Introduction
Nasopharyngeal carcinoma (NPC) is a head and neck
cancer with a relatively high rate of malignancy. The tumor cells
in NPC are derived from nasopharynx epithelium, and invade the
surrounding tissues with the tumor growth. Facilitated by the
progress of radiotherapy, the curative effect on NPC has increased.
However, there are certain patients with advanced stages of disease
with a poor prognosis, owing to recurrence or distant metastasis
(1,2).
Therefore, understanding the molecular mechanisms governing NPC
tumor cells proliferation and metastasis will aid in identifying
novel and more effective therapeutic methods.
MicroRNA (miRNA), which is widely expressed in
numerous organisms, is a small non-coding RNA that is 19–25 nt in
length. Usually, miRNA takes part in the post-transcriptional
regulation of gene expression through base-pairing 3′-untranslated
region (UTR) of the target mRNAs (3–5). The
important regulatory role of miRNAs during biological processes
have been revealed in recent decades. Recent studies also
demonstrated that certain miRNAs were aberrantly expressed in
different types of cancer, and served critical roles in
carcinogenesis (6–8), indicating that miRNAs can serve
clinically as an important indicator of cancer developmental stage.
Furthermore, manipulating miRNA expression was an effective
therapeutic strategy for cancer treatment (9–12). miR-141
was first identified to function in prostate cancer (13). Subsequent studies also revealed that
it served critical roles in gastric and ovarian cancer (14,15).
However, no previous studies has focused on miR-141 in NPC.
The present study investigated the expression and
function of miR-141 in NPC, and revealed its significance in early
diagnosis and clinical treatment. It was demonstrated that miR-141
was a tumor repressor and was downregulated in the NPC biopsy
samples. Functional studies revealed that ectopic expression of
miR-141 inhibited NPC tumor cell proliferation and invasion.
Further analysis revealed that BMI1 served as the direct target of
miR-141. This miR-141/BMI1 signaling cascade provided a novel
therapeutic strategy for NPC treatment.
Materials and methods
Ethics statement
The Research Ethics Committee of the Nanshan
Affiliated Hospital of Guangdong Medical College provided ethical
approval for the present study, and all patients provided written
informed consent. All specimens were handled and stored anonymously
according to ethical and legal standards.
Patients and specimens
Tumor samples were obtained from patients (average
age of 51 years, ranging from 22 to 74 years old, consisting of 38
males and 13 females) with pathologically confirmed NPC
(metastatic, n=10; non-metastatic, n=41) or nasopharyngeal mucosa
chronic inflammation (n=4) at the Department of
Otorhinolaryngology, Nanshan Affiliated Hospital of Guangdong
Medical College between June 2015 and August 2016. No patient had
undergone any antitumor therapy prior to sampling. All patients
were staged according to the 7th edition of the American Joint
Committee on Cancer Staging Manual (16).
Cell lines
The human immortalized nasopharyngeal epithelial
NP69 cell line was maintained in Keratinocyte/serum-free medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
bovine pituitary extract (BD Biosciences, Franklin Lakes, NJ, USA).
Human NPC 6-10B, C666, 5-8F and SUNE1 cell lines were cultured in
Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.). For transfection, the cells were seeded
into 6-well plates the day before transfection using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). 150 pM (pmol/l)
miR-ctrl (5′-GGUCUGGGUAGAUCACAAUCUAC-3′) or miR-141 oligo mimics
(5′-UAACACUGUCUGGUAAAGAUGG-3′) was transfected per well. BMI1
coding sequence was cloned into pcDNA3.1 plasmid. 2.5 µg plasmids
were transfected in each well. Approximately 36 h later, the cells
were harvested for the subsequent experiments.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from NPC cell lines using an
EasyPure RNA kit (Beijing Transgen Biotech Co., Ltd., Beijing,
China), according to the manufacturer's protocol. RNA (2 µg) was
reverse transcribed at 42°C for 1 h using M-MLV reverse
transcriptase reagents (Promega Corporation, Madison, WI, USA). For
the qPCR assay, complementary DNA (cDNA) was PCR-amplified using a
GoTaq qPCR Master mix (Promega Corporation) in the Light Cycler 480
II PCR system (Roche Diagnostics GmbH, Basel, Switzerland). GAPDH
was used as the internal control. The sense and anti-sense primers
of miR-141 were 5′-TGGGTCCATCTTCCAGTA-3′ and
5′-GGGAGCCATCTTTACCAG-3′, respectively. The GAPDH sense and
anti-sense primers were 5′-GAAGGTGAAGGTCGGAGT-3′ and
5′-GAAGATGGTGATGGGATTTC-3′, respectively. The thermocycling
condition of qPCR: initial denaturation 95°C 30 sec, followed by 45
cycles: 95°C 15 sec, 65°C 1 min. Relative gene expression was
presented as the comparative threshold cycle (2−∆∆Cq)
values (17) and was representative
of at least three independent experiments.
