Introduction
The endoplasmic reticulum (ER) is the main cellular
organelle for post-transcriptional modifications, including folding
and assembly of most secretory and membrane proteins (1,2). If cells
are exposed to stress conditions such as hypoxia and nutrient
deprivation, misfolded or unfolded proteins accumulate in the ER
lumen, a condition that has been called ‘ER stress’ (1). In ER stress conditions, the cells induce
an adaptive response known as the ‘unfolded protein response
(UPR).’ UPR activation can enhance cell survival by removing
misfolded proteins from the ER via autophagy (3,4). However,
prolonged UPR activation can also lead to apoptosis (4).
ER stress is considered to be involved in the
pathogenesis of various conditions including diabetes mellitus,
neurodegenerative disease, atherosclerosis, inflammation, and
cancer (5,6). Lack of oxygen and nutrient are common
conditions in cancer, and enhanced proliferation and metabolism of
cancer cells result in increased protein production (3,7) this all
leads to ER stress and UPR. Many investigators have researched the
role of ER stress in cancer. In fact, we reported on the expression
of ER stress-related molecules in non-small cell lung cancer
(NSCLC) patients (8).
Protein disulfide isomerase (PDI) is one of the most
common proteins in the ER; it acts as a molecular chaperone by
catalyzing disulfide bond oxidation, reduction, and isomerization
(9,10). Disulfide bonds are extremely important
for the folding and stability of secretory proteins, which comprise
approximately 30% of all proteins. Therefore, PDI function is
essential for cell viability, and PDI dysfunction can lead to UPR
and cell death. Recent studies suggest that PDI expression is
increased in cancer and it was associated with adverse clinical
outcomes in patients with cancers such as glioblastoma, breast
carcinoma, and hepatocellular carcinoma (HCC) (11–14).
However, studies using clinical specimens in NSCLC have not yet
been reported.
While catalyzing disulfide bond formation in nascent
proteins, the active sites of PDI are reduced (10), and for the reduced PDI to regain
catalytic function for disulfide bond formation, the active sites
of PDI must be reoxidized (10).
Endoplasmic reticulum oxidoreductin 1-α (ERO1A) is an
oxidoreductase in the ER (15); by
oxidizing PDI, ERO1A acts as a major regulator of PDI (16). Although there are few published
papers, ERO1A has also been suggested to be poor prognostic factor
for cancer patients (17–20). However, little is known about the
prognostic impact of the correlative expression of PDI and ERO1A in
NSCLC patients.
Cigarette smoke (CS) is known to be an important
cause of lung cancer, but the precise mechanism involved in the
development of cancer remains unclear. To explain the mechanism by
which smoking causes lung cancer, studies on the relationship
between smoking and ER stress and UPR in lung cancer are
increasing.
In the current study, we investigated the clinical
significance of PDI and ERO1A expression by immunohistochemical
staining in NSCLC patients. With further analysis we evaluated the
prognostic value of the combined expression of PDI and ERO1A in
NSCLC. To understand the mechanism of how smoking is involved in
NSCLC pathogenesis, we also investigated the relationship between
smoking status and expression of these proteins in NSCLC
patients.
Materials and methods
Patients and follow-up
We studied 198 NSCLC patients who had undergone
surgery between 2007 and 2010 at Chonbuk National University
Hospital. We reviewed diagnosis and pathologic staging according to
the American Joint Committee on Cancer staging system (21) and WHO classification (22), and we obtained the patients' clinical
and pathologic information by reviewing medical records. In our
group of 198 patients, the male to female ratio was 3.12:1 (150
male and 48 female) and the mean age was 65.2 years (range 47–81
years) at diagnosis. One hundred and five patients with tumor size
of 3 cm or greater received adjuvant chemotherapy (84 patients;
paclitaxel + carboplatin and 21 patients; paclitaxel + cisplatin).
We classified smoking status following the Centers for Disease
Control and Prevention guidelines of never, former (adults who have
smoked at least 100 cigarettes in their lifetime but say they
currently do not smoke), or current (adults who have smoked 100
cigarettes in their lifetime and currently smoke cigarettes;
(23). This study was approved by the
institutional review board of Chonbuk National University Hospital
(CUH 2016-05-003-001) and was performed according to Declaration of
Helsinki. Based on the retrospective and anonymous character of the
study the approval contained a waiver for written informed
consent.
