Introduction
Breast cancer (BC) is the most frequent tumor and
the second leading cause of cancer death among women, with an
estimate rate of about 250.000 new cases in 2016 (1). Despite the increase in chances of cure,
approximately 20–45% of affected patients will develop metastases
(2), particularly bone metastases in
60–80% of cases (3), bone-only
metastases in 17–37% (4), and from 25
to 40% of patients have bone metastases at the diagnosis (3). Study data suggest that bone-only
patients survive more than others (5). In a recent ‘real-life’ study of
metastatic BC (MBC) patients, the median overall survival (OS) was
37.22 months (6), while some studies
reported a median OS up to 72 months for bone-only patients
(7).
Microarray analyses identified different gene
expression profiles in BC, thus differentiating molecular subgroups
with different clinical course; several studies evaluated the
association between subgroups and metastatic spread, showing that
bone is the most common metastatic site in the luminal A (66.6%),
luminal B (71.4%) and luminal/HER2+ tumors (65%), while it is less
frequently involved in basal-like tumors (39%) (8). So, the expression of hormone receptors
in BC is associated with a higher risk of developing bone
metastases, but their presence does not necessarily imply that the
metastatic involvement remains confined exclusively to the bone
(9,10). Indeed, experimental evidences prove
that BC metastasis to the bone is mediated by a specific set of
genes beyond those involved in the development of the primary tumor
(11).
Osteoporosis, a process of bone mineral density
(BMD) reduction, is accelerated by estrogen deficiency in
postmenopausal women. Tamoxifen reduces BMD in premenopausal women,
while promotes bone formation in postmenopausal patients. On the
other hand, adjuvant aromatase inhibitors (AIs) therapy enhances
the BMD decrease to about 2.5% per year, due to a long-lasting
significant deprivation of circulatory and tissue estrogens
(12). In the bone companion study of
the MA.17 trial, patients treated with anastrozole reported a
significant decreases in BMD (~4%), compared to those treated with
tamoxifen (13). Due to osteoporosis,
the bone microarchitecture is impaired and the microenvironment is
modified. The ‘seed and soil’ hypothesis, proposed by Paget more
than a hundred years ago (14),
speculates that pro-metastatic tumor cells (the ‘seed’) take root
in specific organ sites (the ‘soil’) where the microenvironment is
favorable for metastasis. The primary tumor can promote metastasis
by inducing the creation of a permissive microenvironment in a
secondary organ site, termed the pre-metastatic niche (15,16). The
alteration of bone health associated with osteoporosis may provide
fertile soil for the activation of the metastatic cascade, from the
seeding of tumor cells to the activation of indolent
micrometastases and finally to the expansion of bone lesions
(17).
Several large randomized trials of adjuvant
bisphosphonates vs. placebo showed an improvement, in both
disease-free survival (DFS) and OS, in women with early BC treated
with endocrine therapy (18–21). The role of denosumab in the prevention
of AI-induced bone resorption was demonstrated in the ABCSG-18
phase III trial, which evaluated the effects of adjuvant denosumab
in postmenopausal patients with early BC receiving AIs (22).
It is well known that bone metastases negatively
affect patients' quality of life. Bisphosphonates showed to improve
it, by reducing pain and the consequent consumption of analgesics,
but they did not demonstrate to prolong survival (23).
Bisphosponates like zoledronic acid, limit the loss
of bone density, by binding and blocking the enzyme farnesyl
diphosphate synthase (FPPS) in the HMG-CoA reductase pathway,
leading to inhibition of both osteoclastogenesis, cell survival,
and cytoskeletal dynamics, which is vital for maintaining the
‘ruffled border’ that is required for contact between a resorbing
osteoclast and a bone surface. Denosumab is a human monoclonal
antibody which acts on ‘pre-osteoclasts’. These precursors express
on their cell surface receptors called RANK (receptor activator of
nuclear factor-kappa B). RANK is activated by RANKL (the
RANK-Ligand), which exists as cell surface molecules on osteoblasts
and this binding promotes the maturation of pre-osteoclasts into
osteoclasts. Denosumab proved to be more effective than zoledronic
acid in terms of pain reduction, allowing a smaller percentage of
patients makes use of strong opioids (24).