Western blotting
Protein was extracted using a protein extraction kit
(KGP250-2100; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
according to the manufacturer's protocol. After examining the
concentration by BCA method, 10 µg protein from samples were
treated with Dual Color Protein Loading Buffer (Thermo Fisher
Scientific, Inc.) containing reducing agent at 100°C for 5 min
respectively, resolved on 10% Tris-HCl polyacrylamide gels, and
transferred to a polyvinylidene fluoride membrane. Then the
membrane was blocked by 5% skim milk for 1 h at room temperature.
Overnight incubation (4°C) with primary antibodies against the
following: BMI1 (dilution, 1:1,000; catalog no. ab14389; Abcam,
Cambridge, UK) and GAPDH (dilution, 1:2,000; catalog no. ab8245;
Abcam) was followed by incubation (37°C) with horseradish
peroxidase (HRP)-conjugated anti-rabbit (dilution, 1:1,000; catalog
no. NBP2-30348H; Novus Biologicals, LLC, Littleton, CO, USA) or
anti-mouse (dilution, 1:1,000; catalog no. NBP2-30347H; Novus
Biologicals) secondary antibodies. Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore, Billerica, MA, USA)
and a Tanon 5200 Luminescent Imaging Workstation (Tanon Science
& Technology Co., Ltd., Shanghai, China) were subsequently
used.
Mice tumor model
Mice are raised in individually ventilated cages
(IVF) in the mouse room with temperature of 18–23°C and 40–60%
humidity. A 12 light/12 dark cycle is used. Water and food are
accessible at all times. 10 BALB/c nude male mice (Biocytogen,
Worcester, MA, USA) aged 4 weeks (18–22 g body weight) were used
for the tumor growth experiments. SUNE1 cells transfected with
miR-141 or scrambled control were resuspended in PBS, and
1×106 cells were subcutaneously injected into the dorsal
flank of the nude mice. Mice were observed and the tumor sizes were
measured every two days. A total of 3 weeks later, the mice were
sacrificed and the tumors were dissected and compared. For H&E
staining, the tumor tissues were fixed by 4% paraformaldehyde at
4°C overnight, and embedded in paraffin. Next, the tissues were
sectioned into 5-µm tissues for H&E staining. For metastasis
analysis, 2×106 SUNE1 cells diluted in 200 µl PBS were
injected into 10 nude mice via the tail vein. Mice were observed
every two days. A total of 8 weeks later, mice were sacrificed and
their lungs were dissected. The general humane endpoints for mice
that required euthanasia are as follows: 20% decrease in normal
body weight, the longest diameter of the tumor exceeded 20 mm, the
inability to reach food or water for more that 24 h. All mice were
euthanized by carbon dioxide with 10–30% chamber volume per minute.
Animal feeding and research procedures were approved by the Animal
Care and Use Ethic Committee of Nanshan Affiliated Hospital of
Guangdong Medical College (Shenzhen, China).
Luciferase reporter assay
The wild-type and mutated 3′-UTR of miR-141 were
cloned into firefly luciferase-expressing vector psiCHECK™ (Promega
Corporation). For the luciferase reporter assay, NPC SUNE1 and 5–8F
cells were co-transfected with miR-141 and BMI1 wt or mutated
3′-UTR reporter vectors by Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). 36 h later, the luciferase activity was
examined using a Dual-Luciferase® Reporter assay system
(catalog no. E1910; Promega Corporation), and the reporter activity
were normalized by comparing with Renilla luciferase
activity.
MTT and colony formation assay
For the MTT assay, NPC SUNE1 and 5-8F cells
transfected with miR-141 or scrambled control were counted and
1,500 of them were seeded into 96-well plates. DMSO was used to
dissolve the formazan in each well. From day 1 to day 5, the
absorbance of the cells was determined at 490 nm using a
spectrophotometric plate reader. For the colony formation assay,
500 cells transfected with miR-141 or scrambled control were seeded
into 6-well plates. The colonies were observed and counted 5–7 days
later.
Cell invasion assay
A total of 5×104 5-8F or SUNE1 cells
following transfection with miR-141 or scrambled control were
resuspended in serum-free medium (DMEM, Thermo Fisher Scientific,
Inc.). The Transwell chambers (Corning Incorporated, Corning, NY,
USA) were placed on the upper surface of the 24-well plate, and the
cells were seeded into the upper chamber. The medium supplemented
with 10% FBS was placed in the lower chambers. After 10–16 h of
culture, the chambers were collected and the cells on the lower
surface of the chambers were fixed by absolute methanol for 20 min
at room temperature and stained by 0.1% crystal violet for 30 min
at room temperature. The cells on the chambers were captured at
×100 magnification by an inverted microscope.