Immunohistochemical staining and
scoring
Tissue microarrays were established using
paraffin-embedded tissues of NSCLC patients. Original H&E
slides were reviewed, and 3.0-mm cores from the representative
solid areas were taken. Antigens were retrieved for 12 min in EDTA
buffer. Tissue sections were then incubated overnight with primary
antibodies for PDI (1:200, clone RL90; Cell Signaling Technology,
Inc., Beverly, MA, USA) and ERO1A (1:200, clone 4G3; Abnova,
Taipei, Taiwan). We performed immunohistochemical staining for two
antibodies on consecutive slides. The slides that had been stained
for PDI and ERO1A were evaluated by two pathologists (Myoung Ja
Chung and Kyoung Min Kim) who had no clinicopathologic information
on the patients. Expression of both PDI and ERO1A was scored
semi-quantitatively by assessing both intensities (0, no; 1, weak;
2, intermediate; or 3, strong staining) and proportion of stained
area (0, no cell staining; 1, 1%; 2, 2–10%; 3, 11–33%; 4, 34–66%;
and 5, 67–100%). Intensity and proportion score were summed to
obtain final expression scores ranging from zero to eight.
Statistical analysis
The cutoff points for the PDI- and ERO1A-positivity
were determined at the point with the highest area under the curve
(AUC) to estimate survival of NSCLC patients. Because the ROC curve
is a plot of the true positive rate (sensitivity) vs. the false
positive rate (1-specificity) for the determination of the death of
patients, the cutoff level for the ideal test is presented as the
sensitivity 1 and specificity 1 (AUC 1.000). Therefore, we choose
the cut-off point at the highest AUC value. The cutoff point for
the combined score of PDI (intensity score + proportion score)
immunostaining was six. The AUC was 0.615 for PDI. The
immunostaining for PDI was scored positive when the combined score
was greater than or equal to six. The cutoff point for the combined
score of ERO1A immunostaining was five. The AUC was 0.616 for
ERO1A. The immunostaining for ERO1A was scored positive when the
combined score was greater than or equal to five.
The date of last follow-up was up to last contact or
death of patients through August 2016. We evaluated the prognosis
of the NSCLC patients by analyzing overall survival (OS), and we
considered patient death from lung cancer was considered an event
for OS analysis.
We performed all statistical univariate and
multivariate Cox proportional hazards regression analyses,
Kaplan-Meier survival analysis, and Pearson's Chi-square testing
using SPSS software version 19.0 (IBM Corp., Armonk, NY, USA), and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Correlation between PDI and ERO1A
expression and clinicopathologic parameters of NSCLC patients
PDI and ERO1A were predominantly expressed in the
cytoplasm of tumor cells (Fig. 1A);
non-neoplastic stromal cells did not express either. The cutoffs
for the PDI and ERO1A expression scores were six and five,
respectively (Fig. 1B). Based on
these cutoffs, we classified the expression of PDI and ERO1A as
positive in 71.2% (141 out of 198) and 69.2% (137 out of 198) of
NSCLC patients, respectively.
PDI expression was significantly associated with
never smoking (P=0.003), and ERO1A expression was significantly
higher in adenocarcinoma and other cancers than in squamous
carcinoma (P=0.004). In addition, PDI expression had a significant
positive correlation with ERO1A expression (P<0.001; Table I). Because PDI and ERO1A work together
within the ER and there is a significant positive correlation
between PDI and ERO1A expressions in NSCLC, we wondered whether the
over-expression of both proteins occurs in the same cell. We
performed immunohistochemical staining for the PDI and ERO1A on
consecutive slides to examine whether the two proteins were
co-expressed in tumor cells. In cases showing positivity in both
PDI and ERO1A, expression of PDI and ERO1A was co-localized in
tumor cells (Fig. 2).
 | Table I.Association between
clinicopathological factors and immunohistochemical expression of
PDI and ERO1A in non-small cell lung cancers. |
Table I.
Association between
clinicopathological factors and immunohistochemical expression of
PDI and ERO1A in non-small cell lung cancers.
