Introduction
Esophageal cancer is a common malignant tumor in the
digestive tract. The incidence rate differs between different
ethnic groups and regions, and it is high in China and South
Africa. With the changes in individuals' lifestyle and diet
structure, the incidence rate of esophageal cancer has been
increasing year by year around the world, and esophageal cancer has
become one of the most serious diseases endangering human health
(1). Dietary factors, especially the
ingestion of large amounts of saturated fat and eating at high
speed, are the main causes of esophageal cancer (2). The treatments for esophageal cancer
include surgery, radiotherapy and chemotherapy. Although surgical
resection is the only cure method, patients are definitely
diagnosed with esophageal cancer in the middle and advanced stage
due to the delay of the early diagnosis, relatively imperfect
screening mechanisms and other factors, and they lose the best time
for surgery. So the early diagnosis is particularly important
(3). Computed tomography (CT) scan is
used to evaluate curative effects and predict prognosis mainly from
tumor size and morphological changes, but its accuracy is not high.
Diffusion-weighted imaging (DWI) is used to evaluate curative
effects from tumor molecular or cell level changes, and its
apparent diffusion coefficient (ADC) can provide quantitative
indicators for esophageal cancer before and after treatment, thus
predicting the curative effect more accurately in an earlier stage
(4,5).
In this study, the diagnosis and treatment of esophageal cancer
were analyzed via CT scan and magnetic resonance imaging (MRI) scan
for patients with esophageal cancer.
Patients and methods
General data
A total of 78 esophageal cancer patients admitted
and treated in Jinan Central Hospital (Jinan, China) from January
2013 to June 2014 were randomly selected for retrospective
analysis. Inclusion criteria: i) patients diagnosed with esophageal
cancer by pathological examination; ii) patients receiving
radiotherapy for the first time; iii) patients undergoing CT and
MRI examinations with clear consciousness; and iv) patients who
signed informed consent. Types of ulcer infiltration and stenosis
were excluded. Patients with contraindications detected via MRI
were excluded. The general data of patients are shown in Table I. This study was approved by the
Ethics Committee of Jinan Central Hospital. Signed informed
consents were obtained from the patients or the guardians.
 | Table I.General data of study subjects. |
Table I.
General data of study subjects.
| Item | Tested subjects
(n=78) |
|---|
| Average age
(years) | 55.78±6.54 |
| Sex
(male/female) | 53/25 |
| Tumor site [n
(%)] |
|
| Neck | 7 (8.97) |
| Upper
thoracic part | 12 (15.39) |
| Middle
and lower thoracic part | 59 (75.64) |
| Pathological type [n
(%)] |
|
| Squamous
cell carcinoma | 63 (80.77) |
|
Adenocarcinoma | 8 (10.26) |
| Small
cell carcinoma | 7 (8.97) |
Preparations before examinations
Patients fasted for 6 h prior to CT scan, and they
were informed of the relevant precautions and received respiratory
training (uniform, calm and slow breathing) to avoid poor image
quality due to the change of respiratory rate. The patients were
asked to have a light diet on the day prior to MRI examination so
as to avoid MRI signal changes due to high-fat foods.
CT examination
The patients were instructed to put their hands on
both sides of the pillow, and they were placed in the supine
position and scanned with a dual-source CT scanner (Siemens AG,
Munich, Germany) (thickness, 3.0 mm; layer distance, 3.0 mm). The
scanning sites were the chest and upper abdomen, ranging from the
chest entrance down to the lower pole of the kidney. The scanned
images were transmitted to the workstation. Two senior imaging
physicians applied Syntegra software (Philips Medical Systems,
Andover, MA, USA) for image fusion, target area delineation and
lesion length calculation based on spinal imaging, skeletal
structure and localized CT images.