Statistical analysis
All data are presented as the mean ± standard
deviation. χ2 test, Fisher's exact test, analysis of
variance with Bonferroni's correction and Student's t-test were
used for comparisons between groups. Spearman's correlation
analysis was used to examine the correlation between gene
expression and disease staging. The prognostic factors were
examined by univariate and multivariate analyses using the Cox
proportional hazards model. P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL,
USA).
Results
miR-141 serves as a tumor repressor in
NPC
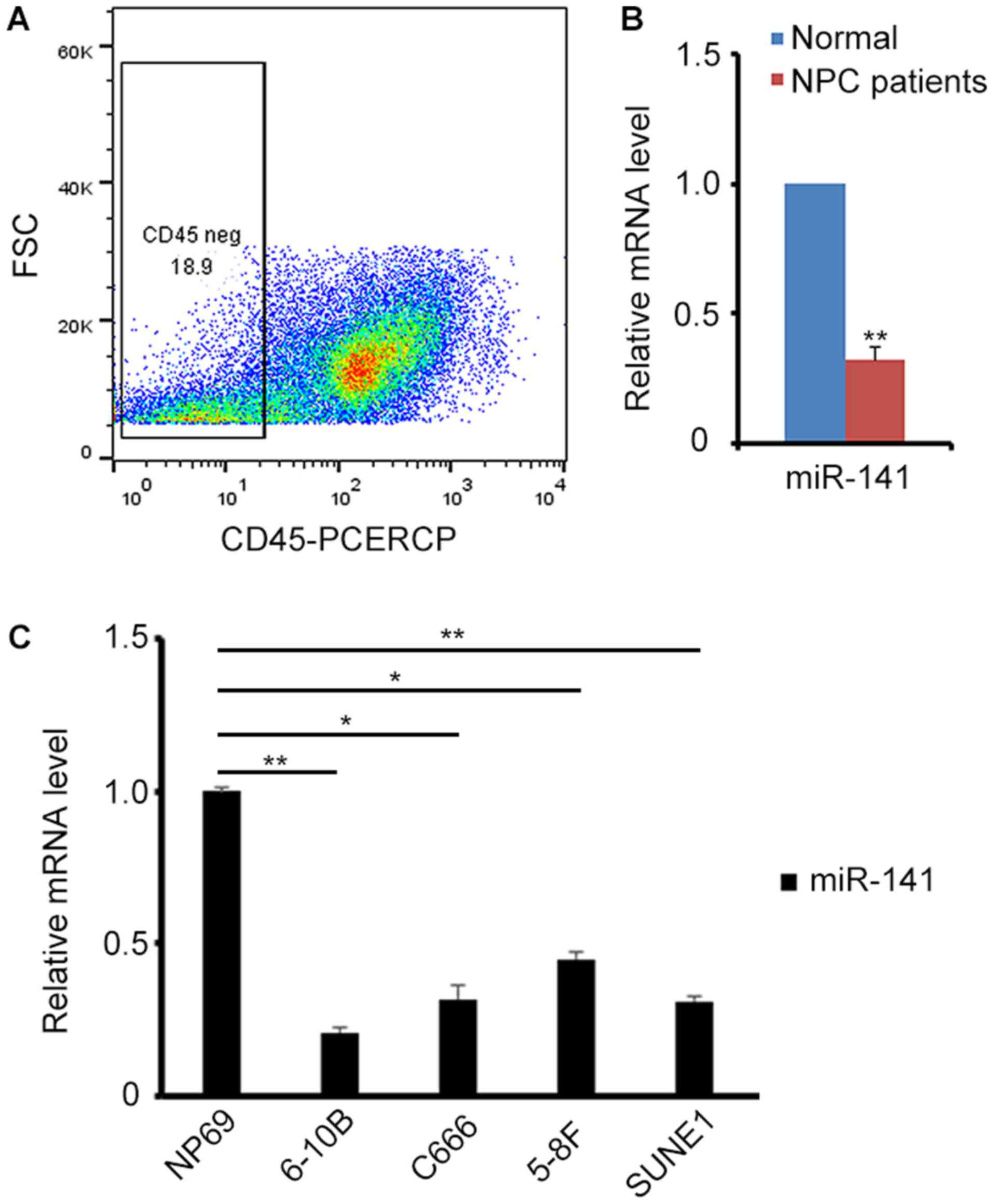
To begin with, the endogenous expression of miR-141
in NPC biopsy samples was detected. To exclude the interference of
lymphocytes in these samples, the CD45− cells were
sorted for the subsequent experiments by flow cytometry (Fig. 1A). A total of 23 patients were
examined. Compared with healthy people, the biopsy samples from
patients with NPC exhibited relatively low expression of miR-141
(Fig. 1B). Next, endogenous miR-141
expression was detected in different NPC cell lines. Consistent
with the results in biopsy samples, miR-141 expression was revealed
to be decreased in NPC cell lines (Fig.