In the phase 3 study published by Stopeck et
al (25), in 2010, 2049 patients
with bone MBC (BMBC), were randomized to receive denosumab or
zoledronic acid. The median time to first skeletal-related event
(SRE) was 26.4 months in the group of patients treated with
zoledronic acid, while it had not yet been reached in the group
treated with denosumab, with a reduction in terms of time to first
SRE by 18% over zoledronic acid (25). The subsequent uploaded data showed a
median time to first SRE in the denosumab arm of 32.4 months
(26). Moreover, denosumab
demonstrated to decrease the risk of occurrence of multiple SREs by
23% and to reduce the skeletal morbidity rate (ratio between the
number of SREs per patient divided by the patient of the time at
risk) by 22% vs. zoledronic acid. Overall survival and disease
progression were similar in the two groups. As to adverse events,
pyrexia, bone pain, arthralgia, renal failure and hypercalcemia
were more frequent during treatment with zoledronic acid, while
hypocalcemia and toothache during treatment with denosumab. The
risk of osteonecrosis of the jaw (ONJ) was not higher with
denosumab compared to zoledronic acid (P=0.39) (25).
Here we report a ‘real life’ multicenter
retrospective analysis of BMBC patients treated with denosumab,
focusing both to clinical outcomes commonly related to the
treatment (safety and efficacy in reduce skeletal related events)
and to the possible correlations between patients/diseases'
features and clinical patterns of recurrence to the bone.
Patients and methods
Study design and statistical
analysis
A retrospective analysis of BMBC patients treated
with denosumab, at the medical oncology departments of St.
Salvatore Hospital in L'Aquila and Campus Bio-medico University
Hospital in Rome, was conducted. Data cut-off was August 2017.
Comorbidities were classified according to the Cumulative Index
Rating Scale (CIRS) (27). Estrogen
and Progesterone Receptor expressions were evaluated by
immunohistochemistry (IHC), using Dako monoclonal antibodies. HER2
analysis was performed by IHC on paraffin embedded tissue from the
primary tumor and/or metastatic site (Hercept-Test®,
Genentech Inc. subject to licenses held by Dako Denmark A/S,
Glostrup, Denmark and F. Hoffmann-La Roche Ltd., Basel,
Switzerland). Fluorescence in situ hybridization (FISH) and
silver in situ hybridization (SISH) were used for cases of
doubtful interpretation. ‘Luminal-like’ disease was defined in any
case of Estrogen and/or Progesterone Receptor expression. Toxicity
was registered according to the National Cancer Institute Common
Toxicity Criteria (v4.0). Clinical evaluation of bone metastases
was performed by radiographic imaging (X-ray, computed tomography
scan or magnetic resonance) every three months or as clinically
indicated up to death or last contact.
Definition of SRE included pathological fractures
(not due to major trauma), radiation therapy on a bone segment,
bone surgery or spinal cord compression (28). Hypercalcemia of malignancy was not
considered among SREs. Subsequent events which occurred within 30
days of each other were not counted as separate events but rather
unique (for example, a surgery to repair a fracture or multiple
doses of radiation therapy during a treatment cycle). Only SREs
occurred during treatment with denosumab were included in the
analysis. Time to first SRE was defined as the interval from the
start of denosumab to the onset of the first SRE (but not within
the first month of treatment). Median time to skeletal recurrence
(TSkR) was defined as the length of time from the surgical
radicalization of the primary tumor to the diagnosis of first
skeletal metastasis (it was calculated only for patients with
metachronous recurrence to the bone). OS was defined as the
interval from the start of denosumab to death or last contact.
