Introduction
Of the worldwide population of patients with gastric
cancer (GC), ~42% of the men and 19% of the women are Chinese, and
the number of patients suffering from GC has increased in recent
years (1,2). Although significant progress has been
made in the comprehensive treatment with surgery, the prognosis for
postoperative patients with GC remains poor (3). A detailed understanding of GC occurrence
and development, and the factors that influence its prognosis are
required.
The barrier-to-autointegration factor 1 (BANF1)
family of proteins has a variety of functions associated with the
maintenance of the intact cellular genome (4). BANF1 is an ~10-kDa, highly conserved
DNA-binding protein that forms homodimers. It was first identified
during the combination of a retrovirus and host, promoting the
fusion of retroviruses and target genes in vitro (5). As an important part of the lamina, BANF1
has essential interactions with numerous cellular proteins,
including transcription factors and DNA damage repair proteins
(6,7).
It regulates gene expression, participates in the formation of
karyotin structures and is associated with cell mitosis (8).
In recent years, with the advent of microarray
technology, novel ideas and insights into the diagnosis and
treatment of tumors have been proposed (9). To understand the effect of BANF1 on GC
progression, its mRNA and protein expression levels in the gastric
tumor tissue were compared with that in adjacent non-tumorous
tissue. BANF1 expression was demonstrated to be upregulated in
patients with GC. In addition, a high expression of BANF1 was
identified as a poor prognostic factor. Furthermore, BANF1
expression correlated with patient age, tumor differentiation and
infiltration depth. Therefore, BANF1 may be an oncogene. The
results of the present study may aid in developing a reference in
prognostic evaluation of patients with GC.
Materials and methods
Data source
The microarray profiles of GC were extracted from
the Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) database under the
accession number of GSE54129. A total of 132 specimens, including
21 gene chips from adjacent non-tumorous tissue and 111 gene chips
from tissues of GC patients, were available for the analysis by
GEO2R. The GEO data was passed through quality control and
homogeneous processing as previously described (9). A total of 876 cases of gastric cancer
used for prognostic analysis were derived from the Kaplan-Meier
Plotter database. Survival analysis was performed using the GC
database with the Kaplan-Meier (K-M) estimator data. For
comparison, the data group (n=876) was segmented into the high
(n=604) and low (n=272) BANF1-expression groups with the median as
the boundary.
Clinical GC specimen collection
Postoperative specimens were collected between June
2012 and January 2013 from The First Affiliated Hospital of Jinzhou
Medical University (Jinzhou, China), which included 23 normal and
118 tumorous tissues. Specimens were placed in 10% neutral buffered
formalin for 24 h at room temperature (RT) and then embedded in a
paraffin wax block for storage. No patients received any other
treatment prior to surgery. Table I
lists the clinicopathological features of all patients. The 8th
edition tumor node metastasis (TNM) staging system of the Union for
International Cancer Control was the standard for tumor staging
(10). The present study was approved
by the Ethics Committees of the First Affiliated Hospital of
Jinzhou Medical University. Written informed consent was obtained
from all patients.
 | Table I.Association between BANF1 expression
and clinicopathological features. |
Table I.
Association between BANF1 expression
and clinicopathological features.