1C), implying that miR-141 may act as a tumor repressor in
NPC.
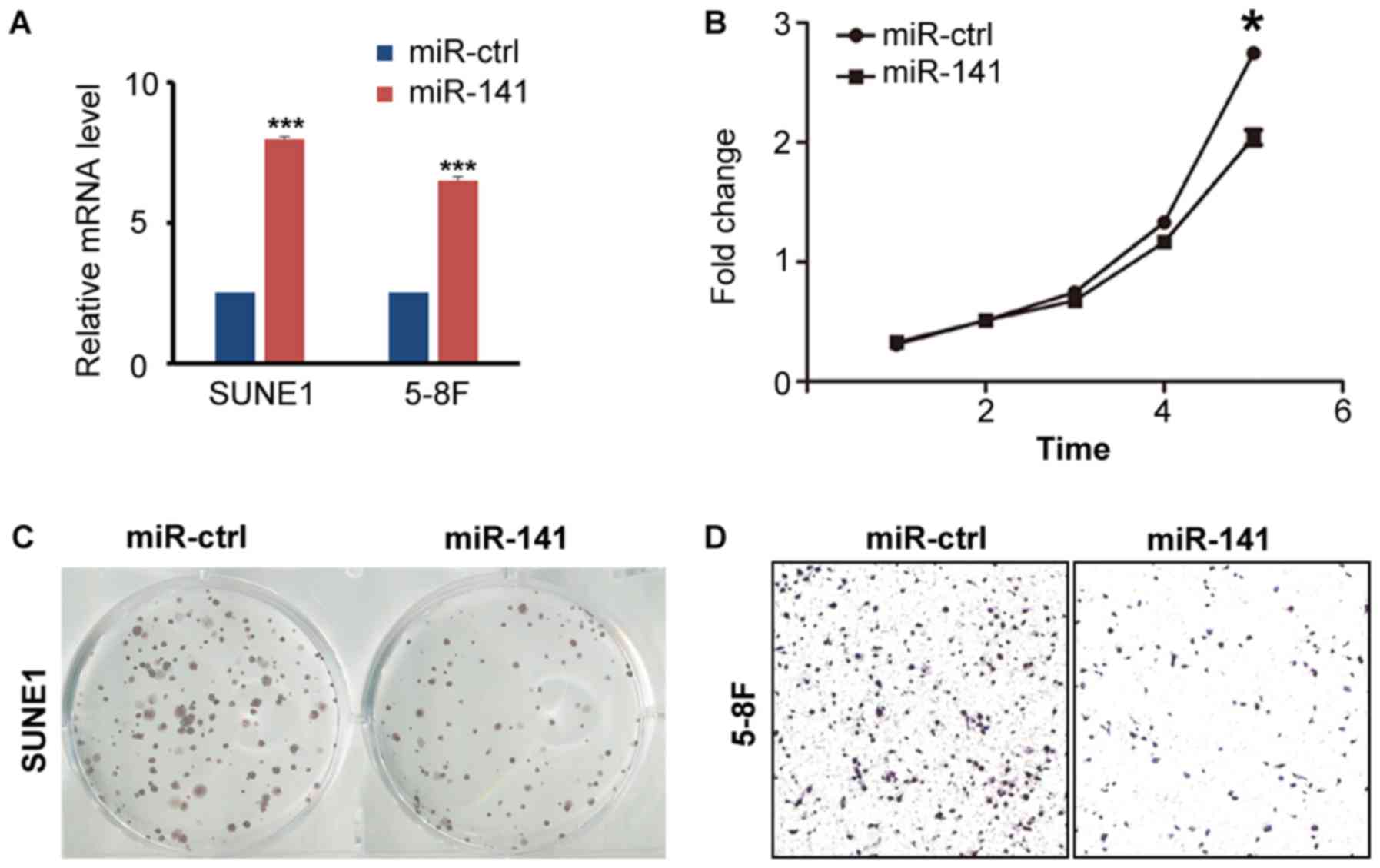
To examine the role of miR-141 in NPC, the miR-141
oligo-mimics were designed to simulate the ectopic expression of
miR-141 in vitro. Following transfection of miR-141 mimics
into NPC SUNE1 and 5-8F cells, increased expression of miR-141 was
detected (Fig. 2A). To identify
whether miR-141 affects NPC cell viability, MTT and colony
formation assays were performed. It was revealed that miR-141
overexpression blocked tumor cells proliferation compared with
their control counterparts (Fig. 2B).
The colony formation assay also revealed that ectopic expression of
miR-141 inhibited tumor cell growth (Fig.
2C). To determine whether miR-141 influenced NPC cell invasive
ability, a Transwell assay was performed. The results revealed that
overexpression of miR-141 decreased the number of invaded tumor
cells (Fig. 2D).