|
|
| PDI |
| ERO1A |
| PDI/ERO1A
expression |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Characteristics | Total | Positive (%) | P-value | Positive (%) | P-value | −/− (%) | −/+ or +/− (%) | +/+ (%) | P-value |
|---|
| All cases | 198 | 141 (71.2) |
| 137 (69.2) |
| 34 (17.2) | 50 (25.3) | 114 (57.6) |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 150 | 103 (68.7) |
| 102 (68) |
| 29 (19.3) | 37 (24.7) | 84 (56) |
|
|
Female | 48 | 38 (79.2) | 0.162 | 35 (72.9) | 0.521 | 5 (10.4) | 13 (27.1) | 30 (62.5) | 0.362 |
| Age (years) |
|
|
|
|
|
|
|
|
|
|
≤65 | 90 | 62 (68.9) |
| 60 (66.7) |
| 17 (18.9) | 24 (26.7) | 49 (54.4) |
|
|
>65 | 108 | 79 (73.1) | 0.51 | 77 (71.3) | 0.482 | 17 (15.7) | 26 (24.1) | 65 (60.2) | 0.706 |
| Smoking
history |
|
|
|
|
|
|
|
|
|
| Never
smoker | 99 | 80 (80.8) |
| 71 (71.7) |
| 11 (11.1) | 25 (25.3) | 63 (63.6) |
|
|
Smoker | 99 | 61 (61.6) | 0.003 | 66 (66.7) | 0.442 | 23 (23.2) | 25 (25.3) | 51 (51.5) | 0.064 |
| Histologic
type |
|
|
|
|
|
|
|
|
|
|
SQCC | 97 | 64 (66) |
| 57 (58.8) |
| 27 (27.8) | 19 (19.6) | 51 (52.6) |
|
|
ADC | 94 | 73 (77.7) |
| 73 (77.7) |
| 7 (7.4) | 28 (29.8) | 59 (62.8) |
|
|
Other | 7 | 4 (57.1) | 0.144 | 7 (100) | 0.004 | 0 (0) | 3 (42.9) | 4 (57.1) | 0.002 |
| Histologic
grade |
|
|
|
|
|
|
|
|
|
| Well or
moderate | 159 | 117 (73.6) |
| 112 (70.4) |
| 25 (15.7) | 39 (24.5) | 95 (59.7) |
|
|
Poor | 39 | 24 (61.5) | 0.137 | 25 (64.1) | 0.442 | 9 (23.1) | 11 (28.2) | 19 (48.7) | 0.403 |
| T stage 8th |
|
|
|
|
|
|
|
|
|
| T
1,2 | 154 | 112 (72.7) |
| 106 (68.8) |
| 26 (16.9) | 38 (24.7) | 90 (58.4) |
|
| T
3,4 | 44 | 29 (65.9) | 0.378 | 31 (70.5) | 0.837 | 8 (18.2) | 12 (27.3) | 24 (54.5) | 0.898 |
| N stage |
|
|
|
|
|
|
|
|
|
| N
0 | 134 | 100 (74.6) |
| 92 (68.7) |
| 19 (14.2) | 38 (28.4) | 77 (57.5) |
|
| N
1–3 | 64 | 41 (64.1) | 0.125 | 45 (70.3) | 0.813 | 15 (23.4) | 12 (18.8) | 37 (57.8) | 0.154 |
| PDI |
|
|
|
|
|
|
|
|
|
|
Negative | 57 |
|
| 23 (40.4) |
|
|
|
|
|
|
Positive | 141 |
|
| 114 (80.9) | <0.001 |
|
|
|
|
| ERO1A |
|
|
|
|
|
|
|
|
|
|
Negative | 61 | 27 (44.3) |
|
|
|
|
|
|
|
|
Positive | 137 | 114 (83.2) | <0.001 |
|
|
|
|
|
|
NSCLC patients with PDI and ERO1A
expression had shorter OS in univariate analysis
Higher tumor stage, PDI expression, and ERO1A
expression had significant impact on the OS of NSCLC patients in
univariate analysis (Table II). The