|
|
| BANF1 expression |
|---|
|
|
|
|
|---|
| Clinicopathological
features | No. | − | + | ++ | +++ | PR (%) | P-value |
|---|
| Sex |
|
|
|
|
|
| 0.260 |
| Male | 76 | 3 | 7 | 26 | 40 | 96.1 |
|
|
Female | 42 | 0 | 1 | 16 | 25 | 100.0 |
|
| Age, years |
|
|
|
|
|
| 0.001a |
| ≥60 | 60 | 2 | 6 | 29 | 23 | 96.7 |
|
|
<60 | 58 | 1 | 2 | 13 | 42 | 98.3 |
|
| Tumor size, cm |
|
|
|
|
|
| 0.648 |
| ≥5 | 51 | 1 | 2 | 21 | 27 | 98.0 |
|
|
<5 | 67 | 2 | 6 | 21 | 38 | 97.0 |
|
|
Differentiation |
|
|
|
|
|
| 0.000a |
|
Poor | 57 | 0 | 0 | 3 | 54 | 100.0 |
|
|
Moderate | 50 | 1 | 4 | 37 | 8 | 98.0 |
|
|
Well | 7 | 2 | 4 | 1 | 0 | 71.4 |
|
| Infiltration
depth |
|
|
|
|
|
| 0.011a |
|
T1/T2 | 28 | 2 | 5 | 10 | 11 | 92.9 |
|
|
T3/T4 | 90 | 1 | 3 | 32 | 54 | 98.9 |
|
| Lymph node
metastasis |
|
|
|
|
|
| 0.154 |
|
With | 86 | 3 | 6 | 33 | 44 | 96.5 |
|
|
Without | 32 | 0 | 2 | 9 | 21 | 100.0 |
|
| Distant
metastasis |
|
|
|
|
|
| 0.214 |
| M0 | 116 | 3 | 8 | 42 | 63 | 97.4 |
|
| M1 | 2 | 0 | 0 | 0 | 2 | 100.0 |
|
| TNM staging |
|
|
|
|
|
| 0.207 |
| I and
II | 37 | 2 | 6 | 10 | 19 | 94.6 |
|
| III and
IV | 81 | 1 | 2 | 32 | 46 | 98.8 |
|
Cell culture
The SGC-7901 and MNK-45 GC cell lines (Hangzhou
Baisi Biotechnology Co., Ltd., Hangzhou, China) were grown in
RPMI-1640 (HyClone; GE Healthcare, Logan, UT, USA) supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin,
at 37°C in 5% CO2.
Western blot analysis
Cells were lysed in RIPA lysis buffer (cat. no.
WLA016a; Wanleibio Co., Ltd., Shenyang, China). Protein
quantification was performed using the Coomassie Brilliant Blue
method. Subsequently, 30 µg protein/lane were separated by 12%
SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The
membrane was blocked with 5% skim milk in Tris-buffered saline with
Tween 20 (TBST, 10 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20) for 1 h
at RT, and then incubated with the rabbit monoclonal anti-BANF1
(cat. no. McAb129184; 1:500; Abcam, Cambridge, UK) and rabbit
anti-GAPDH (cat. no. WL01114; 1:2,000; Wanleibio Co., Ltd)
antibodies overnight at 4°C. The membranes were washed with TBST
and incubated with the goat anti-rabbit IgG secondary antibody
(cat. no. WLA023a; 1:5,000; Wanleibio Co., Ltd. Shenyang, China).
The bands were visualized with a LAS4010 imager (GE Healthcare Life
Sciences, Little Chalfont, UK) using ECL-Plus detection reagent
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA). The
densitometric quantification of protein bands was performed with
GAPDH as a control using ImageJ software version 1.8.0 (National
Institutes of Health, Bethesda, MD, USA). To validate the
specificity of anti-BANF1 antibodies, rabbit
anti-BANF1-peptide-specific antibodies (cat. no. 4019P; 1:100;
ProSci Inc., Poway, CA, USA) were used against the 15 amino acids
near the carboxy-terminus of human BANF1. After the diluted
anti-BANF1 antibodies were preincubated with (+) and without (−)
0.5 mM anti-BANF1-peptide-specific antibodies for 2 h at RT, they
were used to determine BANF1 expression in SGC-7901 and MNK-45 GC
cells (11). The aforementioned
western blotting protocol was performed in the same way following
this incubation.