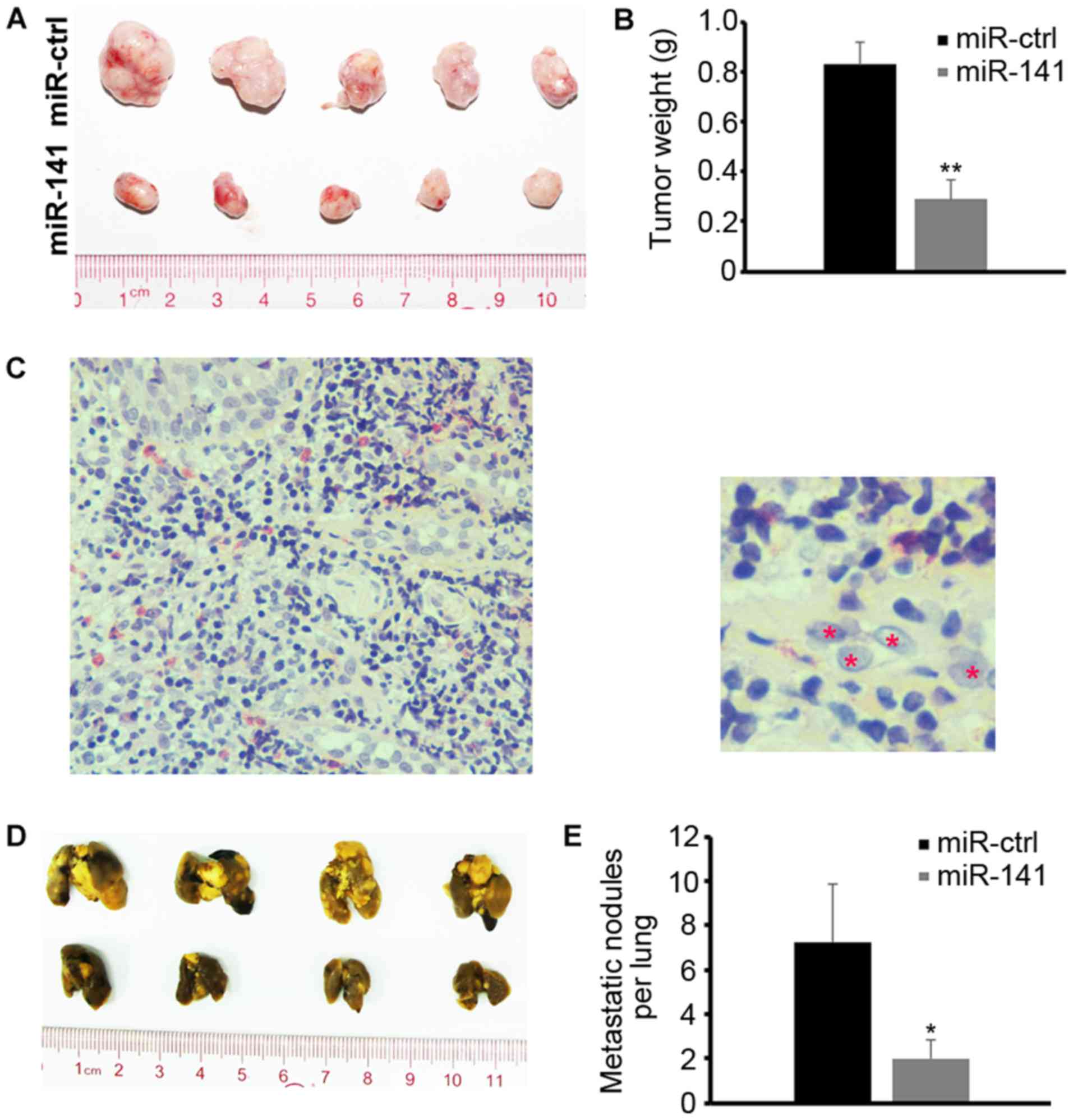
Next, it was determined whether ectopic expression
of miR-141 affects tumor growth in vivo. SUNE1 cells
transfected with miR-141 mimics or scrambled control were
subcutaneously injected into the dorsal flank of nude mice (n=10).
A total of 3 weeks later, the mice were sacrificed and the tumors
were dissected. Compared with the control, miR-141-overexpression
significantly inhibited tumor growth in vivo (Fig. 3A and B). H&E staining confirmed
that the dissected tissues were derived from NPC tumor cells
(Fig. 3C). To evaluate the inhibitory
effect of miR-141 on metastasis in vivo, the SUNE1 cells
were injected into nude mice via the tail vein. A total of 8 weeks
later, the mice were sacrificed and their lungs were dissected.
Compared with the control, ectopic expression of miR-141
effectively repressed the metastasis of NPC cells in vivo
(Fig. 3D and E).