MRI examination
The patients were guided to lie down in the supine
position and scanned by conventional plain MRI [T1-weighted imaging
(T1WI) and T2WI] and DWI using the Avanto 1.5T magnetic resonance
scanner (Siemens AG). The scanning sites were the chest and upper
abdomen: i) T1WI axial scan [repetition time (TR), 140 msec; time
to echo (TE), 2.5 msec; matrix, 256×256; field of view (FOV)
squared, 36×36 cm; flip angle, 70°; bandwidth (BW), 280 Hz; layer
thickness, 6.0 mm; and interlayer interval, 20%) for 2 min and 15
sec. ii) T2WI axial scan (TR, 1,580 msec; TE, 72 msec; matrix,
384×276; FOV, 35×35 cm; flip angle, 140°; BW, 315; layer thickness,
6.0 mm; and interlayer interval, 20%) for 1 min and 30 sec. iii)
DWI axial scan (TR, 6,800 msec; TE, 70 msec; matrix, 128×128; FOV,
40×40 cm; BW, 260; layer thickness, 4.0 mm; and interlayer
interval, 0%) for 9 min and 30 sec using the single-shot
spin-echo-echo-planar imaging (SE-EPI) sequence. Four different
diffusion-sensitive factors were simultaneously collected during
the scans: b=0, 600, 800, and 1,000 sec/mm2. The DWI
images were fused to obtain the corresponding ADC images, and the
lesion lengths at different b values were calculated. The target
area was delineated with b=600 sec/mm2. Patients were
divided into high ABC (>1.5×10−3 mm2/sec)
group and low ADC (≤1.5×10−3 mm2/sec) group.
The length of the lesion was selected for providing a more accurate
location for surgical treatment, to improve the effectiveness of
surgery, to effectively predict the prognosis and to make
reasonable operation plan and postoperative radiotherapy and
chemotherapy. Before a pathological examination, the length of the
lesion can be obtained by imaging examination, which has certain
guiding value for the diagnosis and treatment of esophageal
cancer.
Indicator evaluation
Evaluation criteria for short-term
curative effects
i) Complete remission (CR): the tumor completely
disappears, no stenosis or slight stenosis occurs in the esophageal
lumen, and mucosa basically returns to normal; ii) partial
remission (PR): partial esophageal lesions disappear, but obvious
stenosis is found in the lumen; and iii) no remission (NR): at the
end of radiotherapy, the lesion is not improved or aggregated, and
there are still residual lesions. Clinical control rate = (CR +
PR)/the total number of cases ×100% (1).
ADC value calculation
DWI images with b=0, 600, 800, and 1,000
sec/mm2 were selected and fused to obtain the
corresponding ADC images. The manifested largest and most clear
sections of esophageal lesions were selected as the region of
interest (ROI), and the ROI of each ADC image should be selected in
the same area. Each ROI was measured 3 times, and the average value
was taken as the final ADC value of each ROI (6).
Statistical methods
Statistical Product and Service Solutions (SPSS)
19.0 analysis software (IBM Corp., Armonk, NY, USA) was used.
Measurement data were expressed as mean ± standard deviation, and
detected by t-test. Count data were expressed as percentage, and
detected by χ2 test. ANOVA was used for comparison of
multiple groups and Dunnett's test was the post hoc test. Receiver
operating characteristic (ROC) curve analyses were conducted for
diagnostic methods (CT and MRI) and efficacy prediction. Survival
curves were drawn using the Kaplan-Meier method, and the long-rank
test was employed for survival analysis. Test significance level
α=0.05.
Results
Comparison of the length of esophageal
lesions measured by different examination methods
The difference in value between the length of
esophageal lesions and the length of pathological specimens
measured by CT scan was significantly different from that detected
via DWI examination with b=600, 800 and 1,000 sec/mm2,
respectively (P<0.05), and the feasibility of DWI with b=600
sec/mm2 was the highest (Table II).
 | Table II.Comparison of the length of esophageal
lesions measured by different examination methods (cm). |
Table II.
Comparison of the length of esophageal
lesions measured by different examination methods (cm).
| Method | Length | Difference value |
|---|
| Pathological entity
measurement | 4.56±1.43 |
|
| CT scan | 5.67±1.65 | 1.15±0.59 |
| DWI examination |
|
|
| b=600
sec/mm2 | 4.41±1.62 |
0.19±0.36a |
| b=800
sec/mm2 | 3.97±1.45 |
0.65±0.42a,b |
| b=1,000
sec/mm2 | 3.84±1.62 |
0.84±0.53a,b,c |
Comparison of the diagnostic rate of
esophageal cancer by different imaging examination methods
The diagnostic rate of esophageal cancer via CT scan
was obviously lower than that via DWI examination (P<0.05). The
area under the ROC curve of diagnostic rate of esophageal cancer by
CT scan was 0.794, with sensitivity of 70.3% and specificity of
69.4%, while that by DWI was 0.845, with sensitivity of 87.6% and
specificity of 82.5% (Table III and
Fig. 1).
 | Table III.Comparison of the diagnostic rate of
esophageal cancer by different imaging examination methods [n
(%)]. |
Table III.