Immunohistochemistry
Tissue specimens were cut into successive 4 µm-thick
sections. Then, antigen retrieval was performed by heating the
specimens using the pressure cooker antigen repairing method at
120°C, followed by washing with xylene and gradual rehydration with
a descending ethanol series. Endogenous peroxidase activity in the
specimens was neutralized via incubation with 3% hydrogen peroxide
for 5 min at room temperature. Subsequently, the specimens were
incubated in 5% bovine serum albumin (cat. no. A8020; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) for 30
min at RT to block nonspecific binding. Then, the tissues were
incubated with rabbit monoclonal anti-BANF1 antibody (1:100)
overnight at 4°C. Following incubation with the horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibodies
(cat. no. WLA023a; 1:500; Wanleibio Co., Ltd.) for 1 h at RT, all
sections were visualized using 3,3′-diaminobenzidine, and the
slides were counterstained with haematoxylin (cat. no. WLA051a;
Wanleibio Co., Ltd.) for 1 min at RT (1,2). The
coverslips were then mounted with anti-fade mounting medium and
observed under a microscope (magnification ×200; Olympus DP73;
Japan).
Staining intensity score
The BANF1 expression-intensity scores were
determined by two independent observers blinded to the
clinicopathological data of patients. In order to calculate the
percentage of BANF1-positive cells, 100 cells were randomly
selected and counted in five representative fields of each section.
The positive expression area was counted as follows: <5%, score
of 0; <30%, score of 1; 30–70%, score of 2; and >70%, score
of 3. The staining intensity score was graded as follows:
Colourless, 0; weak, 1; intermediate, 2; and strong, 3. The
positive expression area and staining intensity scores were
multiplied (2).
Statistical analysis
All data were analysed with the SPSS 20.0 software
program (IBM Corp., Armonk, NY, USA). Results were expressed as the
mean ± standard deviation. Student's t-tests were performed to
evaluate the differences in BANF1 mRNA expression between the
gastric tumor and normal tissues. The Spearman's rank correlation
coefficient test was used to analyze the correlation between BANF1
expression and clinicopathological features. The Mann-Whitney
U-test was used to calculate the statistical significance. Survival
