman LS, Long JM, Kempf
RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone
P, et al: Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors:
Rationale and importance to inhibiting these pathways in human
health. Oncotarget. 2:135–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bitting RL and Armstrong AJ: Targeting the
PI3K/Akt/mTOR pathway in castration-resistant prostate cancer.
Endocr Relat Cancer. 20:R83–R99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bayascas JR and Alessi DR: Regulation of
Akt/PKB Ser473 phosphorylation. Mol Cell. 18:143–145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong BCY, Lam SK, Wong WM, Chen JS, Zheng
TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY, et al: China Gastric
Cancer Study Group: Helicobacter pylori eradication to prevent
gastric cancer in a high-risk region of China: A randomized
controlled trial. JAMA. 291:187–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei J, Nagy TA, Vilgelm A, Zaika E, Ogden
SR, Romero-Gallo J, Piazuelo MB, Correa P, Washington MK, El-Rifai
W, et al: Regulation of p53 tumor suppressor by Helicobacter pylori
in gastric epithelial cells. Gastroenterology. 139:1333–1343. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu Y, Qiao L, Wang S, Rong SB, Meuillet
EJ, Berggren M, Gallegos A, Powis G and Kozikowski AP:
3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid
analogues and carbonate surrogates block PI3-K, Akt, and cancer
cell growth. J Med Chem. 43:3045–3051. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Page C, Lin HJ, Jin Y, Castle VP, Nunez G,
Huang M and Lin J: Overexpression of Akt/AKT can modulate
chemotherapy-induced apoptosis. Anticancer Res. 20A:1–416.
2000.
|