Subgroup analyses were performed among patients
according to the following variables: elderly status (< vs. ≥ of
70 years) (29), ECOG-PS (Eastern
Cooperative Oncology Group Performance Status) (0/1 vs. 2), CIRS
stage (primary/intermediate vs. secondary), ‘luminal-like’ disease
(yes vs. no), HER2 status (positive vs. negative), menopausal
status, extension of disease (bone only vs. non-bone only),
visceral involvement (excluding lymph nodes) (yes vs. no), previous
bisphosphonates (yes vs. no), number of bone metastases (1 vs. ≥1),
involvement of axial bones (yes vs. no), exclusively-osteolytic
type of metastases (yes vs. no).
Patient eligibility
Patients were eligible if they had histologically
confirmed diagnosis of BC, radiological confirmation of at least
one bone metastasis; age ≥18 years; adequate haematological, renal
and hepatic functions; albumin-adjusted serum calcium between 8.1
and 10.4 mg/dl. Previous intravenous bisphosphonate therapy was
allowed. Exclusion criteria were recent (<3 months) surgery of
the oral cavity or inflammatory untreated dental-periodontal or
peri-implant disease. All patients performed an orthopantomography
and a dental examination at baseline and twice a year thereafter.
All patients provided written informed consent to the proposed
treatment and to participate to this analysis. To guarantee the
confidentiality of personal data for deceased patients, all the
available procedures to ensure anonymity have been used. The
procedures followed were in accordance with the precepts of Good
Clinical Practice and the ethical standards of local responsible
committee on human experimentation (Comitato Etico per le province
di L'Aquila e Teramo).
Treatment
All patients received a subcutaneous injection of
denosumab (XGEVA®, Amgen Europe B.V. Breda, The
Netherlands) 120 mg every 4 weeks. A daily calcium (≥500 mg) and
vitamin D (≥1,000 U) oral supplement was recommended. All patients
received concomitant specific antineoplastic treatment,
chemotherapy or endocrine therapy (Table
I).
 | Table I.Patients' features. |
Table I.
Patients' features.
| Clinical
feature | No. of patients
(%) |
|---|
| Total no. of
patients | 90 (100.0) |
| Sex |
|
|
Male | 1 (1.1) |
|
Female | 89 (98.9) |
| Age |
|
| Non
elderly | 69 (76.7) |
|
Elderly | 21 (23.3) |
| ECOG PS |
|
|
0–1 | 84 (93.3) |
| ≥2 | 6 (6.7) |
| CIRS
(Comorbidity) |
|
|
Primary/intermedieate | 73 (81.1) |
|
Secondary | 17 (18.9) |
| Luminal-like | 86 (95.6) |
| HER2 positive | 13 (14.4) |
| Triple
negative | 4 (4.4) |
| Type of
disease |
|
|
Synchronous | 36 (40.0) |
|
Metachronous | 54 (60.0) |
| Menopausal
status |
|
|
Yes | 74 (82.2) |
| No | 16 (17.8) |
| Onset localization
of metastases |
|
|
Bone | 72 (80.0) |
|
Visceral | 18 (20.0) |
| >1 bone
metastases | 80 (88.9) |
| Bone-only
disease | 35 (38.9) |
| Visceral
disease | 38 (42.2) |
| Axial bone
metastases | 81 (90.0) |
| Type of bone
metastases |
|
|
Osteolytic exclusively | 48 (53.3) |
|
Others | 42 (46.7) |
| Concomitant
treatments |
|
|
Chemotherapy | 43 (47.8) |
|
Hormonal therapy | 70 (77.8) |
|
Aromatase inhibitors | 52 (57.8) |
|
Anti-HER2 therapy | 13 (14.4) |
|
Everolimus | 29 (32.2) |
|
Bevacizumab | 14 (15.6) |
| CDK
inhibitors | 5 (5.6) |
| Previous
bisphosphonates | 27 (30.0) |
Statistical analysis
Median TSkR, median time to first SRE and median OS
were evaluated using the Kaplan-Meier method (30). Median period of follow-up was
calculated according to the reverse Kaplan-Meier method (31). χ2 and Fisher's exact test
were used to compare the incidence of SREs among subgroups, using
the appropriate test according to the sample size in contingency
tables for each comparison (32,33).
