Introduction
Hepatocellular carcinoma (HCC) is the second leading
cause of cancer-associated mortality worldwide, although the
survival rate of patients with HCC has improved due to curative
treatments, including surgical techniques, perioperative management
and a targeted drug (sorafenib) (1).
However, long-term survival following surgical resection remains
difficult to achieve owing to the high rate of cancer cell invasion
and metastasis (2). The
epithelial-mesenchymal transition (EMT) is a complex cellular
process, and may be one of the underlying molecular mechanisms for
enabling cancer cell invasion and metastasis, which are considered
to be malignant phases of tumor progression. Furthermore, EMT has
been widely reported to serve a central function in the process of
HCC metastasis (3). Therefore, the
development of novel agents targeting the EMT in HCC is an urgent
requirement.
Silent information regulator 1 (SIRT1), a member of
the mammalian sirtuin family (SIRT1-SIRT7), is involved in numerous
biological processes, including drug resistance, aging, apoptosis,
and tumor development and progression (4–8). Notably,
previous studies have revealed that SIRT1 is associated with the
EMT of HCC. The overexpression of SIRT1, which is frequently
detected in human HCC specimens, promotes HCC metastasis through
the EMT (9,10). SIRT1 has been proposed as a key
regulator of cancer metastasis by promoting EMT. As SIRT1 is a
nicotinamide-adenine dinucleotide (NAD+)-dependent
histone deacetylase, the abundance of NAD+ directly
regulates the activity of SIRT1 (11). Nicotinamide phosphoribosyltransferase
(NAMPT) is the rate-limiting enzyme in the synthesis of
NAD+ via a salvage pathway (12); its expression directly determines
NAD+ levels (13). Thus,
the NAMPT/NAD+/SIRT1 pathway may be a potential
alternative target in the treatment of HCC.
Previous studies have identified that FK866, a novel
small-molecule NAMPT inhibitor, possesses an anticancer function in
numerous types of cancer, including colon cancer, HCC, breast
cancer, Ewing sarcoma, lung cancer and pancreatic cancer (12,14–18). FK866
markedly decreases the NAMPT activity and NAD+ content
in HCC cells and leads to the decrease of adenosine 5′-triphosphate
(ATP) levels, which is associated with an increased rate of cell
death (14). The inhibitory effects
of FK866 on NAD+ and ATP activity, and
NAD+/SIRT1 signaling, have been well-studied and
reported (19–21). However, to the best of our knowledge,
the effect of FK866 on the invasion and metastasis of HCC cells, in
particular through regulating the NAMPT/NAD+/SIRT1
pathway, has not yet been reported.
The aim of the present study was to investigate
whether FK866 inhibited the EMT, migration and invasion of HCC
cells by mediating the NAMPT/NAD+ signaling pathway. The
inhibition of the viability of HCC cell line MHCC97-H by FK866
through the decrease in NAMPT activity and NAD+ levels
was demonstrated. Furthermore, the FK866-induced suppression of the
SIRT1 expression and metastatic capability of MHCC97-H cells via
the NAMPT/NAD+ pathway was revealed, as well as the
decrease in vimentin levels and increase in epithelial (E-)cadherin
levels. These results indicate that the NAMPT/NAD+/SIRT1
pathway may be a potential alternative therapeutic target and that
FK866 may be an effective drug targeting HCC metastasis and
invasion.
Materials and methods
Cells and treatments
The human liver tumor cell line MHCC97-H was
obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd.
(Shanghai, China) and maintained in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) containing 10% fetal bovine serum (FBS; Excell Biology, Inc.,
Shanghai, China), penicillin (100 U/ml) and streptomycin (100
µg/ml) (Gibco; Thermo Fisher Scientific, Inc.) under 95% air and 5%
CO2 at 37°C. For cell treatments, MHCC97-H cells were
cultured and treated with FK866 (Beyotime Institute of
Biotechnology; Shanghai, China) at concentrations of 0, 1.25, 2.5,
5, 10, 20 and 40 nM.
Cell Counting Kit-8 (CCK-8) assay
In total, ~5×103 cells/well were seeded
into 96-well plates (Corning Life Sciences Inc., Midland, MI, USA)
and treated with various concentrations of FK866 (0, 1.25, 2.5, 5,
10, 20 and 40 nM). Following incubation for 24, 48 and 72 h at
37°C, CCK-8 (5 mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was added to each well and cells were incubated for an
additional 1 h. Subsequently, the absorbance of the colored product
was measured at 450 nm using a plate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA). Each experiment was performed at least
three times.
NAD+ assay
Total cellular NAD+ was measured using a
Microdetermination assay kit (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China). In total, 5×106 cells were
seeded in a cell culture bottle and treated with various
concentrations of FK866 (0, 2.5, 5 and 10 nM) for 24 h at 37°C.
Following the addition of 1 ml alkaline extract (Beijing Solarbio
Science & Technology, Co., Ltd., Beijing, China) the mixture
was ultrasonicated for 1 min and heated at 95°C for 5 min in a
water bath. From the mixture, 500 µl supernatant was collected and
centrifuged at 10,000 × g for 10 min at 4°C. Subsequently, 500 µl
acid extract was added to the supernatant of the previous
centrifugation and centrifuged at 10,000 × g for 10 min at 4°C;
then extracted the supernatant and preserved on ice. Of this
supernatant, 200 µl was used for downstream procedures according to
the protocol for the NAD+ detection kit, using microdetermination.