patients with PDI expression showed 2.215 times greater risk of
death from NSCLC [95% confidence interval (95% CI): 1.348–3.64,
P=0.002]. ERO1A expression predicted a 1.932 times greater risk of
NSCLC death (95% CI: 1.208–3.09, P=0.006). The survival curves for
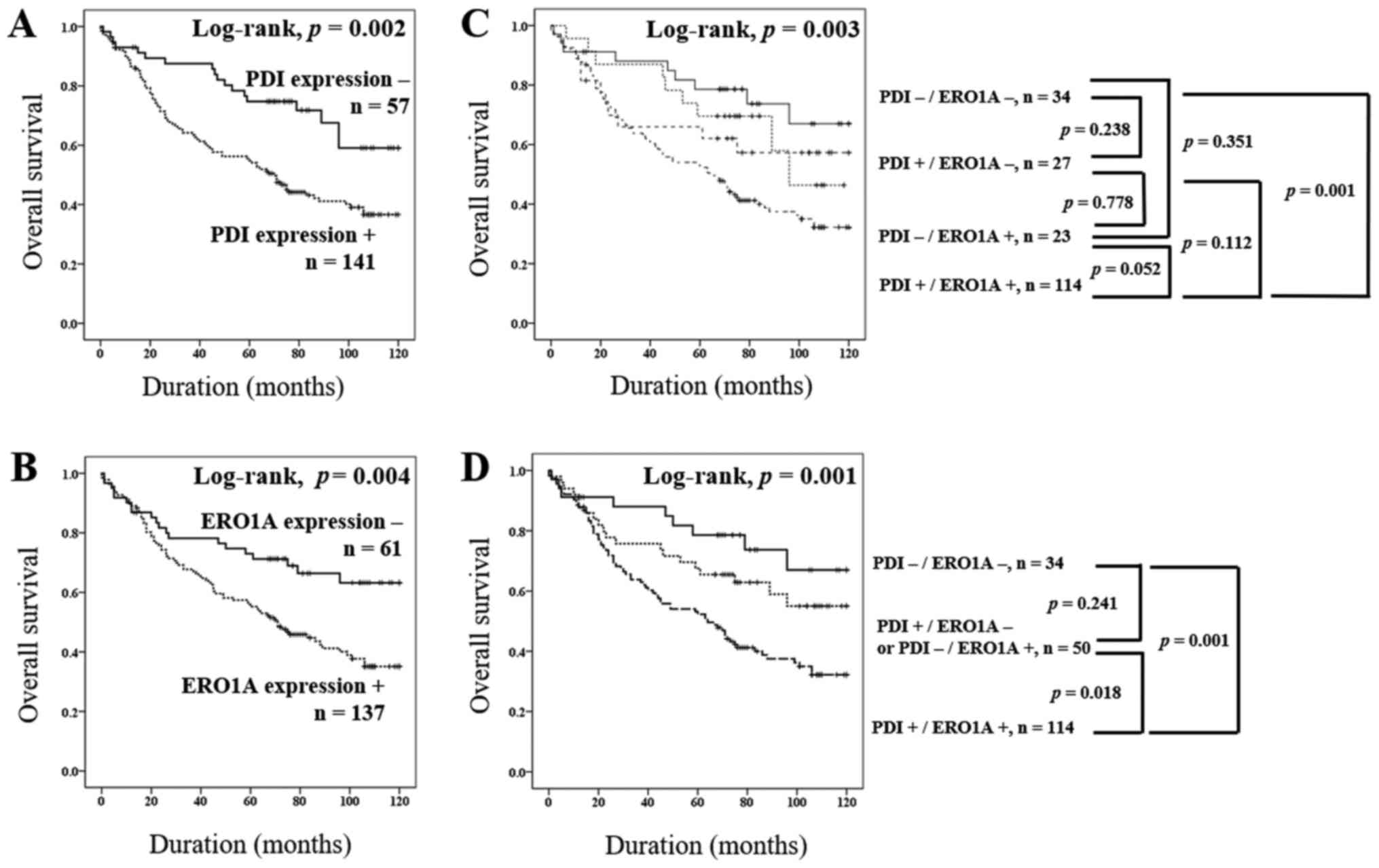
OS for each marker are presented in Fig.
3.
 | Table II.Univariate Cox regression analysis
for the overall survival of patients with non-small cell lung
cancer. |
Table II.
Univariate Cox regression analysis
for the overall survival of patients with non-small cell lung
cancer.
| Variables | Risk ratio | 95% confidence
interval | P-value |
|---|
| Sex, female (vs.
male) | 0.634 | 0.389–1.031 | 0.066 |
| Age, >65 (vs.
≤65) | 1.436 | 0.974–2.118 | 0.068 |
| Smoking history,
smoker (vs. never smoker) | 1.402 | 0.955–2.059 | 0.085 |
| Histologic grade,
poor (vs. well or moderate) | 0.607 | 0.539–1.436 | 0.607 |
| T stage, T 3,4 (vs.
T 1,2) | 1.630 | 1.062–2.503 | 0.025 |
| N stage, N 1,2 (vs.
N 0) | 1.203 | 0.802–1.805 | 0.372 |
| PDI expression,
positive (vs. negative) | 2.215 | 1.348–3.640 | 0.002 |
| ERO1A expression,
positive (vs. negative) | 1.932 | 1.208–3.090 | 0.006 |
| PDI/ERO1A
expression pattern, −/− | 1 |
|
|
| −/+ or
+/− | 1.634 | 0.767–3.480 | 0.203 |
|
+/+ | 2.798 | 1.445–5.417 | 0.002 |
| PDI/ERO1A
expression pattern, -/+ or +/- | 1 |
|
|
|
+/+ | 1.702 | 1.047–2.767 | 0.032 |
Due to a significant positive correlation between
PDI and ERO1A expressions in NSCLC (Table
I), we wonder whether the prediction of NSCLC prognosis was
more accurate based on the combined expression of PDI and ERO1A
than with the expression of either alone. First, we grouped NSCLC
into four-groups according to the expression types of PDI and ERO1A
(PDI-/ERO1A-, PDI+/ERO1A-, PDI-/ERO1A+, and PDI+/ERO1A+) and
performed a Kaplan-Meier survival analysis (Fig. 3C). Based on these results, we
re-grouped the PDI/ERO1A expression pattern into three sub-groups
(PDI-/ERO1A-, PDI+/ERO1A- or PDI-/ERO1A+, and PDI+/ERO1A+). We then
compared the OS between the three groups by univariate analysis and
Kaplan-Meier survival analysis. The type of co-expression pattern
(PDI+/ERO1A+) was significantly associated with shorter OS (overall
P=0.002) by univariate analysis (Table
II). The patients with PDI+/ERO1A+ had the poorest prognoses,
and the patients with PDI-/ERO1A- had the best (Table II and Fig.