Log-rank (34) was used to compare
TSkR among subgroups. Cox proportional hazards regression (35) was used for univariate and multivariate
analyses of clinical outcomes among subgroups (time to first SRE
and OS). For statistical analysis MedCalc Statistical Software,
v18.6 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018) was used.
Results
Patients' features
From July 2012 to August 2017 90 consecutive BMBC
patients were treated with denosumab. Clinical features of patients
are summarized in Table I. One out of
90 patients was male, median age was 61 years (range 26–91).
Twenty-one patients (23.3%) were elderly, 6 (6.7%) had ECOG-PS ≥2,
17 (18.9%) had a secondary CIRS stage and 74 (82.2%) were in
menopausal status. Eighty-six patients (95.6%) had ‘luminal-like’
disease, 72 (80%) had bone metastases at the time of diagnosis of
metastatic disease, 18 (20%) had the firs recurrence of disease to
visceral organs. Disease-oriented systemic treatments were
concomitantly administered to all patients; 27 (30%) patients
received previous bisphosphonate therapy. Fifty-four patients (60%)
had a metachronous disease; clinical features of these patients are
listed in Table II. Among 50
patients with metachronous ‘luminal-like’ disease, 40 (80%) had
first recurrence to the bone, 10 (20%) to the visceral organs.