The absorbance was measured at 570 nm using a plate reader and the
concentration of NAD+ was determined.
ATP measurement
Total cellular ATP levels were measured using the
Microdetermination assay kit. In total, 5×106 cells were seeded and
treated with various concentrations of FK866 (0, 2.5, 5 and 10 nM)
for 24 h at 37°C. Following the addition of 1 ml acid extract,
cells were ultrasonicated for 1 min and 500 µl supernatant was
collected and centrifuged at 8,000 × g for 10 min at 4°C.
Subsequently, 500 µl alkaline extract was added to the supernatant
of the previous centrifugation, the mixture was centrifuged at
8,000 × g for 10 min at 4°C, and the supernatant was preserved on
ice. Finally, 200 µl supernatant was used for downstream
microdetermination experiments using the ATP detection kit.
According to the manufacturer's protocol, the absorbance was
measured at 700 nm using a plate reader and the concentration of
ATP was determined accordingly.
Wound healing assay
In total, 1×106 cells were seeded into 6-well plates
(Corning Life Sciences, Inc.) and incubated at 37°C until the cells
reached a confluence of ≥90%. A scratch was created using a sterile
10-µl pipette tip. Subsequently, the cells were treated with
various concentrations of FK866 (0, 2.5, 5 and 10 nM) for 24 h at
37°C. Images were acquired at 0 h and 24 h using an inverted light
microscope (CKX41; Olympus Corporation, Tokyo, Japan). Thus,
relative migration distance=(the gap at 0 h - the gap at 24 h)/the
gap at 0 h. Cell healing and migration was observed by comparing
the relative migration distance of cells treated with different
concentrations of FK866 (0 and 10 nM) for 24 h.
Cell migration assay
In total, 2×104 cells were plated in 200 µl DMEM
without serum and containing different concentrations of FK866 (0,
2.5, 5 and 10 nM) in the upper chamber of a Transwell chamber
(Corning Life Sciences, Inc.). A 500 µl volume of DMEM containing
20% FBS and similar concentrations of FK866 were added to the lower
chamber, and the cells were incubated for 24 h at 37°C.
Subsequently, the cells on the upper surface of the membrane were
removed using a cotton swab. The cells that migrated through the
pores to the lower surface of the filter were fixed in 4%
formaldehyde for 20 min and stained with 0.1% crystal violet dye
for 20 min at 20°C. Following staining, the cells were washed three
times with PBS (Sigma-Aldrich; Merck KGaA). The outside membrane
cells were observed at ×200 magnification using an upright light
microscope (Eclipse 80i; Nikon Corporation, Tokyo, Japan) and 5
fields were randomly selected to calculate an average cell count.
This procedure was performed three times.
Transwell invasion assay
Matrigel invasion chambers installed with an 8.0 µm
polyethylene terephthalate membrane in 24-well plates (Corning Life
Sciences, Inc.) were used. In total, 5×104 cells in 200 µl DMEM
without serum were plated in each upper chamber, and 500 µl DMEM
containing 20% FBS was added to the lower chamber as a
chemoattractant; the upper and lower chambers contained similar
concentrations of FK866 (0, 2.5, 5 and 10 nM). After 24 h of
conventional incubation at 37°C, the cells on the upper surface
were wiped with a cotton swab. The cells that invaded through the
Matrigel and pores to the lower surface of the filter were fixed in
4% formaldehyde for 20 min and stained with 0.1% crystal violet for
20 min at 20°C, and then washed three times with PBS. The outside
membrane cells were observed at ×200 magnification using an upright
light microscope and 5 fields were selected randomly to calculate
an average cell count. This procedure was performed three
times.
Western blot analysis
In total, 5×106 cells were seeded in a cell culture
bottle and treated with various concentrations of FK866 (0, 2.5, 5
and 10 nM) for 24 h at 37°C. All cells were washed three times (5
min each) with ice-cold PBS and MHCC97-H cells suspended in
radioimmunoprecipitation assay lysis buffer (50 mM Tris/HCl, pH
7.4, 2 mM EDTA, 1 mM dithiothreitol and 150 mM NaCl), containing 1%
protease inhibitor cocktail (Thermo Fisher Scientific, Inc.),
followed by centrifugation at 12,000 × g for 5 min at 4°C to remove
the cellular debris. Protein concentrations were determined using
the enhanced Bincinchoninic Acid Protein assay kit (Pierce; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
An equivalent of 50 µg protein extract was separated by SDS-PAGE
(10% gel) and transferred onto polyvinylidene fluoride membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked in
Tris-buffered saline with 0.1% Tween-20 containing 5% non-fat milk
for 1 h at room temperature. Subsequently, the membranes were
probed with the primary antibodies anti-E-cadherin (mouse;
dilution, 1:1,000; cat. no. ab1416; Abcam, Cambridge, UK),
anti-SIRT1 (rabbit; dilution, 1:1,000; cat. no ab32441; Abcam) and
anti-vimentin (rabbit; dilution, 1:1,000; cat. no. ab92547; Abcam)
at 4°C overnight, and detected using appropriate horseradish
peroxidase-conjugated secondary antibody (Goat anti-rabbit;
dilution, 1:2,000. no. ab97051; Abcam) and enhanced
chemiluminescence reagent (Pierce; Thermo Fisher Scientific, Inc.).