curves were derived using the K-M estimator method and compared
using the log-rank test. Cox's proportional hazards model was used
to analyze the effect of clinicopathological parameters on patient
survival. P<0.05 was considered to indicate a statistically
significant difference.
Results
High expression of BANF1 mRNA in GC
tissues
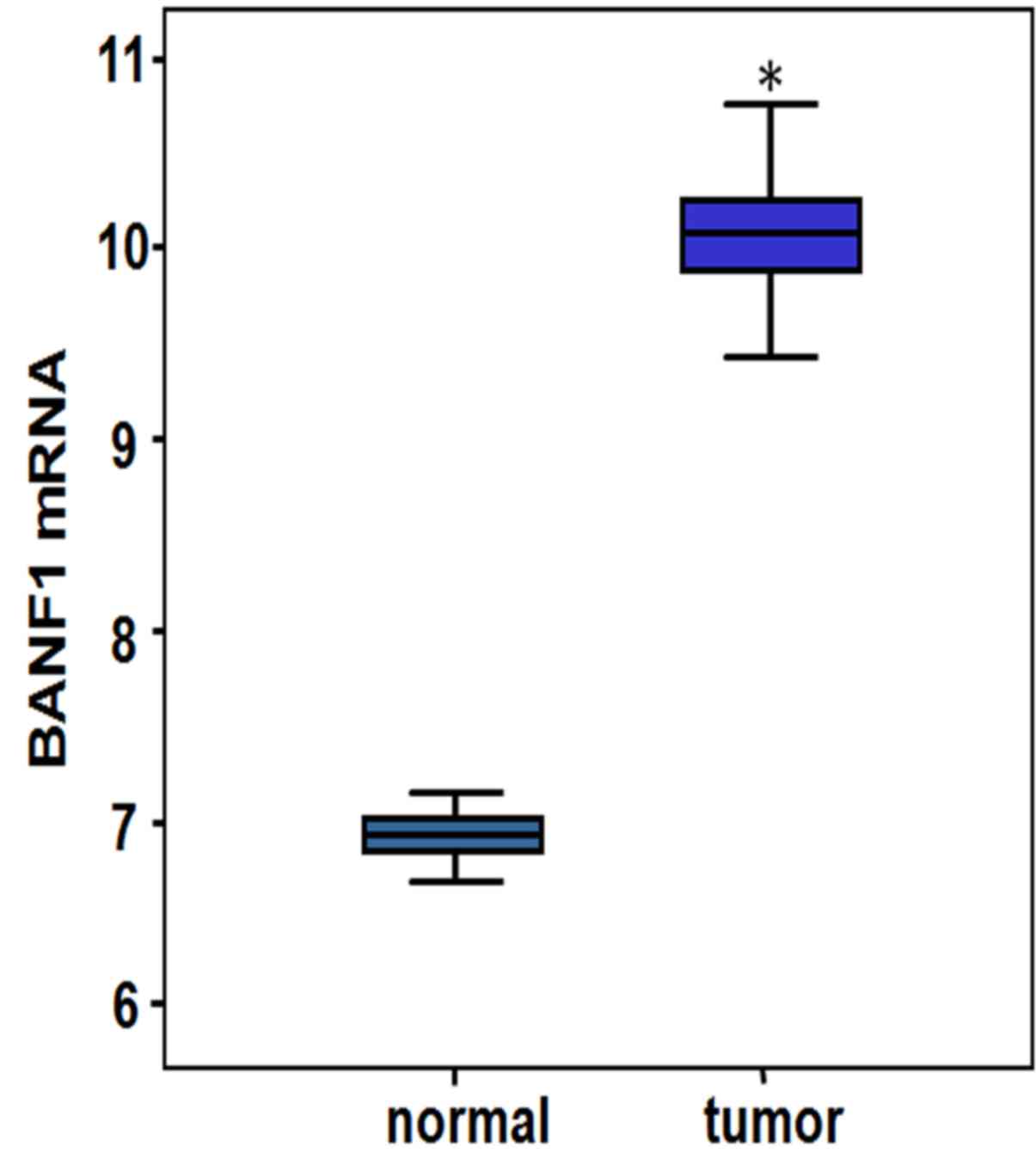
In the GSE54129 data sets, BANF1 expression in the
gastric tumor tissues was identified to be significantly higher
compared with in the adjacent non-tumorous tissues (Fig. 1), as demonstrated by statistical
parameters for the BANF1 gene expression profiles in Table II (P=1.50×10−87).
 | Table II.Statistical parameters of BANF1 gene
expression profiles. |
Table II.
Statistical parameters of BANF1 gene
expression profiles.
| Gene name | ID | Normal mean | Tumor mean | logFC | t | P-value | Adjust P-value |
|---|
| BANF1 | 210125_s_at | 6.93204 | 10.05825 | 3.126 | 49.7 |
1.50×10-87a |
8.20×10-83a |
Expression of BANF1 in GC cells
After the diluted anti-BANF1 antibodies were
preincubated with the anti-BANF1-peptide-specific antibodies, the
latter were used for western blotting. Examination of total
extracts of SGC-7901 and MNK-45 GC cells by immunoblotting revealed
that the antibodies specifically recognized a 10-kDa protein
(Fig. 2, lanes 1 and 2), which was
not present at the same positions in the lanes with
anti-BANF1-peptide-specific antibody preincubation (Fig. 2, lanes 3 and 4). This indicates that
BANF1 protein is expressed in SGC-7901 and MNK-45 GC cells and the
anti-BANF1 antibody can specifically recognize the 10 kDa BANF1
protein.
Difference in BANF1 protein expression
between the normal and gastric tumor tissues
The intensity of BANF1 expression in the nucleus of
GC cells varied (Fig. 3). The total
of 118 GC tissues included three cases of negative BANF1
expression, eight weakly positive (+) samples, 42 moderately
positive (++) samples and 65 strongly positive (+++) samples. By
contrast, negative (−) and weakly positive (+) BANF1 staining was
detected in 18 samples and five samples of 23 normal tissues,
respectively, while no moderately positive or strongly positive
staining was detected. The protein expression of BANF1 in GC
specimens was significantly higher compared with that in normal
tissues (P<0.001; Table
III).
 | Table III.BANF1 expression in gastric
cancer. |
Table III.