Nineteen (38%) were previously treated with tamoxifen, 26 patients
(52%) with AIs adjuvant therapy (including 10 to both tamoxifen and
AIs).
 | Table II.Clinical features of patients with
metachronous disease. |
Table II.
Clinical features of patients with
metachronous disease.
| Clinical
feature | No. of patients (%)
(n=54) |
|---|
| Triple
negative | 4 (7.4) |
| Luminal-like | 50 (92.6) |
| Adjuvant hormonal
therapy |
|
|
Aromatase inhibitors | 26 (52) |
|
Tamoxifen | 19 (38) |
| Onset localization
of metastases |
|
|
Bone | 40 (80) |
|
Visceral | 10 (20) |
Clinical patterns of disease
recurrence to the bone
Among 40 patients with metachronous ‘luminal-like’
disease and first recurrence to the bone, fisher exact test did not
show any statistically significant difference between previously
exposed to AIs vs. not (P=0.4896) and to tamoxifen vs. not
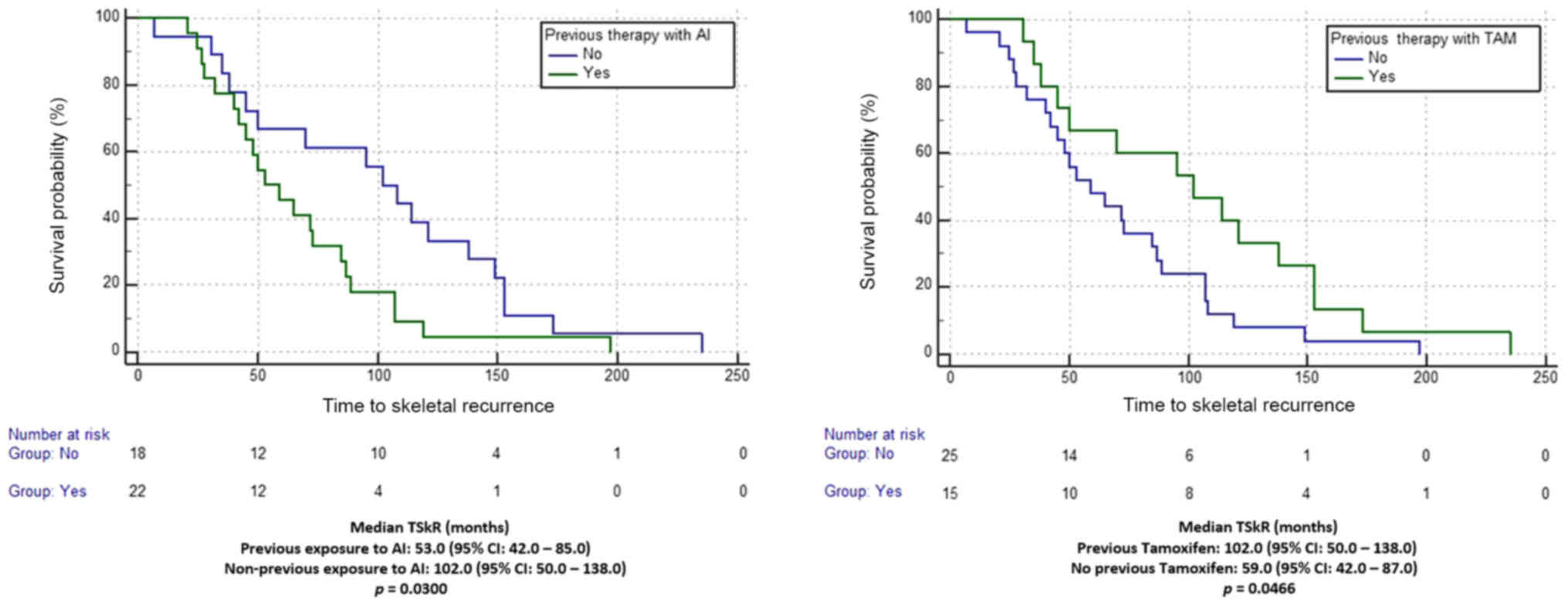
(P=1.0000). Among these patients median TSkR was 70.0 months (95%
CI: 50.0–95.0). At univariate analysis with log-rank test, median
TSkR was significantly shorter for patients who were previously
exposed to AIs compared to those who were not (53.0 vs 102.0
months, respectively; P=0.0300). Median TSkR was also significantly
longer for patients previously treated with tamoxifen compared to