The expression level of the proteins was normalized to that of
GAPDH (dilution, 1:1,000; cat. no. ab9485; Abcam), which served as
an endogenous control. Western blots were subjected to
densitometric analysis using Quantity One software (version 4.6.2;
Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5 software (version 6.02; GraphPad Software, La Jolla, CA,
USA). Data from 3 independent experiments were expressed as the
mean ± standard deviation. Statistical significance between the
groups was determined using one-way analysis of variance, followed
by Student-Newman-Keuls analyses. P<0.05 for a two-tailed test
was considered to indicate a statistically significant
difference.
Results
FK866 inhibits the viability of the
HCC cell line MHCC97-H
As a targeted NAMPT inhibitor, FK866 inhibits the
viability of the HCC cells and induces cancer cell apoptosis
(14). In the present study, the
effects of FK866 on HCC cell viability were investigated, using the
highly metastatic human MHCC97-H cell line. The inhibitory effects
of FK866 on the cell viability of MHCC97-H cells were observed
using the CCK-8 cell viability assay. Following incubation for
various durations (24, 48 and 72 h), the cell viability was
determined by measuring the absorbance at 450 nm. No significant
difference was observed among the FK866-untreated group and the
FK866-treated groups at lower concentrations (1.25 and 2.5 nM).
Compared with the FK866-untreated group, FK866 at medium and high
doses (5, 10, 20 and 40 nM) suppressed the cell viability
significantly. However, no significant difference was observed
among the FK866-treated groups at medium (10 nM) and high doses (20
and 40 nM). We also think that high concentration of FK866 has a
greater toxic effect on normal cells; so, we screened a more
reasonable range of drug concentration (0, 2.5, 5 and 10 nM) for
subsequent experiments. Furthermore, no significant difference was
detected in the level of cell viability using the CCK-8 assay at
various time points (24–72 h). The data suggest that FK866 inhibits
the viability of MHCC97-H cells in a dose-dependent, but not a
time-dependent, manner (Fig. 1).
FK866 decreases the levels of