BMI1 functions as the direct target of
miR-141 in NPC
To investigate the mechanism of miR-141 in
repressing NPC growth, the present study aimed to identify the
direct downstream target of miR-141. BMI1 is known as an important
regulator in NPC (18). A previous
study revealed that miR-141 represses BMI1 expression in diploid
fibroblasts (19). Therefore, we
hypothesized that BMI1 may serve as a potential miR-141 target. To
verify this, BMI1 3′-UTR target sequences with and without
mutations were constructed into the luciferase reporter vector
(Fig. 4A). Next, the luciferase
reporter assay was performed, and the results confirmed that BMI1
served as the direct target of miR-141 (Fig. 4B).
Since miR-141 is a NPC tumor repressor gene, it was
investigated whether miR-141 functioned through inhibiting BMI1
expression. BMI1 expression was first detected in NPC biopsy
samples and cell lines, and it was revealed that BMI1 was highly
activated (Fig. 4C and D). Next, BMI1
expression was examined in SUNE1 cells with ectopic miR-141
expression, and the results demonstrated that Bmi1 was inhibited
accordingly (Fig. 4E and F).
Furthermore, overexpressing BMI1 partially rescued the inhibitory
effect of NPC cells with ectopic miR-141 expression using Transwell
and MTT assays (Fig. 4G-I),
indicating that BMI1 functions downstream of miR-141.
The expression of miR-141 and BMI1
correlate with the staging status of patients with NPC
To evaluate the clinical significance of miR-141 and
BMI1, their expression level was detected in 51 NPC biopsy samples.
As demonstrated in Table I, it was
demonstrated that miR-141 expression was correlated with the
clinical stage of patients with NPC (P=0.03, Table I), and univariable and multivariable
analyses confirmed this correlation (Table II). Patients with advanced stages of
disease or metastasis exhibited relatively lower expression of
miR-141 than patients with earlier stages of disease (Fig. 5A). Furthermore, patients with low
expression of miR-141 had high expression of BMI1 (Fig. 5A and B), indicative of the regulatory
role of miR-141 and BMI1 in vivo.
 | Table I.Correlation between miR-141 expression
and clinicopathological characteristics of nasopharyngeal
carcinoma. |
Table I.
Correlation between miR-141 expression
and clinicopathological characteristics of nasopharyngeal
carcinoma.
|
|
| Expression of
miR-141 |
|
|---|
|
|
|
|
|
|---|
| Characteristic | No. patients | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.96 |
|
≤45 | 22 | 10 | 12 |
|
|
>45 | 29 | 13 | 16 |
|
| Sex |
|
|
| 0.67 |
|
Male | 39 | 19 | 20 |
|
|
Female | 12 | 5 | 7 |
|
| VCA-IgA |
|
|
| 0.74 |
|
<1:80 | 11 | 6 | 5 |
|
|
≥1:80 | 40 | 24 | 16 |
|
| EA-IgA |
|
|
| 0.54 |
|
<1:10 | 14 | 7 | 7 |
|
|
≥1:10 | 37 | 15 | 22 |
|
| TNM stage |
|
|
| 0.04 |
|
I–II | 22 | 10 | 12 |
|
|
III–IV | 27 | 20 | 7 |
|
| Metastasis |
|
|
| 0.03 |
|
Yes | 10 | 9 | 1 |
|
| No | 41 | 22 | 19 |
|
 | Table II.Univariable and multivariable
analyses of prognostic parameters in patients with nasopharyngeal
carcinoma. |
Table II.
Univariable and multivariable
analyses of prognostic parameters in patients with nasopharyngeal
carcinoma.
| Variable | HR (95% CI) | P-value |
|---|
| Univariate
analysis |
|
|
| Age,
≤45 years/>45 years | 1.212
(0.932–2.819) | 0.082 |
| Sex,
male/female | 1.878
(1.241–3.752) | 0.241 |
|
VCA-IgA, <1:80/≥1:80 | 2.131
(1.381–3.543) | 0.080 |
| EA-IgA,
<1:10/≥1:10 | 1.772
(1.373–2.949) | 0.088 |
| TNM
stage, I–II/III–IV | 2.308
(1.365–3.658) | 0.028a |
| miR-141
expression, low/high | 0.525
(0.035–1.384) | 0.014a |
| Multivariate
analysis |
|
|
| TNM
stage, I–II/III–IV | 2.015
(1.103–3.223) | 0.021a |
| miR-141
expression, low/high | 0.423
(0.103–1.186) | 0.018a |
Discussion
The present study revealed that miR-141 was
downregulated in NPC clinical samples and cell lines. Ectopic
expression of miR-141 blocked tumor cell proliferation and
invasion. Mechanistic analysis identified that BMI1 served as the
direct target of miR-141, and overexpressing BMI1 partially rescued
the tumor suppressive phenotype of miR-141. Furthermore, the
miR-141/BMI1 signaling cascade was correlated with NPC clinical
staging. Patients with metastases have relatively lower expression
levels of miR-141 and higher expression levels of BMI1, which
suggested that ectopic expression of BMI1 facilitated NPC tumor
cell metastasis in vivo.