Comparison of the diagnostic rate of
esophageal cancer by different imaging examination methods [n
(%)].
| Method | n | Diagnostic rate |
|---|
| CT scan | 78 | 69 (88.46) |
| DWI examination | 78 | 77 (98.72) |
| χ2 |
| 5.236 |
| P-value |
| 0.022 |
Comparison of the curative effects in
the two groups of patients
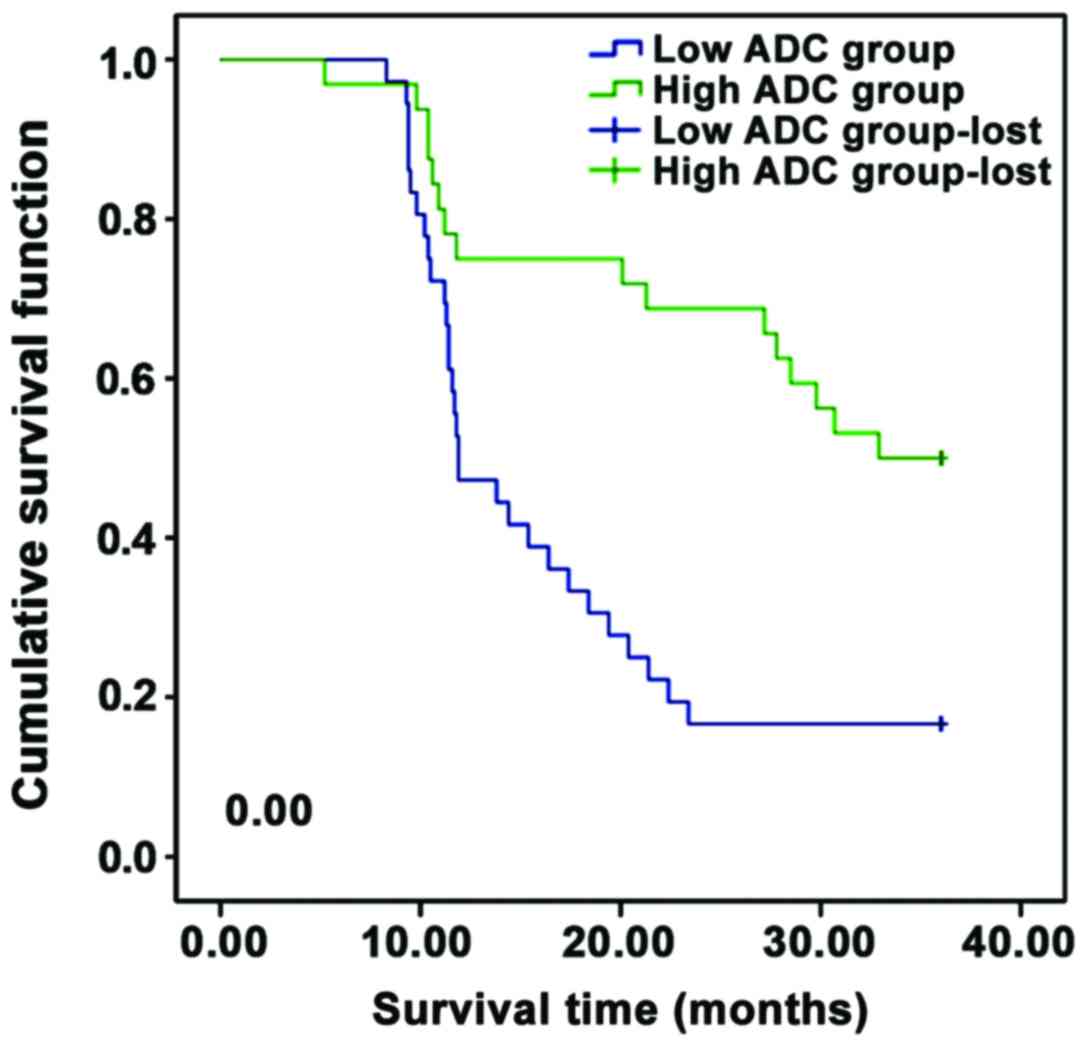
After radiotherapy, the 1-year clinical control rate
in the high ADC value group before radiotherapy was significantly
higher than that in the low ADC value group, and the 3-year
survival rate in the former was significantly higher than that in
the latter (Table IV and Fig. 2).
 | Table IV.Comparison of the curative effects in
the two groups of patients [n (%)]. |
Table IV.