NAD+ and ATP in MHCC97-H cells
NAD+ is the reaction substrate of ATP and is
predominantly synthesized via the salvage pathway in normal and
tumor cells. NAMPT is the rate-limiting enzyme in the salvage
pathway, affecting the synthesis rate and NAD+ level (20). It was hypothesized that FK866
suppresses the viability of MHCC97-H cells by inhibiting NAMPT and
decreasing the levels of NAD+ and ATP. Therefore, various
concentrations of FK866 (0, 2.5, 5 and 10 nM) were added to the
cells for 24 h. Compared with the FK886-untreated group, the levels
of ATP and NAD+ in all the FK866-treated groups (2.5, 5 and 10 nM)
were significantly lower (P<0.05), as presented in Fig. 2. Furthermore, it was identified that
FK866 led to the decrease in NAD+ and ATP levels in a
dose-dependent manner. At the highest concentration of FK866, the
levels of NAD+ and ATP were at their lowest. These results suggest
that FK886 decreases the NAMPT activity, which causes a decline in
the levels of NAD+ and ATP in MHCC97-H cells.
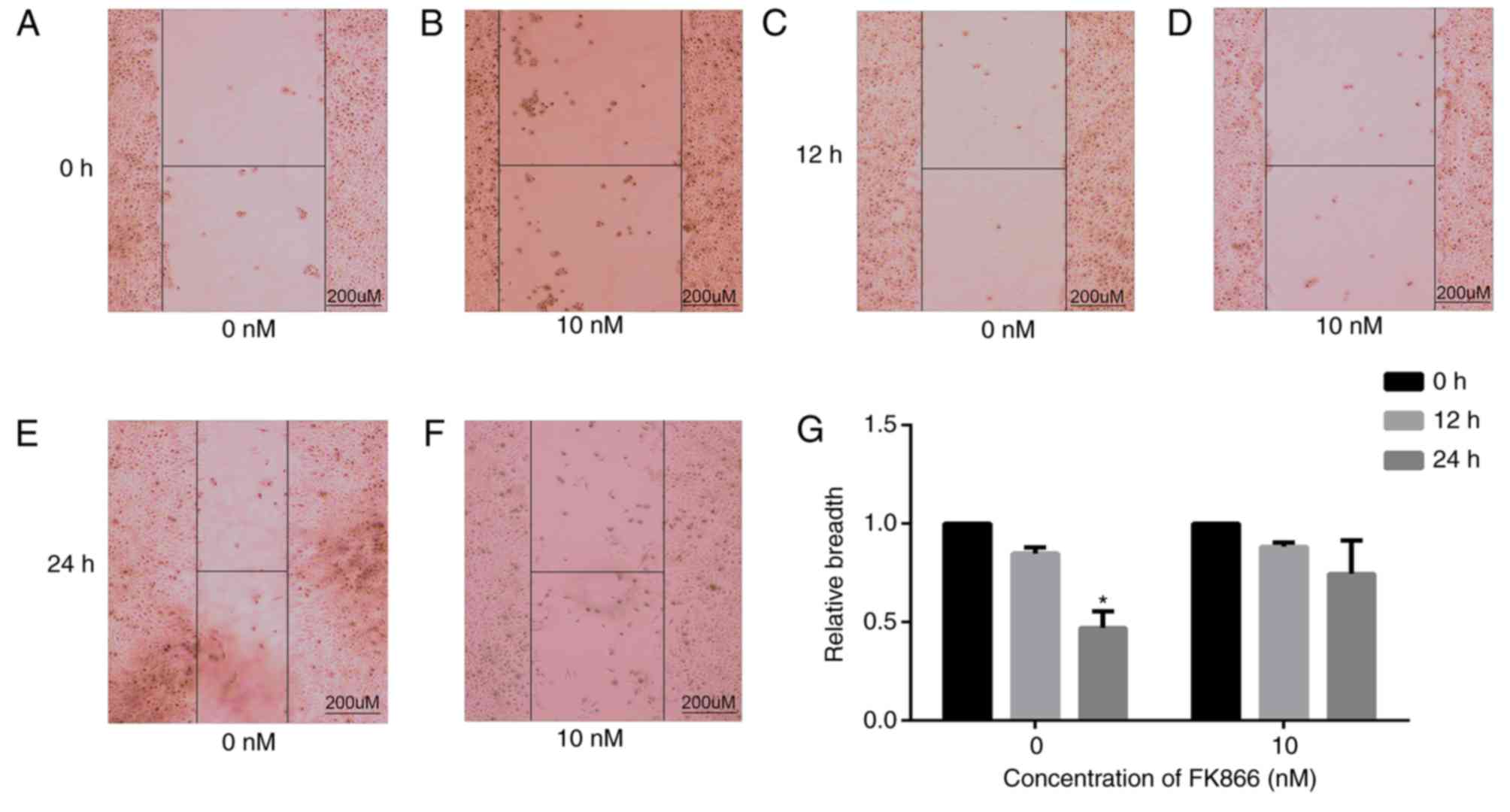
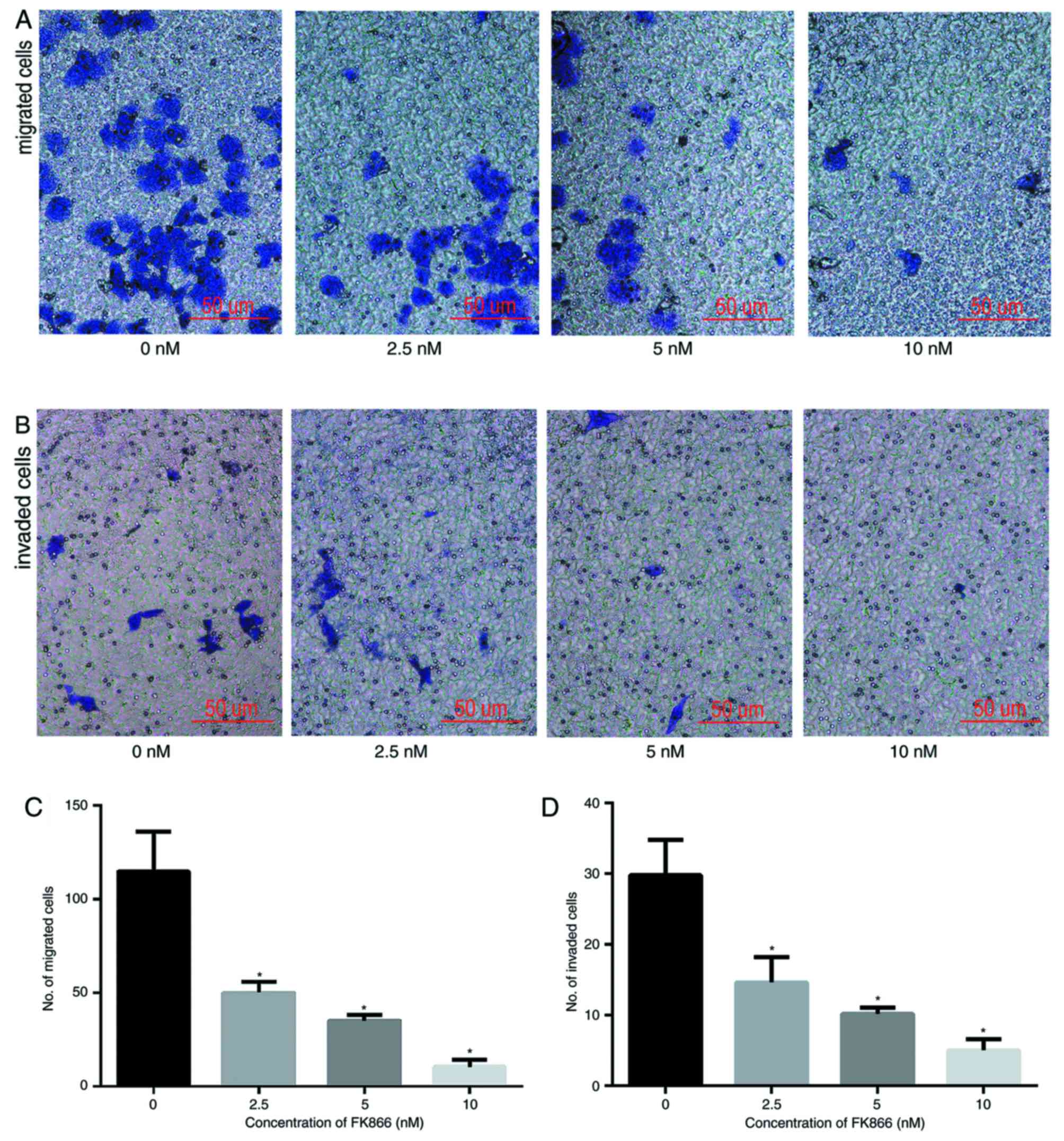
FK866 inhibits the invasion and
migration of MHCC97-H cells
The effects of FK866 on the invasion and metastasis
of MHCC97-H cells were investigated using wound healing, invasion
and migration assays. MHCC97-H cells were treated with FK866 (10
nM) and those untreated served as a negative control. After 24 h,
the wound closures of control group were significantly decreased
(P<0.05) compared with those at 0 h; however, the wound closures
of FK866 (10 nM)-treated cells were not significantly decreased
compared with those at 0 h (Fig. 3)
(FK866 at 2.5 and 5 nM did not exhibit a significant effect
compared with the control group; therefore, we chose two groups (0
and 10 nM) with significant differences in the results). This
indicates that FK866 inhibits the healing ability of MHCC97-H
cells. The migratory and invasive capabilities of MHCC97-H cells
were measured using Transwell assays. The number of cells invading
into the lower chambers was significantly lower upon treatment with
FK866 for 24 h compared with those in the vehicle-treated control
group and the decrease occurred in a concentration-dependent manner