BANF1 expression in gastric
cancer.
|
|
| BANF1
expression |
|---|
|
|
|
|
|---|
| Group | No. | − | + | ++ | +++ | PR (%) | P-value |
|---|
| Normal | 23 | 18 | 5 | 0 | 0 | 21.7 |
<0.001a |
| Cancer | 118 | 3 | 8 | 42 | 65 | 97.5 |
|
BANF1 expression is associated with
patient age, and the degree of GC differentiation and depth of
invasion
The immunohistochemistry results are summarized in
Table I. The rates of positive BANF1
expression in GC tissues with low, medium and high differentiation
statuses were 100, 98 and 71.4%, respectively. The BANF1 expression
in poorly differentiated GC tissues was significantly higher
compared with that in moderately and highly differentiated tissues,
and was associated with the differentiation degree of tumors
(P<0.001). In addition, BANF1 expression correlated with the
patient age (P=0.001) and tumor infiltration depth (P=0.011).
Effect of BANF1 expression on the
overall survival (OS) of postoperative patients
Online survival analysis was performed using the GC
database in K-M Plotter, which revealed that the survival times for
patients with high BANF1 mRNA expression was significantly reduced
compared with that for the low-expression patients [log-rank test,
P<0.001; hazard ratio (HR), 2.37; 95% confidence interval (CI),
1.93–2.92; Fig. 4A]. Subsequently,
the K-M estimator method was used to analyze the effect of BANF1
expression on the postoperative overall survival (OS) time of
patients, the follow-up period was 150 months, and the mortality
was considered as the end point during the follow-up period. The
survival curve suggested that the OS of the patients with low BANF1
expression was significantly increased compared with the OS of high
expression patients (log-rank test, P<0.001; Fig. 4B). Thus, the level of BANF1 expression
may be used as a prognostic indicate for patients with GC. Next,
the age, the TNM stage and other clinical features were
incorporated into the Cox regression model. It was revealed that
the tumor differentiation status and the TNM stage were independent
factors affecting the OS of patients with GC (Table IV).
 | Table IV.Cox multivariate regression analysis
of the association between the clinical factors and overall
survival. |
Table IV.
Cox multivariate regression analysis
of the association between the clinical factors and overall
survival.
| Clinicopathological
factors | Relative risk (95%
CI) | P-value |
|---|
| BANF1 | 0.686
(0.305–1.542) | 0.362 |
|
Negative (−) |
|
|
|
Positive (+) |
|
|
| Age, years | 0.749
(0.457–1.227) | 0.25 |
|
≥60 |
|
|
|
<60 |
|
|
| Sex | 0.899
(0.550–1.470) | 0.672 |
|
Male |
|
|
|
Female |
|
|
| Tumor size, cm | 1.578
(0.948–2.628) | 0.079 |
| ≥5 |
|
|
|
<5 |
|
|
|
Differentiation |
|
|
|
Poor |
|
|
|
Moderate | 7.495
(1.448–38.809) | 0.016a |
|
Poor | 4.989
(1.129–22.052) | 0.034a |
|
Well |
|
|
| Infiltration
depth | 0.872
(0.402–1.889) | 0.728 |
|
T1/T2 |
|
|
|
T3/T4 |
|
|
| Lymph node
metastasis | 0.913
(0.479–1.742) | 0.783 |
|
With |
|
|
|
Without |
|
|
| TNM staging | 0.230
(0.109–0.485) |
<0.001a |
| Phase I
and II |
|
|
| Phase
III and IV |
|
|
Discussion
The five-year survival rates of postoperative
patients with GC vary between 15 and 60%, with GC diagnosis and
detection lacking effective biomarkers (1,12). Despite
the significant advances in the understanding of GC pathogenesis,
there is no effective therapy available for GC treatment.
Therefore, the identification of effective biomarkers is required
to significantly improve the early diagnosis rate and prognosis for
patients with GC.
BANF1 encodes a highly conserved BAF protein
consisting of 89 amino acids and binding to double-stranded DNA
with high-affinity. It was discovered due to its involvement in the
integration of retroviral DNA (13).