those who were not (102.0 vs. 59.0 months, respectively; P=0.0466;
Fig. 1). At multivariate analysis,
neither previous exposure to AIs (HR=1.66, 95% CI: 0.48–5.72) nor
treatment with tamoxifen (HR = 0.78, 95% CI: 0.22–2.75) were
confirmed as independent predictors for TSkR.
Clinical outcomes
All 90 patients were evaluable for clinical
outcomes, as summarized in Table
III. A median number of 18.5 cycles of denosumab was
administered. In the overall population, 17 first SREs were
observed, represented by 16 radiation therapy on a bone segment and
1 pathological fracture, with an incidence of 18.8% (95% CI:
11.4–28.5). Among patients who developed a SRE, 5 (29.4%) developed
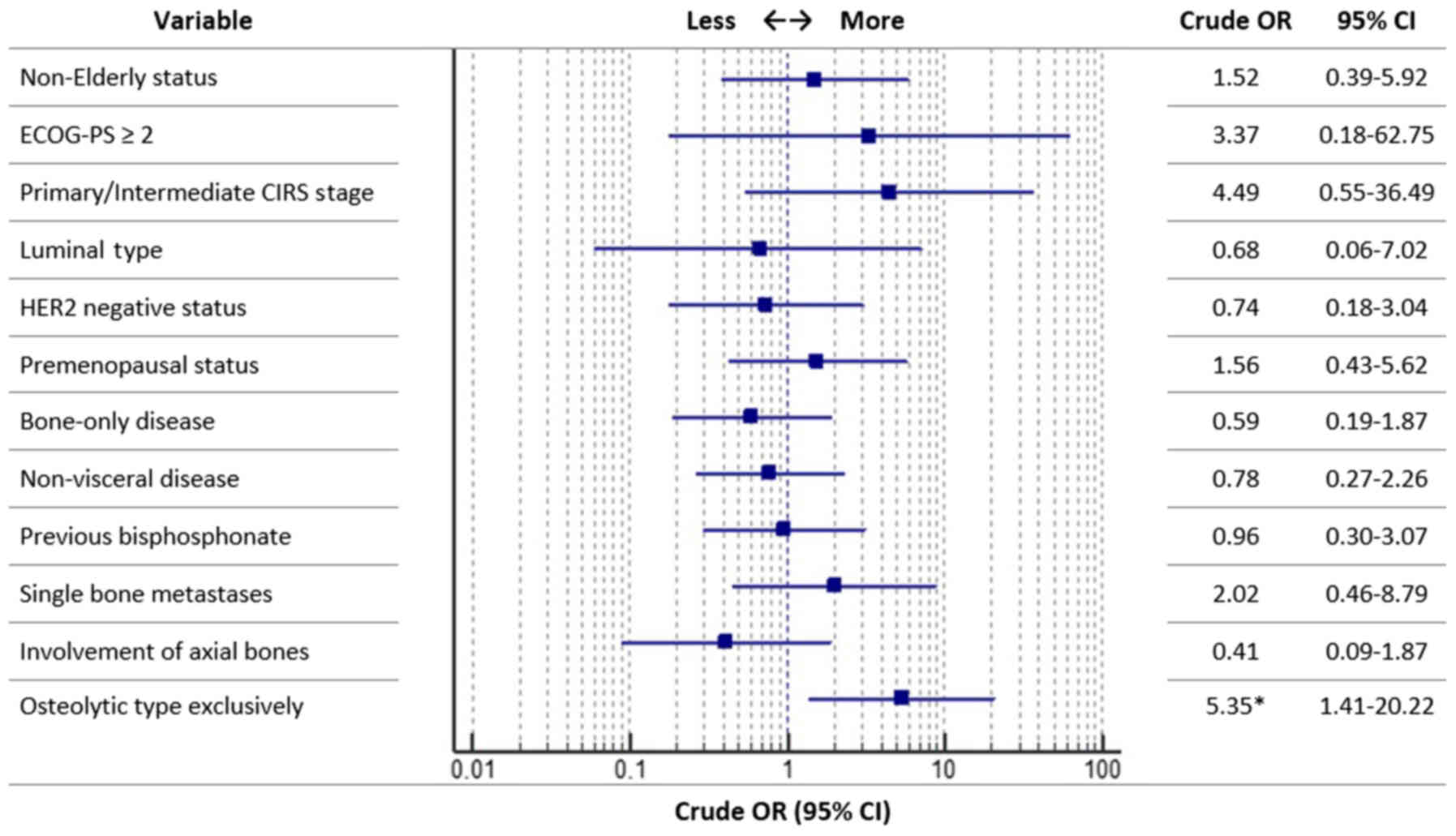
a subsequent SRE (5 radiation therapy on a bone segment). As shown
in Fig. 2, a statistically
significant difference in the incidence of SREs was detected only
between patients with exclusively-osteolytic bone metastases vs.
not (P=0.013). Specifically, among the first group (48 patients
with exclusively-osteolytic metastases) 14 (29.2%) SREs were
observed (95% CI: 15.9–48.9) compared to 3 SREs (7.1%) among the
second group (95% CI: 1,4–20.8) (42 patients with other types of
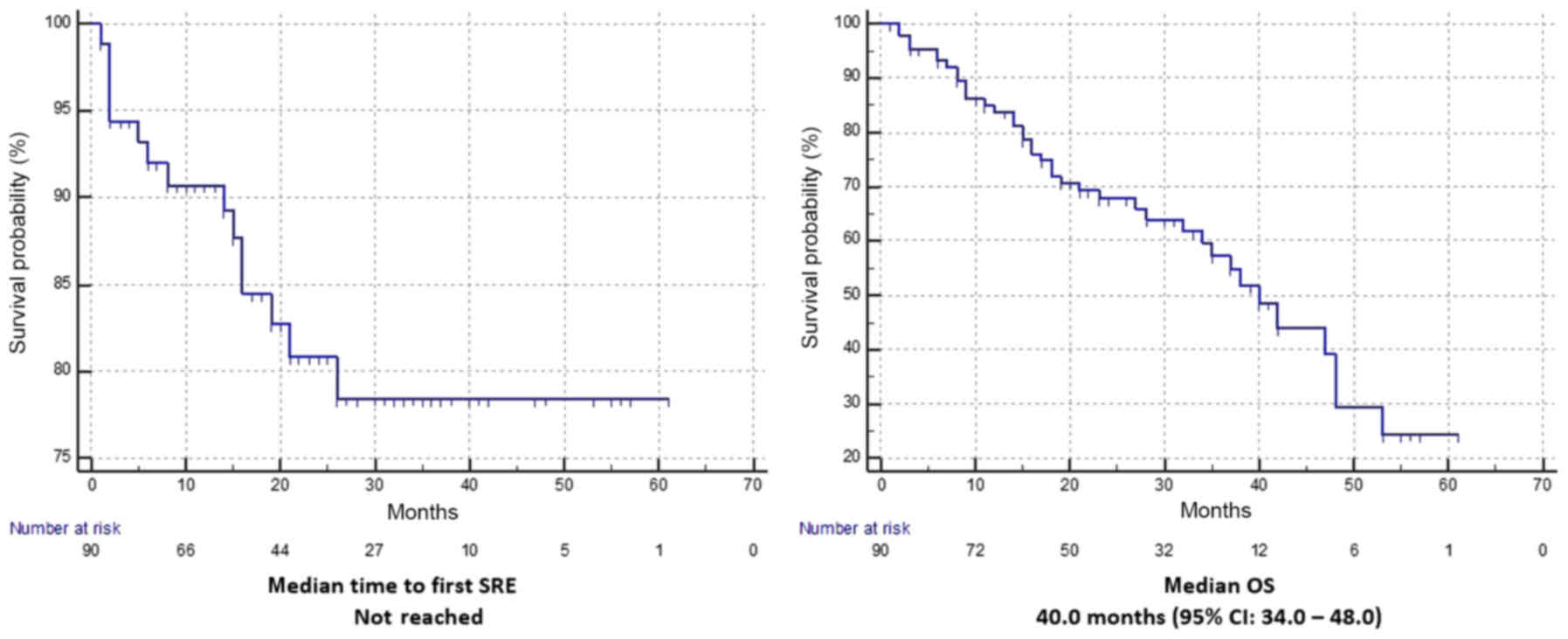
bone metastases). After a median follow up of 33 months, median
time to first SRE was not reached (Fig.