(Fig. 4). These data suggest that
FK866 treatment for 24 h results in a significant decrease in the
invasive and migratory capabilities of MHCC97-H cells in a
concentration-dependent manner compared with control cells.
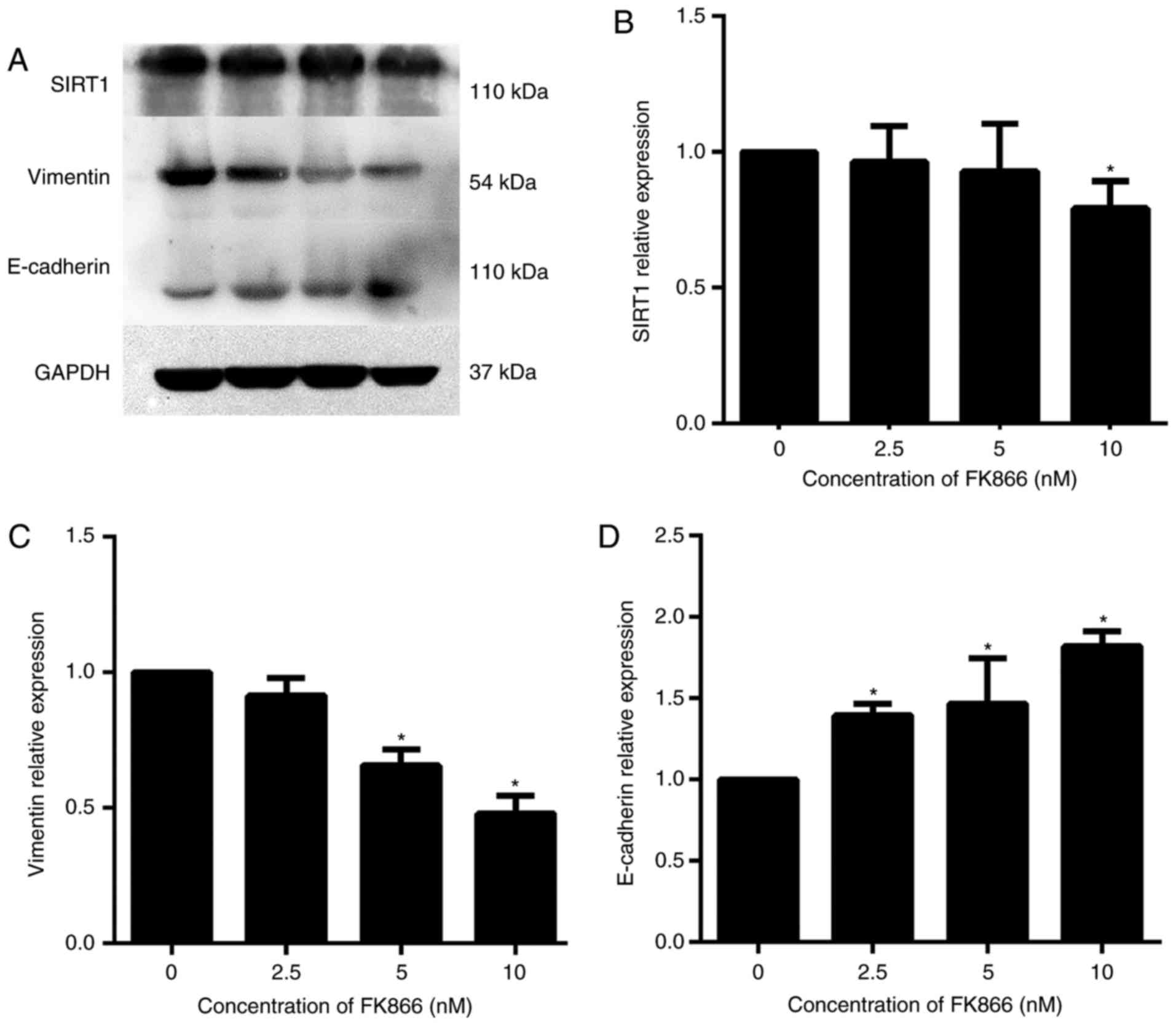
FK866 inhibits the expression of SIRT1
and reverses the EMT in the HCC cell line MHCC97-H
Subsequently, the molecular mechanism underlying
FK866-mediated inhibition of MHCC97-H cell invasion was
investigated. SIRT1 participates in the EMT process of HCC, and
E-cadherin and vimentin, as EMT markers, serve critical functions
in cancer cell invasion (9,10). The results of the present study
indicated that the expression of SIRT1 and vimentin was
downregulated significantly and that of E-cadherin was upregulated
significantly at the protein level by 10 nM FK866, as compared with
the vehicle-treated control group (Fig.
5).
Discussion
HCC is one of the most common causes of malignant
tumors, with a poor prognosis and a clinical 5-year postoperative
recurrence rate of 70%, due primarily to high invasion and
migration rates of the HCC cells (1,2). HCC cell
invasion and migration are two of the most clinically important
characteristics of the tumor, and EMT serves a crucial function in
these processes (3). Therefore,
identifying potential novel targeted agents that are effective
against EMT in HCC is an urgent requirement. In the present study,
novel small-molecule NAMPT inhibitor FK866 was revealed to inhibit
NAMPT/NAD+/SIRT1-mediated EMT, suggesting that FK866 may
be a novel targeted drug for the treatment of patients with
metastatic HCC.
In normal and tumor cells, NAD+
participates in several critical biological processes, including
transcription, cell cycle progression, DNA repair, and circadian
rhythm and metabolic regulation (22). NAD+ is predominantly
synthesized via the salvage pathway. NAMPT is the rate-limiting
enzyme in the salvage pathway of NAD+ and catalyzes the
conversion of nicotinamide (a main precursor of NAD+)
into nicotinamide mononucleotide. The survival of the tumor cells
requires more NAD+ and NAMPT compared with that of the
normal cells (23). One of the most