3C). The prognosis of ‘PDI+/ERO1A- or PDI-/ERO1A+’ sub-group
was intermediate to the PDI-/ERO1A- and PDI+/ERO1A+ sub-groups
(Fig. 3D). The patients with
PDI+/ERO1A- or PDI-/ERO1A+ pattern showed a better prognosis than
did those with PDI+/ERO1A+ (P=0.032; Table II). However, there was no significant
difference in OS between PDI-/ERO1A- and PDI+/ERO1A- or PDI-/ERO1A+
(P=0.203).
Multivariate analysis revealed that
individual PDI expression and co-expression of PDI and ERO1A are
independent poor prognostic indicators for NSCLC patients
We included in the multivariate analysis the factors
that were significant or borderline significant (P<0.1) in
univariate analysis. We determined current or former smoker, higher
tumor stage, and PDI expression to be independent poor prognostic
factors for OS in NSCLC patients. Patients with PDI expression had
2.33 times greater risk of death from NSCLC (95% CI: 1.322–4.105,
P=0.003; model 1, Table III).
Additionally, we inserted the combined PDI/ERO1A expression pattern
instead of individual expression of PDI and ERO1A in multivariate
analysis. Current or former smoker, higher tumor stage, and
co-expression of PDI+/ERO1A+ were independent poor prognostic
indicators for OS in NSCLC patients, patients with co-expression of
PDI+/ERO1A+ showed a 3.517 times greater risk of death from NSCLC
(95% CI: 1.736–7.122, P<0.001) than did the patients who showed
PDI-/ERO1A- expression pattern (model 2, Table III).
 | Table III.Multivariate Cox regression analysis
for the overall survival of patients with non-small cell lung
cancer. |
Table III.
Multivariate Cox regression analysis
for the overall survival of patients with non-small cell lung
cancer.
| Variables | Risk ratio | 95% confidence
interval | P-value |
|---|
| Model
1a |
|
|
|
|
Smoking, smoker (vs. never
smoker) | 1.594 | 1.063–2.388 | 0.024 |
| T
stage, T 3,4 (vs. T 1,2) | 1.862 | 1.194–2.903 | 0.006 |
| PDI
expression, positive (vs. negative) | 2.33 | 1.322–4.105 | 0.003 |
| Model 2b |
|
|
|
|
Smoking, smoker (vs. never
smoker) | 1.536 | 1.031–2.288 | 0.035 |
| T
stage, T 3,4 (vs. T 1,2) | 1.831 | 1.174–2.854 | 0.008 |
| PDI/ERO1A
expression, −/− | 1 |
| <0.001 |
| −/+ or
+/− | 1.81 | 0.82–3.999 | 0.142 |
|
+/+ | 3.517 | 1.736–7.122 | <0.001 |
Discussion
In the present study, we investigated
immunohistochemical expression of PDI and ERO1A in human NSCLC
tissues. To the best of our knowledge, this is the first study of
the correlative expression of PDI and ERO1A in NSCLC. Our results
show: i) expression of PDI and ERO1A in 71.2 and 69.2% of NSCLC,
respectively; ii) significant positive correlation (P<0.001) for
expression of PDI and ERO1A; iii) significant association of
individual and co-expression PDI and ERO1A with shorter OS in
univariate and Kaplan-Meier survival analysis; and iv) poor
independent prognosis value of individual PDI expression and
PDI+/ERO1A+ co-expression for OS in NSCLC patients in multivariate
analysis.
PDI is a 57-kDa sized molecular chaperone and one of
the most abundant proteins in the ER (9). The main function of PDI is to catalyze
disulfide bond formation, breakage, and rearrangement of its
protein and peptide substrates. Disulfide bonds are formed between
the sulfhydryl cysteine groups and are involved in the
stabilization of the protein tertiary structure which is essential
for the proteins' function (10).
Therefore, PDI dysfunction results in accumulation of unfolded and
misfolded proteins in the ER. Aberrant PDI expression or function
is reported to be involved in many human diseases such as
neurodegenerative (24) and
cardiovascular diseases (25) and
also in cancer (10).
PDI expression was significantly higher in various
cancer types including lung cancer (10) and was associated with adverse clinical
outcomes in various cancer types (11–14).