Tumor cell growth requires a series of gene
transcription and modification networks. Understanding these
regulatory networks may aid in identifying the mechanism of tumor
cell initiation and growth, so as to develop precise medical
strategies for clinical use. Previous studies have demonstrated
that several miRNAs were aberrantly expressed and functioned in NPC
tumor cells, as well as being involved in cell proliferation,
migration and metabolism processes (6,7,9). Furthermore, recent studies have
demonstrated that circulating free nucleic acid (cfNA) in patients
with cancer were more highly concentrated (20–22).
Detecting circulating miRNAs has been emerging as a novel early
diagnosis strategy to identify the clinical stage of patients with
cancer (22,23). Therefore, it would be of great
importance to study the whole miRNAs regulation network during NPC
development.
Recent studies have revealed that miR-141 was active
in prostate cancer and ovarian cancer (14,15), and
served an inhibitory role in gastric cancer growth (13). Several miRNAs were reported to serve
roles in manipulating NPC development (24–30).
However, the expression and function of miR-141 in NPC has not been
identified. The results of the present study established the tumor
repressor effect of miR-141 in NPC, and confirmed that miR-141
functioned through regulating its target, BMI1. Previous results
confirmed that BMI1 was an important oncogene in NPC. Inhibiting
BMI1 was regarded as the effective method to block NPC
proliferation and invasion (16,31,32). The
results provided a direct BMI1 regulator in NPC, which may aid in
shedding light on the clinical application of pharmacological
inhibition of BMI1.
Acknowledgements
The authors would like to thank Dr Lei Wang from the
Central Laboratory and Dr Wenchao Yu from the Clinical Laboratory
of Nanshan Affiliated Hospital of Guangdong Medical College for
their help in data analysis.
Funding
The present study was supported by a grant from the
Knowledge Innovation Project of Shenzhen (grant no.
JCYJ20150402152130190). The funder did not participate in study
design, data analysis and manuscript publication.
Authors' contributions
FL and CG conceived the project. FL and WW carried
out most of the experiments. SL, QY, JH, and NZ participated in
data analysis and interpretation of results. All authors read and
approved the manuscript.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Ethics approval and consent to
participate
This study was performed in accordance with the
ethical standards and according to the Declaration of Helsinki and
according to national and international guidelines and has been
approved by the ethics committee of Nanshan Affiliated Hospital of
Guangdong Medical College. Patients provided written informed
consent.
Patient consent for publication
All patients have provided written informed consent
for the publication of this manuscript and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NPC
|
nasopharyngeal carcinoma
|
|
miRNA
|
microRNA
|
References
|
1
|
Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu
LZ, Sun Y, Lin AH, Liu MZ and Ma J: How does intensity-modulated
radiotherapy versus conventional two-dimensional radiotherapy
influence the treatment results in nasopharyngeal carcinoma
patients? Int J Radiat Oncol Biol Phys. 80:661–668. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Sun Y, Liang SB, Zong JF, Li WF,
Chen M, Chen L, Mao YP, Tang LL, Guo Y, et al: Progress report of a
randomized trial comparing long-term survival and late toxicity of
concurrent chemoradiotherapy with adjuvant chemotherapy versus
radiotherapy alone in patients with stage III to IVB nasopharyngeal
carcinoma from endemic regions of China. Cancer. 119:2230–2238.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Plaisance-Bonstaff K and Renne R: Viral
miRNAs. Methods Mol Biol. 721:43–66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
He L, Thomson JM, Hemann MT,
Hernando-Monge E, Mu D, Goodson S, Powers S, Cordon-Cardo C, Lowe
SW, Hannon GJ and Hammond SM: A microRNA polycistron as a potential
human oncogene. Nature. 435:828–833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krützfeldt J, Rajewsky N, Braich R, Rajeev
KG, Tuschl T, Manoharan M and Stoffel M: Silencing of microRNAs in
vivo with ‘antagomirs’. Nature. 438:685–689. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elmén J, Lindow A, Silahtaroglu M, Bak M,
Christensen A, Lind-Thomsen M, Hedtjärn M, Hansen HF, Hansen EM,
Straarup EM, et al: Antagonism of microRNA-122 in mice by
systemically administered LNA-antimiR leads to up-regulation of a
large set of predicted target mRNAs in the liver. Nucleic Acids
Res. 36:1153–1162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Obad S, dos Santos CO, Petri A, Heidenblad
M, Broom O, Ruse C, Fu C, Lindow M, Stenvang J, Straarup EM, et al:
Silencing of microRNA families by seed-targeting tiny LNAs. Nat
Genet. 43:371–378. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elmén J, Lindow M, Schütz S, Lawrence M,
Petri A, Obad S, Lindholm M, Hedtjärn M, Hansen HF, Berger U, et
al: LNA-mediated microRNA silencing in non-human primates. Nature.