Comparison of the curative effects in
the two groups of patients [n (%)].
| Group | n | CR | PR | Clinical control
rate | 3-year survival
rate |
|---|
| High ADC value
group | 41 | 21 (51.22) | 16 (39.02) | 37 (90.24) | 24 (58.54) |
| Low ADC value
group | 37 | 11 (29.73) | 14 (37.84) | 25 (67.57) | 12 (32.43) |
| χ2 |
|
|
| 4.822 | 4.334 |
| P-value |
|
|
| 0.028 | 0.037 |
Comparison of the ADC value of primary
esophageal foci of patients in the CR and the PR groups in
different time-points
There was no significant difference in the ADC value
of primary esophageal foci before radiotherapy between the CR and
the PR group (P>0.05). At the 2nd week during radiotherapy and
at the end of radiotherapy, ADC values in the CR group were
significantly higher than those in the PR group (P<0.05). At the
2nd week during radiotherapy and at the end of radiotherapy, ADC
values were used to predict the CR rate of radiotherapy for
esophageal cancer, and the areas under the ROC curve were 0.776 and
0.935, respectively, with critical values of 1.38 ×10−3
and 1.46 ×10−3 mm2/sec, sensitivities of 62.3
and 91.5%, and specificities of 98.3% and 83.7% (Table V and Fig.
3).
 | Table V.Comparison of the ADC values of
primary esophageal foci at different time-points of patients of the
CR and the PR groups (10−3 mm2/sec). |
Table V.
Comparison of the ADC values of
primary esophageal foci at different time-points of patients of the
CR and the PR groups (10−3 mm2/sec).
| Group | n | Before
radiotherapy | At 2 weeks after
radiotherapy | At the end of
radiotherapy | F | P-value |
|---|
| CR group | 32 | 1.63±0.65 | 2.23±0.53 | 2.84±0.56 | 52.163 | <0.001 |
| PR group | 30 | 1.46±0.57 | 1.68±0.48 | 1.96±0.47 | 48.826 | <0.001 |
| t |
| 1.092 | 4.273 | 6.679 |
|
|
| P-value |
| 0.279 | <0.001 | <0.001 |
|
|
Discussion
Esophageal cancer is a malignant tumor occurring in
esophageal epithelial tissues, which is caused by abnormal cell
proliferation. The invasion ability of esophageal cancer is strong,
and it is mostly located in the middle part of the chest, but less
distributed in the upper and lower parts of the chest. The majority
are esophageal squamous cell carcinoma, but adenocarcinoma and
small cell carcinoma are rare (7).
Esophageal cancer is a malignant tumor that occurs in esophageal
epithelial tissues. Its clinical stage can be divided into stage I,
II and III. Esophageal cancer occurs frequently at the age of
>40 years, with higher frequency in men than women, and its
incidence and mortality rates rank 4th among malignant tumors
(8). Esophageal cancer is induced by
many factors, including excessively high eating speed, ingestion of
too hot foods, foods with mycotoxins or large amounts of
nitrosamines, smoking, excessive drinking, poor oral hygiene,
nutritional deficiencies, human papillomavirus infections and
genetic factors (9). Patients are
often diagnosed with esophageal cancer in the middle and advanced
stage with poor prognosis and low survival rate, so improving the
early screening and diagnosis of esophageal cancer has important
clinical significance (10).
Endoscopic examination is usually used in clinical
diagnosis of esophageal carcinoma, but it is invasive and patient
compliance is poor, so other imaging techniques, such as CT and
MRI, are often used. CT scan has the advantages of high scanning
speed, clear images and little susceptibility to peripheral organs.