Functional studies also indicate that PDI is involved in cancer
progression and cancer cell survival (26–28). There
are several proposed mechanisms to explain the relevance of PDI
over-expression and adverse clinical outcomes. First, PDI
over-expression may protect cancer cells from apoptosis. Inhibiting
PDI increased chemotherapy-induced apoptosis in melanoma (26). A PDI inhibitor induced apoptosis in
HCC cells and ovarian cancer cells (10). Second, PDI is suggested to be
associated with neutralization of the transforming growth factor-β1
(TGF-β1) effect. TGF-β1 is a strong cell proliferation inhibitor
and frequently inhibits cancer growth (27). Lastly, PDI can affect cancer invasion
and metastasis. Matrix metallopeptidase 9 (MMP-9) is an important
protein for cancer cell metastasis (28). PDI-mediated disulfide bond formation
is essential for MMP secretion and gelatinolytic activity. Our
result showed that PDI expression was associated with adverse OS in
NSCLC patients on univariate and Kaplan-Meier survival analysis and
was an independent poor prognostic indicator in NSCLC patients on
multivariate Cox regression analysis. Our results do not explain
the mechanism of the relationship between PDI over-expression and
short OS. In previous reports, however, it was suggested that PDI
protects tumor cells from oxidative damage in (10). Therefore, we assume that cancer cells
with PDI over-expression can be protected from apoptosis and
survival can be promoted, which may explain the relationship
between PDI over-expression and poor prognosis. Additional studies
are needed to clarify the role of PDI over-expression in poor NSCLC
prognosis.
Exposure to CS is a major risk factor for the
development of lung cancer. A previous study reported that PDI was
up-regulated in smokers, and it is a primary ER-resident target of
smoking-induced oxidation (29).
However, another study showed that smoking-induced radicals can
lead to PDI unfolding, inhibiting PDI activity (30). We wondered how smoking status in
patients with NSCLC might be related to PDI expression. Therefore,
we evaluated the relationship between smoking history and PDI
expression, and expression was significantly higher in never
smokers than in former or current smokers (P=0.003). In this study,
we cannot explain the mechanism for the relationship between
non-smokers and high PDI expression, but based on the results of
other researchers, we have inferred some possibilities. CS exposure
leads to post-translational modifications and changes in the 3D
structure of PDI and then render it inactive (30). Therefore, PDI over-expression may be
less relevant to the pathogenesis of NSCLC in smokers given that
smoking can impair PDI generation and function. Other investigators
reported that UPR-related proteins (eIF2α, phospho-eIF2α, and BiP)
were increased in NSCLC but not directly related to smoking
exposure, and these were the common traits observed in lung cancer,
regardless of the triggering factors (31). We believe that the association between
never smoking and PDI over-expression in our study should be
understood through additional studies.
ERO1 is one of the essential proteins for protein
disulfide bond formation in the ER in cooperation with PDI. While
PDI plays a role in protein folding and isomerization, it takes a
reduced form and is then re-oxidized so regulators can oxidize PDI
efficiently. ERO1A is known as a major regulator in PDI oxidation.
Recent reports have suggested that ERO1A was over-expressed in
cancer and had an oncogenic role in cancer (22–25). ERO1A
knock-down suppressed the proliferation, migration, invasiveness,
and chemoresistance in gastric cancer cells through controlling the
phosphorylation state of Akt and JNK. In breast cancer cells, ERO1A
promoted immune escape by increasing PD-L1 expression (20). Additionally, ERO1A can play an
important role in vascular endothelial growth factor secretion and
contributes the neovascular formation of cancer (32). ERO1A over-expression was associated
with poor OS among lung adenocarcinoma patients (33). In line with previous reports, our
results also show that NSCLC patients with positive ERO1A
expression had significantly shorter OS than did patients with
negative ERO1A expression. However, more studies are needed to
identify the mechanisms that explain the association between ERO1A
over-expression and poor prognosis in NSCLC.
Many functional studies have shown that ERO1A is an
important regulator of PDI and that the dysregulation of these two
proteins is involved in the progression of cancer. However, to our
best knowledge, no study authors to date have investigated the
correlative role of PDI and ERO1A expression using clinical cancer
samples. Our data revealed that the expression of PDI and ERO1A
were very significantly (P<0.001) related. The patient group
with co-expression of PDI+/ERO1A+ showed the shortest OS than did
the PDI-/ERO1A- group in Kaplan Meier and univariate survival
analysis (P<0.05). The multivariate analysis revealed that
PDI+/ERO1A+ were an independent poor prognostic indicator of OS in
NSCLC patients. Our results support the previous findings that the
PDI and ERO1A are closely related with each other functionally and
that the co-expression of PDI and ERO1A can be utilized as a
stronger prognostic indicator than the single expression of either
alone. However, little is known about the prognostic effect of the
combined expression of PDI and ERO1A in cancer patients; given our
small sample, a definitive conclusion on the prognosis of NSCLC
patients would be premature. In order for PDI and/or ERO1A
expression to be used as an important prognostic indicator for
patients with NSCLC, we believe that confirmation is necessary
through large-scale studies and accumulated results. Modalities of
treatment also affect the OS in NSCLC patients. However, the
information on the postoperative treatment of the patients was not
included in the OS analysis in the present study and we think it is
a limitation of this study. Additional studies supplementing this
may be helpful in confirming the role as a prognostic marker of PDI
and/or ERO1A expression.