3) and median OS was 40.0 months (95% CI: 34.0–48.0). At
univariate analysis, only type of bone metastases was significantly
correlated to time to first SRE in favor of other than
exclusively-osteolytic ones (P=0.011) (Table IV). As to OS, at univariate analysis
ECOG-PS 0/1 (P=0.027), bone-only disease (P=0.004) and non-visceral
disease (P=0.023) were significantly correlated to a better OS,
while multivariate analysis confirmed just ECOG-PS (P=0.006) and
bone-only disease (P=0.035) as independent predictors for OS
(Table V).
 | Table III.Efficacy data. |
Table III.
Efficacy data.
| Variables | Value |
|---|
| Evaluable patients,
no. of patients (%) | 90 (100) |
| Incidence of SRE,
(%) | 18.8a |
| Radiation therapy,
no. of patients (%) | 16 (17.8) |
| Pathological
fracture, no. of patients (%) | 1 (1.1) |
| Median time to
first SRE, months (range) | Not reached
(1–61) |
| Median OS time,
months | 40.0
(1–61)b |
| No. of
mortalities | 38 |
 | Table IV.Univariate analysis for time to first
SRE. |
Table IV.
Univariate analysis for time to first
SRE.
|
|
| Univariate analysis
for time to first SRE |
|---|
|
|
|
|
|---|
| Clinical
features | No. of
patients | HR (95% CI) | P-value |
|---|
| Age |
| Non
elderly vs. elderly | 69 vs. 21 | 0.87
(0.25–3.06) | 0.836 |
| ECOG-PS |
| 0–1 vs.