With the rapid development of medical imaging technology, CT has
been developed from 4 rows in the past to 64 rows and even more,
thus resulting in shorter scanning time, higher resolution and less
motion artifact interference. Therefore, CT scan has become a main
clinical diagnostic method for many diseases (11). MRI is a new type of medical imaging
technology developed on the basis of magnetic resonance display. It
has the advantages of no radiation damage, multi-orientation and
multi-sequence imaging and high spatial resolution, and it is
widely used in the examination of various diseases (12). The results of this study showed that
the diagnostic rate of esophageal cancer by CT scan was
significantly lower than that by DWI examination. The difference in
value between the length of esophageal lesions and the length of
pathological specimens measured by CT scan was significantly
different from that detected via DWI examination with b=600, 800
and 1,000 sec/mm2, respectively (P<0.05). This is
because CT scan examines the morphological changes in tumors, and
there are deficiencies in the determination of the esophageal
lesion range and lymph node metastasis of esophageal lesions. The
length of esophageal lesions measured by CT scan is too large,
since tumor necrosis occurs followed by secondary infection, so
that edema occurs in normal esophageal tissues, and CT scan cannot
be effectively performed for differential diagnosis. When
esophageal cancer is in stage III, lymph node metastasis and
esophageal wall adhesion occur, so it is difficult for CT scan to
distinguish, which is also influenced by the partial volume effect
of CT, resulting in the poor accuracy of the doctor outlining the
target area, and thus affecting the curative effect of radiotherapy
(13). DWI, as a new MRI technique,
utilizing the dispersed phase effect caused by a special magnetic
resonance sequence to reflect the microscopic diffusion motion of
water molecules in tissues at the macro-imaging, so as to form DWI
images, which can reflect the function state of the exchange of
water molecules in diseased tissues. DWI can distinguish lymph
nodes and blood vessels without enhancers, so the length of
esophageal lesions measured by DWI is relatively accurate.
Therefore, the sensitivity and specificity of the diagnosis of
esophageal cancer by DWI are significantly higher than those by CT
scan, thus providing important information for delineating the
target area (5,14). b is one of the important parameters of
DWI technique. The quality of DWI images and the authenticity of
water molecule diffusion are directly related to b. The smaller the
b is, the lower the degree of diffusion, the smaller the influence
on signals and the higher the image quality will be. Therefore, DWI
has the highest credibility when b=600 sec/mm2, which
can be used to draw the target area (15).
ADC value is one of the quantitative indicators of
DWI, which can quantitatively evaluate the diffusion state of water
molecules in the tissue structure (16). The study results revealed that the
1-year clinical control rate in the high ADC value group before
radiotherapy was significantly higher than that in the low ADC
value group, and the 3-year survival rate in the former was
significantly higher than that in the latter. There was no
significant difference in the ADC value of esophageal primary foci
between the CR and PR group (P>0.05). At the 2nd week during
radiotherapy and at the end of radiotherapy, the ADC values in the
CR group were significantly higher than those in the PR group
(P<0.05). This is because the more active the proliferation of
tumor cells in esophageal cancer is, the larger the number of tumor
cells and the closer the arrangement are, which leads to the
smaller extracellular space and seriously restricted diffusion of
water molecules, and the lower the ADC value will be (17). In addition, ADC value can reflect the
degree of tumor load. With the progression of treatment, esophageal
lesions are shrinking; tumor cells continue to apoptosis, and thus
the number of cells and density are decreased, the extracellular
space is increased; the limitation of diffusion of water molecules
is alleviated, so ADC value is elevated (18). Changes in the ADC value during and
after treatment reflect the different sensitivities of tumor
tissues to treatment responses. The more obvious the curative
effect is, the higher the ADC value, the better the prognosis of
patients, and the higher the survival rate will be (19).
In summary, compared with CT scan, DWI examination
is more objective and accurate in the diagnosis of esophageal
cancer, and DWI is of great significance in predicting the curative
effect of radiotherapy on esophageal cancer. ADC value can be used
as a quantitative index to predict the sensitivity of esophageal
cancer to radiotherapy. Due to the small sample size and short
follow-up period, there is still a need to further expand the
sample size and conduct long-term research in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG collected and analyzed the general data of the
patients. LZ assisted with ADC value calculation. LG and JZ were
responsible for interpreting the scan results. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Jinan Central Hospital (Jinan, China). Signed informed consents
were obtained from the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Probst A, Aust D, Märkl B, Anthuber M and
Messmann H: Early esophageal cancer in Europe: Endoscopic treatment
by endoscopic submucosal dissection. Endoscopy. 47:113–121.
2015.PubMed/NCBI
|
|
2
|
Oyama T, Hotta K and Tomori A: Endoscopic
submucosal dissection for superficial esophageal squamous cell
carcinoma and adenocarcinoma. Gastrointest Endosc. 65:AB1102007.