In conclusion, this study has demonstrated that the
immunohistochemical expression of PDI and ERO1A might be helpful
for predicting the prognosis of NSCLC patients.
Acknowledgements
The biospecimens for this study were provided by the
Chonbuk National University Hospital Biobank, a member of the Korea
Biobank Network, which is supported by the Ministry of Health,
Welfare and Family Affairs, Republic of Korea. All samples derived
from the National Biobank of Korea were obtained with informed
consent under institutional review board-approved protocols.
Funding
The present study was supported by the Biomedical
Research Institute, Chonbuk National University Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KMK, HSP, KYJ, WSM, MJK, YCL, JHK and MJC
participated in the study design. KMK, ARA, HSP, KYJ, WSM, MJK and
MJC performed the experiments and interpreted the patient data from
immunohistochemistry. YCL and JHK organized and analyzed the
patient's clinical data. ARA, KMK, HSP, KYJ, WSM, MJK, YCL, JHK and
MJC were involved in drafting the manuscript. KMK, ARA, HSP, KYJ,
WSM, MJK, YCL, JHK and MJC revised the manuscript critically for
important intellectual content and provided final approval of the
version to be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
Review Board of Chonbuk National University Hospital (approval no.
CUH 2016-05-003-001) and was performed according to the Declaration
of Helsinki. Based on the retrospective and anonymous character of
the study the approval contained a waiver for written informed
consent.
Patient consent for publication
Based on the retrospective, anonymous character of
the study, the IRB approval contained a waiver for written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee AS and Hendershot LM: ER stress and
cancer. Cancer Biol Ther. 5:721–722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoseki J, Ushioda R and Nagata K:
Mechanism and components of endoplasmic reticulum-associated
degradation. J Biochem. 147:19–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alasiri G, Fan LY, Zona S, Goldsbrough IG,
Ke HL, Auner HW and Lam EW: ER stress and cancer: The FOXO forkhead
transcription factor link. Mol Cell Endocrinol. 462:67–81. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rashid HO, Yadav RK, Kim HR and Chae HJ:
ER stress: Autophagy induction, inhibition and selection.
Autophagy. 11:1956–1977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida H: ER stress and diseases. FEBS J.
274:630–658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hosoi T and Ozawa K: Endoplasmic reticulum
stress in disease: Mechanisms and therapeutic opportunities. Clin
Sci (Lond). 118:19–29. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozcan U, Ozcan L, Yilmaz E, Düvel K, Sahin
M, Manning BD and Hotamisligil GS: Loss of the tuberous sclerosis
complex tumor suppressors triggers the unfolded protein response to
regulate insulin signaling and apoptosis. Mol Cell. 29:541–551.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim KM, Yu TK, Chu HH, Park HS, Jang KY,
Moon WS, Kang MJ, Lee DG, Kim MH, Lee JH and Chung MJ: Expression
of ER stress and autophagy-related molecules in human non-small
cell lung cancer and premalignant lesions. Int J Cancer.
131:E362–E370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferrari DM and Söling HD: The protein
disulphide-isomerase family: Unravelling a string of folds. Biochem
J. 339:1–10. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu S, Sankar S and Neamati N: Protein
disulfide isomerase: A promising target for cancer therapy. Drug
Discov Today. 19:222–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cancer Genome Atlas Research Network, .
Comprehensive genomic characterization defines human glioblastoma
genes and core pathways. Nature. 455:1061–1068. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shai R, Shi T, Kremen TJ, Horvath S, Liau
LM, Cloughesy TF, Mischel PS and Nelson SF: Gene expression
profiling identifies molecular subtypes of gliomas. Oncogene.
22:4918–4923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thongwatchara P, Promwikorn W, Srisomsap
C, Chokchaichamnankit D, Boonyaphiphat P and Thongsuksai P:
Differential protein expression in primary breast cancer and
matched axillary node metastasis. Oncol Rep. 26:185–191.
2011.PubMed/NCBI
|
|
14
|
Xia W, Zhuang J, Wang G, Ni J, Wang J and
Ye Y: P4HB promotes HCC tumorigenesis through downregulation of
GRP78 and subsequent upregulation of epithelial-to-mesenchymal
transition. Oncotarget. 8:8512–8521. 2017.PubMed/NCBI
|
|
15
|
Sevier CS and Kaiser CA: Ero1 and redox
homeostasis in the endoplasmic reticulum. Biochim Biophys Acta.