Regulation of BAF phosphorylation, mediated by cell or virus,
regulates numerous cellular activities, including protein
dimerization, its binding to DNA, and subcellular localization of
the protein (14–16). Additionally, BAF participates in
karyomitosis, karyon assembly, karyoplasm regulation and the DNA
damage response (17). As the first
protein that is recruited to the core area of karyolemma, the role
of BAF in mitosis is important. Once BAF is located at the core of
chromatin, other karyotheca anchoring and membrane-spanning
proteins are recruited to complete the nuclear membrane
reformation, which is a key step in cell mitosis (18,19).
Previous studies have demonstrated that the absence of BAF or the
blockage of its phosphorylation results in abnormal morphological
abnormalities of the caryotheca (13). This causes aberrant nuclear
architecture and disrupts normal cell mitosis process (20). Abnormal mitosis may cause chromosomal
instability, which is considered a characteristic change in
malignant tumor cells (21).
BAF is able to positively or negatively regulate
cell activities, although the specific mechanisms remain unclear.
At the early stages of Caenorhabditis elegans embryogenesis, BAF
regulates seam cell fusion by suppressing the expression of
epithelial fusion failure-1-fused protein and the development of
germ cells (22). It has been
reported that BAF inhibits transcription in vaccinia virus
deficiency in the viral B1 kinase (23,24).
Activation of BANF1 may inhibit skin inflammation in psoriatic
lesions by inactivation of S100A9 and c-Jun, but it may also
promote keratinocyte proliferation (5). A coding mutation of the BANF1 alanine-12
to threonine was reported to be associated with BANF1-protein
instability and abnormalities of the caryotheca, which has been
confirmed as the hereditary fundamental of Néstor-Guillermo
Progeria syndrome (NGPS) (25). The
disruption of the interaction between Prelamin A and BAF may be the
cause of NGPS (26). In addition,
certain studies have reported that BANF1 expression is increased in
breast and oesophageal cancer, and is associated with the poor
prognosis of patients (27,28). Furthermore, its role is essential in
regulating the S-phase process, stem cell self-renewal, and
differentiation and proliferation of keratinocytes (5,29).
Targeted therapy and individualized tumor treatment
are directed against specific gene mutations and proteins. Based on
the notion of tumor therapy treatment, it is essential to identify
suitable oncogenes to develop small molecules or monoclonal
antibodies to control the disease. BANF1 has been demonstrated to
be a target for the treatment of tumors with a specialized
inhibitor aimed at BAF phosphorylation mediated by vaccinia related
kinase 1, which blocks cell cycle progression in tumor cells by
interfering with the caryomitotic phase (30).
In the present study, the expression level of BANF1
was demonstrated to be significantly increased in GC tissues, and
was inversely correlated with the patient age, tumor
differentiation status and infiltration depth. Furthermore, the
survival curve analysis revealed that the prognosis for patients
with high BANF1 expression was poor at the mRNA and protein levels.
To further investigate the association between the clinical
characteristics and prognosis, Cox regression analysis was
performed. The results suggested that tumor differentiation status
and TNM stage were independent risk factors affecting the OS of
patients with GC. The HR of BANF1 was 0.686 (95% CI, 0.305–1.542;
P=0.362) in the Cox regression analysis, but BANF1 significantly
impacts poor survival of GC in the K-M analysis (log-rank
P<0.001). This indicates that BANF1 has an impact on prognosis
together with other factors, but is not an independent prognostic
factor by itself. A limitation of the present study was that the
BANF1 protein levels in the tissues from patients with GC were only
detected by immunohistochemistry, and that western blot analysis is
required to support the immunohistochemistry results.
In summary, BANF1 expression was upregulated in
clinical GC tissues, and was associated with the tumor age,
infiltration depth and differentiation. Additionally, the results
of the present study confirmed that BANF1 closely correlates with
adverse outcomes in GC patients undergoing surgery. Taken together,
the present study is the first to propose that high BANF1
expression may be used as a novel indicator of poor prognosis or as
a therapeutic target for patients with GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Liaoning
Province Science and Technology Project (grant no.