View Article : Google Scholar
|
|
3
|
van der Sluis PC, Ruurda JP, Verhage RJ,
van der Horst S, Haverkamp L, Siersema PD, Rinkes Borel IH, Ten
Kate FJ and van Hillegersberg R: Oncologic long-term results of
robot-assisted minimally invasive thoraco-laparoscopic
esophagectomy with two-field lymphadenectomy for esophageal cancer.
Ann Surg Oncol. 22 Suppl 3:S1350–S1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drummond MB, Lambert AA, Hussien AF, Lin
CT, Merlo CA, Wise RA, Kirk GD and Brown RH: HIV infection is
independently associated with increased CT scan lung density. Acad
Radiol. 24:137–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boone D, Taylor SA and Halligan S:
Diffusion weighted MRI: Overview and implications for rectal cancer
management. Colorectal Dis. 15:655–661. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nozaki I, Hato S, Hori S, Nishide N and
Kurita A: Incidence of food residue interfering with postoperative
endoscopic examination for gastric pull-up after esophagectomy.
Esophagus. 13:195–199. 2016. View Article : Google Scholar
|
|
7
|
Tachibana M, Kinugasa S, Hirahara N and
Yoshimura H: Lymph node classification of esophageal squamous cell
carcinoma and adenocarcinoma. Eur J Cardiothorac Surg. 34:427–431.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arnal Domper MJ, Ferrández Arenas Á and
Lanas Arbeloa Á: Esophageal cancer: Risk factors, screening and
endoscopic treatment in Western and Eastern countries. World J
Gastroenterol. 21:7933–7943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu W, Liu Z, Bao Q and Qian Z: Viruses,
other pathogenic microorganisms and esophageal cancer. Gastrointest
Tumors. 2:2–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin G, Han SY, Xu YP and Mao WM:
Increasing the interval between neoadjuvant chemoradiotherapy and
surgery in esophageal cancer: A meta-analysis of published studies.
Dis Esophagus. 29:1107–1114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB
and Chen YL: Prediction of microvascular invasion of hepatocellular
carcinoma: Preoperative CT and histopathologic correlation. AJR Am
J Roentgenol. 203:W253–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ganten MK, Schuessler M, Bäuerle T,
Muenter M, Schlemmer HP, Jensen A, Brand K, Dueck M, Dinkel J,
Kopp-Schneider A, et al: The role of perfusion effects in
monitoring of chemoradiotherapy of rectal carcinoma using
diffusion-weighted imaging. Cancer Imaging. 13:548–556. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tchelebi L and Ashamalla H: Overcoming the
hurdles of using PET/CT for target volume delineation in curative
intent radiotherapy of non-small cell lung cancer. Ann Transl Med.
3:1912015.PubMed/NCBI
|
|
14
|
Anderson SW, Barry B, Soto JA, Ozonoff A,
O'Brien M and Jara H: Quantifying hepatic fibrosis using a
biexponential model of diffusion weighted imaging in ex vivo liver
specimens. Magn Reson Imaging. 30:1475–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giganti F, Salerno A, Ambrosi A, Chiari D,
Orsenigo E, Esposito A, Albarello L, Mazza E, Staudacher C, Del
Maschio A, et al: Prognostic utility of diffusion-weighted MRI in
oesophageal cancer: Is apparent diffusion coefficient a potential
marker of tumour aggressiveness? Radiol Med (Torino). 121:173–180.
2016. View Article : Google Scholar
|
|
16
|
Purushotham A, Campbell BC, Straka M,
Mlynash M, Olivot JM, Bammer R, Kemp SM, Albers GW and Lansberg MG:
Apparent diffusion coefficient threshold for delineation of
ischemic core. Int J Stroke. 10:348–353. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mori N, Ota H, Mugikura S, Takasawa C,
Ishida T, Watanabe G, Tada H, Watanabe M, Takase K and Takahashi S:
Luminal-type breast cancer: Correlation of apparent diffusion
coefficients with the Ki-67 labeling index. Radiology. 274:66–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
PLOS ONE Staff: Correction: Correlation of
the apparent diffusion coefficient (ADC) with the standardized
uptake value (SUV) in lymph node metastases of non-small cell lung
cancer (NSCLC) patients using hybrid 18F-FDG PET/MRI. PLoS One.
10:e01206062015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boesen L, Chabanova E, Løgager V, Balslev
I and Thomsen HS: Apparent diffusion coefficient ratio correlates
significantly with prostate cancer gleason score at final
pathology. J Magn Reson Imaging. 42:446–453. 2015. View Article : Google Scholar : PubMed/NCBI
|