1783:549–556. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Araki K and Nagata K: Functional in vitro
analysis of the ERO1 protein and protein-disulfide isomerase
pathway. J Biol Chem. 286:32705–32712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou B, Wang G, Gao S, Chen Y, Jin C, Wang
Z, Yang Y, Ma Z, Zhang W and Feng X: Expression of ERO1L in gastric
cancer and its association with patient prognosis. Exp Ther Med.
14:2298–2302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Seol SY, Kim C, Lim JY, Yoon SO, Hong SW,
Kim JW, Choi SH and Cho JY: Overexpression of endoplasmic reticulum
oxidoreductin 1-α (ERO1L) is associated with poor prognosis of
gastric cancer. Cancer Res Treat. 48:1196–1209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kutomi G, Tamura Y, Tanaka T, Kajiwara T,
Kukita K, Ohmura T, Shima H, Takamaru T, Satomi F, Suzuki Y, et al:
Human endoplasmic reticulum oxidoreductin 1-α is a novel predictor
for poor prognosis of breast cancer. Cancer Sci. 104:1091–1096.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tanaka T, Kutomi G, Kajiwara T, Kukita K,
Kochin V, Kanaseki T, Tsukahara T, Hirohashi Y, Torigoe T, Okamoto
Y, et al: Cancer-associated oxidoreductase ERO1-α promotes immune
escape through up-regulation of PD-L1 in human breast cancer.
Oncotarget. 8:24706–24718. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. 8th. Springer
International Publishing; New York, NY: 2017, View Article : Google Scholar
|
|
22
|
Travis WD, Brambilla E, Burke A, Marx A
and Nicholson AG: WHO classification of tumours of the lung,
pleura, thymus and heart. 4th. IARC press; Lyon: 2015
|
|
23
|
Schoenborn CA and Adams PE: Health
behaviors of adults: United States, 2005–2007. Vital Health Stat.
10:1–132. 2010.
|
|
24
|
Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z,
Ma Y, Masliah E, Nomura Y and Lipton SA: S-nitrosylated
protein-disulphide isomerase links protein misfolding to
neurodegeneration. Nature. 441:513–517. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Severino A, Campioni M, Straino S, Salloum
FN, Schmidt N, Herbrand U, Frede S, Toietta G, Di Rocco G, Bussani
R, et al: Identification of protein disulfide isomerase as a
cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll
Cardiol. 50:1029–1037. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lovat PE, Corazzari M, Armstrong JL,
Martin S, Pagliarini V, Hill D, Brown AM, Piacentini M,
Birch-Machin MA and Redfern CP: Increasing melanoma cell death
using inhibitors of protein disulfide isomerases to abrogate
survival responses to endoplasmic reticulum stress. Cancer Res.
68:5363–5369. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sipes NJ, Miller DA, Bascom CC, Winkler
JK, Matrisian LM and Moses HL: Altered regulation of protein
disulfide isomerase in cells resistant to the growth-inhibitory
effects of transforming growth factor beta 1. Cell Growth Differ.
1:241–246. 1990.PubMed/NCBI
|
|
28
|
Khan MM, Simizu S, Suzuki T, Masuda A,
Kawatani M, Muroi M, Dohmae N and Osada H: Protein disulfide
isomerase-mediated disulfide bonds regulate the gelatinolytic
activity and secretion of matrix metalloproteinase-9. Exp Cell Res.
318:904–914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kenche H, Ye ZW, Vedagiri K, Richards DM,
Gao XH, Tew KD, Townsend DM and Blumental-Perry A: Adverse outcomes
associated with cigarette smoke radicals related to damage to
protein-disulfide isomerase. J Biol Chem. 291:4763–4778. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kenche H, Baty CJ, Vedagiri K, Shapiro SD
and Blumental-Perry A: Cigarette smoking affects oxidative protein
folding in endoplasmic reticulum by modifying protein disulfide
isomerase. FASEB J. 27:965–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jorgensen E, Stinson A, Shan L, Yang J,
Gietl D and Albino AP: Cigarette smoke induces endoplasmic
reticulum stress and the unfolded protein response in normal and
malignant human lung cells. BMC Cancer. 8:2292008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
May D, Itin A, Gal O, Kalinski H,
Feinstein E and Keshet E: Ero1-L alpha plays a key role in a
HIF-1-mediated pathway to improve disulfide bond formation and VEGF
secretion under hypoxia: Implication for cancer. Oncogene.
24:1011–1020. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsu CH, Hsu CW, Hsueh C, Wang CL, Wu YC,
Wu CC, Liu CC, Yu JS, Chang YS and Yu CJ: Identification and
characterization of potential biomarkers by quantitative tissue
proteomics of primary lung adenocarcinoma. Mol Cell Proteomics.
15:2396–2410. 2016. View Article : Google Scholar : PubMed/NCBI
|