important functions of NAD+ is to act as an acetylation
substrate of SIRT1, whose activity is directly regulated by the
NAD+ salvage pathway, in particular by the rate-limiting
enzyme NAMPT (20). Consistent with
these studies, the results of the present study revealed that
inhibiting the expression of NAMPT suppresses the levels of
NAD+ and SIRT1. It has been identified previously that
SIRT1 is associated with the EMT process of HCC (24). The level of SIRT1 increases in HCC, a
phenomenon that is associated with poor prognosis in patients with
HCC (25). SIRT1 also serves
important functions in HCC stem cell self-renewal and promotes the
invasiveness of tumor cells (25,26).
E-cadherin and vimentin are EMT marker proteins that serve a
central function in cancer cell invasion; SIRT1 is associated with
the expression of invasive proteins in the tumor (9,10). SIRT1
is a critical regulator of cancer progression, by participating in
HCC metastasis via EMT promotion (24). However, to the best of our knowledge,
the precise molecular mechanism of how SIRT1 affects the invasion
and metastasis of HCC cells has not yet been elucidated. In the
present study, the function of SIRT1 in the EMT process of HCC
cells was investigated using cell invasion and metastasis assays.
Inhibiting the level of SIRT1 leads to the suppression the EMT, as
demonstrated by the upregulation of E-cadherin and downregulation
of vimentin. Furthermore, SIRT1 inhibits the EMT process by
inhibiting the NAMPT/NAD+ pathway.
Subsequently, the inhibitory regulation of the
NAMPT/NAD+/SIRIT1 pathway required further
investigation. FK866, as an NAMPT inhibitor, significantly
decreases the NAD+ levels by inhibiting NAMPT; it also
markedly inhibits the viability of different types of tumor cells
(7,13–16). The
results of the present study further confirm that FK866 decreases
the levels of NAD+ and ATP and suppresses the viability
of HCC cells by inhibiting NAMPT in MHCC97-H cells.
FK866 has been used in a Phase II clinical trial as
an antitumor drug (27). A recent
study identified that FK866 provides a novel therapeutic strategy
to enhance the efficacy of chemotherapeutic agents, including
etoposide, against leukemia (28).