2010010280-401).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available in the GEO repository (http://www.ncbi.nlm.nih.gov/geo/) and the
Kaplan-Meier Plotter database (http://kmplot.com/analysis/index.php?p=service&cancer=gastric).
Authors' contributions
JL, BH, LF, YG, SS and HH performed the experiments
and analyzed the data. JL, LF, XL and CY designed the study and
co-wrote the manuscript. JL, YG, LF and HH were involved in
revising the manuscript. All the authors have read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of the First Affiliated Hospital of Jinzhou Medical
University. Written informed consent was obtained from all
patients.
Patient consent for publication
Written informed consent was obtained from all
patients regarding the publication of the data and associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the human
development index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu D, Zhu S, Li J, Ji G, Wang W, Wu G and
Zheng J: CD147 expression in human gastric cancer is associated
with tumor recurrence and prognosis. PLoS One. 9:e1010272014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun J, Jiang J, Lu K, Chen Q, Tao D and
Chen Z: Therapeutic potential of ADAM17 modulation in gastric
cancer through regulation of the EGFR and TNF-α signalling
pathways. Mol Cell Biochem. 426:17–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shen Q, Eun JW, Lee K, Kim HS, Yang HD,
Kim SY, Lee EK, Kim T, Kang K, Kim S, et al: Barrier to
autointegration factor 1, procollagen-lysine, 2-oxoglutarate
5-dioxygenase 3, and splicing factor 3b subunit 4 as early-stage
cancer decision markers and drivers of hepatocellular carcinoma.
Hepatology. 67:1360–1377. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takama H, Sugiura K, Ogawa Y, Muro Y and
Akiyama M: Possible roles of barrier-to-autointegration factor 1 in
regulation of keratinocyte differentiation and proliferation. J
Dermatol Sci. 71:100–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mekhail K and Moazed D: The nuclear
envelope in genome organization, expression and stability. Nat Rev
Mol Cell Biol. 11:317–328. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutchison CJ: Lamins: Building blocks or
regulators of gene expression? Nat Rev Mol Cell Biol. 3:848–858.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brachner A, Braun J, Ghodgaonkar M, Castor
D, Zlopasa L, Ehrlich V, Jiricny J, Gotzmann J, Knasmüller S and
Foisner R: The endonuclease Ankle1 requires its LEM and GIY-YIG
motifs for DNA cleavage in vivo. J Cell Sci. 125:1048–1057. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pastorino U: Lung cancer screening. Br J
Cancer. 102:1681–1686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu JY, Peng CW, Yang XJ, Huang CQ and Li
Y: The prognosis role of AJCC/UICC 8th edition staging system in
gastric cancer, a retrospective analysis. Am J Transl Res.
10:292–303. 2018.PubMed/NCBI
|
|
11
|
Furukawa K: LAP2 binding protein 1
(L2BP1/BAF) is a candidate mediator of LAP2-chromatin interaction.
J Cell Sci. 112:2485–2492. 1999.PubMed/NCBI
|
|
12
|
Sotgia F and Lisanti MP: Mitochondrial
biomarkers predict tumor progression and poor overall survival in
gastric cancers: Companion diagnostics for personalized medicine.
Oncotarget. 8:67117–67128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Molitor TP and Traktman P: Depletion of
the protein kinase VRK1 disrupts nuclear envelope morphology and
leads to BAF retention on mitotic chromosomes. Mol Biol Cell.
25:891–903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wiebe MS and Jamin A: The barrier to
autointegration factor: Interlocking antiviral defense with genome
maintenance. J Virol. 90:3806–3809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Snyers L, Erhart R, Laffer S, Pusch O,
Weipoltshammer K and Schöfer C: LEM4/ANKLE-2 deficiency impairs
post-mitotic re-localization of BAF, LAP2α and LaminA to the
nucleus, causes nuclear envelope instability in telophase and leads
to hyperploidy in HeLa cells. Eur J Cell Biol. 97:63–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou W, Ngo T and Gershon PD: An overview
of the vaccinia virus infectome: A survey of the proteins of the
poxvirus-infected cell. J Virol. 86:1487–1499. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samwer M, Schneider MWG, Hoefler R,
Schmalhorst PS, Jude JG, Zuber J and Gerlich DW: DNA cross-bridging
shapes a single nucleus from a set of mitotic chromosomes. Cell.