452:896–899. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agaoglu Yaman F, Kovancilar M, Dizdar Y,
Darendeliler E, Holdenrieder S, Dalay N and Gezer U: Investigation
of miR-21, miR-141, and miR-221 in blood circulation of patients
with prostate cancer. Tumour Biol. 32:583–588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mateescu B, Batista L, Cardon M, Gruosso
T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P,
Sastre-Garau X and Mechta-Grigoriou F: miR-141 and miR-200a act on
ovarian tumorigenesis by controlling oxidative stress response. Nat
Med. 17:1627–1635. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zuo QF, Zhang R, Li BS, Zhao YL, Zhuang Y,
Yu T, Gong L, Li S, Xiao B and Zou QM: MicroRNA-141 inhibits tumor
growth and metastasis in gastric cancer by directly targeting
transcriptional co-activator with PDZ-binding motif, TAZ. Cell
Death Dis. 6:e16232015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song LB, Zeng MS, Liao WT, Zhang L, Mo HY,
Liu WL, Shao JY, Wu QL, Li MZ, Xia YF, et al: Bmi-1 is a novel
molecular marker of nasopharyngeal carcinoma progression and
immortalizes primary human nasopharyngeal epithelial cells. Cancer
Res. 66:6225–6232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dimri M, Carroll JD, Cho JH and Dimri GP:
microRNA-141 regulates BMI1 expression and induces senescence in
human diploid fibroblasts. Cell Cycle. 12:3537–3546. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gormally E, Caboux E, Vineis P and Hainaut
P: Circulating free DNA in plasma or serum as biomarker of
carcinogenesis: Practical aspects and biological significance.
Mutat Res. 635:105–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kosaka N, Iguchi H and Ochiya T:
Circulating microRNA in body fluid: A new potential biomarker for
cancer diagnosis and prognosis. Cancer Sci. 101:2087–2092. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang K, Peng X, Luo J and Gou D:
Identification of circulating miRNA biomarkers based on global
quantitative real-time PCR profiling. J Anim Sci Biotechnol.
3:42012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu J, Luo H, Liu X, Peng Y, Zhang B, Wang
L, Xu X, Peng X, Li G, Tian W, et al: miR-9 targets CXCR4 and
functions as a potential tumor suppressor in nasopharyngeal
carcinoma. Carcinogenesis. 35:554–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu J, He ML, Wang L, Chen Y, Liu X, Dong
Q, Chen YC, Peng Y, Yao KT, Kung HF and Li XP: MiR-26a inhibits
cell growth and tumorigenesis of nasopharyngeal carcinoma through
repression of EZH2. Cancer Res. 71:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu N, Tang LL, Sun Y, Cui RX, Wang HY,
Huang BJ, He QM, Jiang W and Ma J: MiR-29c suppresses invasion and
metastasis by targeting TIAM1 in nasopharyngeal carcinoma. Cancer
Lett. 329:181–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang LY, Lee Ho-Fun V, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY and Fu L:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xia H, Cheung WK, Sze J, Lu G, Jiang S,
Yao H, Bian XW, Poon WS, Kung HF and Lin MC: miR-200a regulates
epithelial-mesenchymal to stem-like transition via ZEB2 and
beta-catenin signaling. J Biol Chem. 285:36995–37004. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu N, Jiang N, Guo R, Jiang W, He QM, Xu
YF, Li YQ, Tang LL, Mao YP, Sun Y and Ma J: MiR-451 inhibits cell
growth and invasion by targeting MIF and is associated with
survival in nasopharyngeal carcinoma. Mol Cancer. 12:1232013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yi C, Wang Q, Wang L, Huang Y, Li L, Liu
L, Zhou X, Xie G, Kang T, Wang H, et al: MiR-663, a microRNA
targeting p21(WAF1/CIP1), promotes the proliferation and
tumorigenesis of nasopharyngeal carcinoma. Oncogene. 31:4421–4433.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qin L, Zhang X, Zhang L, Feng Y, Weng GX,
Li MZ, Kong QL, Qian CN, Zeng YX, Zeng MS, et al: Downregulation of
BMI-1 enhances 5-fluorouracil-induced apoptosis in nasopharyngeal
carcinoma cells. Biochem Biophys Res Commun. 371:531–535. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alajez NM, Shi W, Hui AB, Yue S, Ng R, Lo
KW, Bastianutto C, O'Sullivan B, Gullane P and Liu FF: Targeted
depletion of BMI1 sensitizes tumor cells to P53-mediated apoptosis
in response to radiation therapy. Cell Death Differ. 16:1469–1479.
2009. View Article : Google Scholar : PubMed/NCBI
|