Furthermore, FK866 also significantly enhances the antitumor
activity of gemcitabine in pancreatic ductal adenocarcinoma cells
and orthotopic xenograft mouse models, suggesting that FK866 may
aid in overcoming gemcitabine resistance by decreasing the
NAD+ level and suppressing the glycolytic activity in
pancreatic cancer treatment (29).
However, the inhibitory effect of FK866 on tumor invasion and
metastasis is rarely reported. In the present study, for the first
time, the inhibitory function of the NAMPT-specific inhibitor FK866
in SIRT1-induced EMT in HCC was revealed, suggesting that FK866 may
be used as a novel SIRT1 inhibitor. Regarding cell phenotype, these
results indicated that FK866 inhibits MHCC97-H cell invasion and
metastasis. In addition, FK866 decreases the SIRT1 protein
expression and reverses that of EMT marker proteins; specifically,
it upregulates E-cadherin and downregulates vimentin protein
expression. The function of FK866 on EMT is demonstrated in a
schematic diagram (Fig. 6).
 | Figure 6.Function of FK866. In normal and tumor
cells, NAD+ is predominantly synthesized via the salvage
pathway. NAMPT, as the rate-limiting enzyme in the salvage pathway
of NAD+, catalyzes the conversion of NAM (a main
precursor substance of NAD+) into NMN. One of the major
functions of NAD+ is an acetylation substrate of SIRT1,
whose activity is directly regulated by the nicotinamide to
NAD+ salvage pathway, in particular the rate-limiting
enzyme NAMPT. FK866, as the NAMPT inhibitor, inhibits the cell
NAMPT/NAD+/SIRT1 pathway and reverses the expression of
EMT marker proteins, by upregulating E-cadherin and downregulating
vimentin protein expression. NAD+, nicotinamide adenine
dinucleotide; NAMPT, nicotinamide phosphoribosyltransferase; NAM,
nicotinamide; NMN, nicotinamide mononucleotide; SIRT1, silent
information regulator 1; E-cadherin, epithelial cadherin; EMT,
epithelial-mesenchymal transition. |
In summary, NAMPT/NAD+/SIRT1 is
associated with energy metabolism in a number of downstream protein
regulatory pathways that together comprise a systemic regulatory
network in HCC cell invasion and migration.
The results of the present study confirm that FK866
significantly decreases the levels of NAD+ and ATP, and
suppresses the cell invasion and metastasis by inhibiting the
NAMPT/NAD+/SIRT1 signaling pathway in MHCC97-H cells.
Overall, these results suggest that FK866 may be an effective
targeted HCC drug and that the NAMPT/NAD+/SIRT1 pathway
may serve as a potential therapeutic alternative for HCC
metastasis.
Acknowledgements
Not applicable.
Funding
The authors received grants from the National
Natural Science Foundation of China (grant nos. 81773337 and
81401653) and the Natural Science Foundation of Shandong Province
(grant no. ZR2015HL127), China.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ, GL, DS and SQ conceived and designed the
experiments. BZ conducted all of the experiments. BZ wrote and
revised the manuscript. XZ and WL analyzed the obtained data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing and treating hepatocellular carcinoma. CA
Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Wang W, Wang Y, Huang X, Zhang Z,
Chen B, Xie W, Li S, Shen S and Peng B: NEK2 promotes
hepatocellular carcinoma migration and invasion through modulation
of the epithelial-mesenchymal transition. Oncol Rep. 39:1023–1033.
2018.PubMed/NCBI
|
|
4
|
Liu T, Liu P Y and Marshall GM: The
critical role of the class III histone deacetylase SIRT1 in cancer.
Cancer Res. 69:1702–1705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jang KY, Noh SJ, Lehwald N, Tao GZ,
Bellovin DI, Park HS, Moon WS, Felsher DW and Sylvester KG: SIRT1
and c-Myc promote liver tumor cell survival and predict poor
survival of human hepatocellular carcinomas. PLoS One.
7:e451192012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bae HJ, Noh JH, Kim JK, Eun JW, Jung KH,
Kim MG, Chang YG, Shen Q, Kim SJ, Park WS, et al: MicroRNA-29c
functions as a tumor suppressor by direct targeting oncogenic SIRT1
in hepatocellular carcinoma. Oncogene. 33:2557–2567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang
X, Liu H, Lu Y, Liao J, Chen X, et al: SIRT1 limits the function
and fate of myeloid-derived suppressor cells in tumors by
orchestrating HIF-1α-dependent glycolysis. Cancer Res. 74:727–737.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang XJ, Finkel T, Shen DW, Yin JJ,
Aszalos A and Gottesman MM: SIRT1 Contributes in part to cisplatin
resistance in cancer cells by altering mitochondrial metabolism.