170:956–972. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haraguchi T, Kojidani T, Koujin T, Shimi
T, Osakada H, Mori C, Yamamoto A and Hiraoka Y: Live cell imaging
and electron microscopy reveal dynamic processes of BAF-directed
nuclear envelope assembly. J Cell Sci. 121:2540–2554. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SH, Lyu HN, Kim YS, Jeon YH, Kim W,
Kim S, Lim JK, Lee HW, Baek NI, Choi KY, et al: Brazilin isolated
from caesalpinia sappan suppresses nuclear envelope reassembly by
inhibiting barrier-to-autointegration factor phosphorylation. J
Pharmacol Exp Ther. 352:175–184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi R, Xu N, Wang G, Ren H, Li S, Lei J,
Lin Q, Wang L, Gu X and Zhang H: The lamin-A/C-LAP2α-BAF1 protein
complex regulates mitotic spindle assembly and positioning. J Cell
Sci. 128:2830–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuang X, Semenova E, Maric D and Craigie
R: Dephosphorylation of barrier-to-autointegration factor by
protein phosphatase 4 and its role in cell mitosis. J Biol Chem.
289:1119–1127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Margalit A, Neufeld E, Feinstein N, Wilson
KL, Podbilewicz B and Gruenbaum Y: Barrier toautointegration factor
blocks premature cell fusion and maintains adult muscle integrity
in C. elegans. J Cell Biol. 178:661–673. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zheng R, Ghirlando R, Lee MS, Mizuuchi K,
Krause M and Craigie R: Barrier-to-autointegration factor (BAF)
bridges DNA in a discrete, higher-order nucleoprotein complex. Proc
Natl Acad Sci USA. 97:8997–9002. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ibrahim N, Wicklund A, Jamin A and Wiebe
MS: Barrier to autointegration factor (BAF) inhibits vaccinia virus
intermediate transcription in the absence of the viral B1 kinase.
Virology. 444:363–373. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paquet N, Box JK, Ashton NW, Suraweera A,
Croft LV, Urquhart AJ, Bolderson E, Zhang SD, O'Byrne KJ and
Richard DJ: Néstor-guillermo progeria syndrome: A biochemical
insight into barrier-to-autointegration factor 1, alanine 12
threonine mutation. BMC Mol Biol. 15:272014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loi M, Cenni V, Duchi S, Squarzoni S,
Lopez-Otin C, Foisner R, Lattanzi G and Capanni C:
Barrier-to-autointegration factor (BAF) involvement in prelamin
A-related chromatin organization changes. Oncotarget.
7:15662–15677. 2015.
|
|
27
|
Li J, Wang T, Pei L, Jing J, Hu W, Sun T
and Liu H: Expression of VRK1 and the downstream gene BANF1 in
esophageal cancer. Biomed Pharmacother. 89:1086–1091. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai TC, Chou HC, Chen YW, Lee TR, Chan HT,
Shen HH, Lee WT, Lin ST, Lu YC, Wu CL and Chan HL: Secretomic and
proteomic analysis of potential breast cancer markers by
two-dimensional differential gel electrophoresis. J Proteome Res.
9:1302–1322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cox JL, Mallanna SK, Ormsbee BD, Desler M,
Wiebe MS and Rizzino A: Banf1 is required to maintain the
self-renewal of both mouse and human embryonic stem cells. J Cell
Sci. 124:2654–2665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim W, Lyu HN, Kwon HS, Kim YS, Lee KH,
Kim DY, Chakraborty G, Choi KY, Yoon HS and Kim KT: Obtusilactone B
from Machilus Thunbergii targets barrier-to-autointegration factor
to treat cancer. Mol Pharmacol. 83:367–376. 2013. View Article : Google Scholar : PubMed/NCBI
|