Mol Cancer Res. 6:1499–1506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen J, Zhang B, Wong N, Lo AW, To KF,
Chan AW, Ng MH, Ho CY, Cheng SH, Lai PB, et al: Sirtuin 1 is
upregulated in a subset of hepatocellular carcinomas where it is
essential for telomere maintenance and tumor cell growth. Cancer
Res. 71:4138–4149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hao C, Zhu PX, Yang X, Han ZP, Jiang JH,
Zong C, Zhang XG, Liu WT, Zhao QD, Fan TT, et al: Overexpression of
SIRT1 promotes metastasis through epithelial-mesenchymal transition
in hepatocellular carcinoma. BMC Cancer. 14:9782014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee Y, Drake AC, Thomas NO, Ferguson LG,
Chappell PE and Shay KP: Dietary resveratrol increases mid-life
fecundity of female Nothobranchius guentheri. Comp Biochem Physiol
C Toxicol Pharmacol. 208:71–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerner RR, Klepsch V, Macheiner S, Arnhard
K, Adolph TE, Grander C, Wieser V, Pfister A, Moser P,
Hermann-Kleiter N, et al: NAD metabolism fuels human and mouse
intestinal inflammation. Gut. 67:1813–1823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Grubisha O, Smith BC and Denu JM: Small
molecule regulation of Sir2 protein deacetylases. FEBS J.
272:4607–4616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schuster S, Penke M, Gorski T, Gebhardt R,
Weiss TS, Kiess W and Garten A: FK866-induced NAMPT inhibition
activates AMPK and downregulates mTOR signaling in hepatocarcinoma
cell. Biochem Biophys Res Commun. 458:334–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alaee M, Khaghani S, Behroozfar K, Hesari
Z, Ghorbanhosseini SS and Nourbakhsh M: Inhibition of nicotinamide
phosphoribosyltransferase induces apoptosis in estrogen
receptor-positive MCF-7 breast cancer cells. J Breast Cancer.
20:20–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mutz CN, Schwentner R, Aryee DNT, Bouchard
EDJ, Mejia EM, Hatch GM, Kauer MO, Katschnig AM, Ban J, Garten A,
et al: EWS-FLI1 confers exquisite sensitivity to NAMPT inhibition
in Ewing sarcoma cells. Oncotarget. 8:24679–24693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HY, Li QR, Cheng XF, Wang GJ and Hao
HP: NAMPT inhibition synergizes with NQO1-targeting agents in
inducing apoptotic cell death in non-small cell lung cancer cells.
Chin J Nat Med. 14:582–589. 2016.PubMed/NCBI
|
|
18
|
Barraud M, Garnier J, Loncle C, Gayet O,
Lequeue C, Vasseur S, Bian B, Duconseil P, Gilabert M, Bigonnet M,
et al: A pancreatic ductal adenocarcinoma subpopulation is
sensitive to FK866, an inhibitor of NAMPT. Oncotarget.
7:53783–53796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song TY, Yeh SL, Hu ML, Chen MY and Yang
NC: A Nampt inhibitor FK866 mimics vitamin B3 deficiency by causing
senescence of human fibroblastic Hs68 cells via attenuation of
NAD(+)-SIRT1 signaling. Biogerontology. 16:789–800. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Imai S: Dissecting systemic control of
metabolism and aging in the NAD World: The importance of SIRT1 and
NAMPT-mediated NAD biosynthesis. FEBS Lett. 585:1657–1662. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang NC, Song TY, Chen MY and Hu ML:
Effects of 2-deoxyglucose and dehydroepiandrosterone on
intracellular NAD(+) level, SIRT1 activity and replicative lifespan
of human Hs68 cells. Biogerontology. 12:527–536. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiarugi A, Dölle C, Felici R and Ziegler
M: The NAD metabolome- a key determinant of cancer cell biology.
Nat Rev Cancer. 12:741–752. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bi TQ and Che XM: Nampt/PBEF/visfatin and
cancer. Cancer Biol Ther. 10:119–125. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choupani J, Mansoori Derakhshan S, Bayat
S, Alivand MR and Shekari Khaniani M: Narrower insight to SIRT1
role in cancer: A potential therapeutic target to control
epithelial-mesenchymal transition in cancer cells. J Cell Physiol.
233:4443–4457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Liu C, Zhang Q, Shen J, Zhang H,
Shan J, Duan G, Guo D, Chen X, Cheng J, et al: SIRT1-mediated
transcriptional regulation of SOX2 is important for self-renewal of
liver cancer stem cells. Hepatology. 64:814–827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng J, Liu C, Liu L, Chen X, Shan J,
Shen J, Zhu W and Qian C: MEK1 signaling promotes self-renewal and
tumorigenicity of liver cancer stem cells via maintaining SIRT1
protein stabilization. Oncotarget. 7:20597–20611. 2016.PubMed/NCBI
|
|
27
|
Esposito E, Impellizzeri D, Mazzon E,
Fakhfouri G, Rahimian R, Travelli C, Tron GC, Genazzani AA and
Cuzzocrea S: The NAMPT inhibitor FK866 reverts the damage in spinal
cord injury. J Neuroinflammation. 9:662012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grohmann T, Penke M, Petzold-Quinque S,
Schuster S, Richter S, Kiess W and Garten A: Inhibition of NAMPT
sensitizes MOLT4 leukemia cells for etoposide treatment through the
SIRT2-p53 pathway. Leuk Res. 69:39–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ju HQ, Zhuang ZN, Li H, Tian T, Lu YX, Fan
XQ, Zhou HJ, Mo HY, Sheng H, Chiao PJ and Xu RH: Regulation of the
Nampt-mediated NAD salvage pathway and its therapeutic implications
in pancreatic cancer. Cancer Lett. 379:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|