≥2 | 84 vs. 6 | Not computable | 0.953 |
| CIRS stage |
|
Primary/intermediate vs.
secondary | 73 vs. 17 | 0.29
(0.03–2.23) | 0.237 |
| Luminal-like
disease |
| Yes vs.
no | 86 vs. 4 | 1.34
(0.17–10.23) | 0.777 |
| HER2 status |
|
Positive vs. negative | 13 vs. 77 | 1.13
(0.32–3.96) | 0.839 |
| Menopausal
status |
| Yes vs.
no | 74 vs. 16 | 0.76
(0.28–2.08) | 0.605 |
| Bone-only
disease |
| Yes vs.
no | 35 vs. 55 | 1.76
(0.62–5.03) | 0.284 |
| Visceral
disease |
| Yes vs.
no | 38 vs. 52 | 1.26
(0.48–3.27) | 0.632 |
| >1 bone
metastases |
| Yes vs.
no | 10 vs. 80 | 0.52
(0.14–1.83) | 0.311 |
| Axial bone
metastases |
| Yes vs.
no | 81 vs. 9 | 0.40
(0.11–1.43) | 0.161 |
| Previous
bisphosphonates |
| Yes vs.
no | 27 vs. 63 | 1.27
(0.44–3.62) | 0.655 |
| Type of bone
metastases |
|
Osteolytic exclusively vs.
others | 48 vs. 42 | 0.19
(0.05–0.69) | 0.011a |
 | Table V.Univariate and multivatiate analyses
for OS. |
Table V.
Univariate and multivatiate analyses
for OS.
|
|
| Univariate analysis
for OS | Multivariate
analysis for OS |
|---|
|
|
|
|
|
|---|
| Clinical
features | No. of
patients | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age |
| Non
elderly vs. elderly | 69 vs. 21 | 1.45
(0.70–2.99) | 0.313 | – | – |
| ECOG-PS |
| 0–1 vs.
≥2 | 84 vs. 6 | 3.30
(1.14–9.55) | 0.027a | 4.90
(1.57–15.30) | 0.006a |
| CIRS stage |
|
Primary/intermediate vs.
secondary | 73 vs. 17 | 1.75
(0.82–3.72) | 0.144 | – | – |
| Luminal-like
disease |
| Yes vs.
no | 86 vs. 4 | 1.54
(0.46–5.08) | 0.476 | – | – |
| HER2 status |
|
Positive vs. negative | 13 vs. 77 | 1.02
(0.44–2.34) | 0.952 | – | – |
| Menopausal
status |
| Yes vs.
no | 74 vs. 16 | 0.93
(0.55–1.55) | 0.792 | – | – |
| Bone-only
disease |
| Yes vs.
no | 35 vs. 55 | 3.09
(1.41–6.76) | 0.004a | 2.86
(1.06–7.69) | 0.035a |
| Visceral
disease |
| Yes vs.
no | 38 vs. 52 | 2.11
(1.10–4.02) | 0.023a | 1.29
(0.55–3.02) | 0.543 |
| >1 bone
metastases |
| Yes vs.
no | 10 vs. 80 | 0.64
(0.22–1.86) | 0.420 | – | – |
| Axial bone
metastases |
| Yes vs.
no | 81 vs. 9 | 1.06
(0.32–3.47) | 0.916 | – | – |
| Previous
bisphosphonates |
| Yes vs.
no | 27 vs. 63 | 0.81
(0.42–1.55) | 0.525 | – | – |
| Type of bone
metastases |
|
Osteolytic exclusively vs.
others | 48 vs. 42 | 1.22
(0.64–2.33) | 0.527 | – | – |
Safety
In the present analysis, we reported only
class-specific adverse events which could be related to denosumab
and not to disease-oriented treatments concomitantly administered
(Table VI). All patients were
evaluable for toxicity. Four patients (4.4%) discontinued denosumab
due to adverse events. The only G3 toxicity reported was
hypocalcemia in a patient, with a history of total thyroidectomy,
completely recovered by strengthening of calcium and vitamin D
support. Decrease in serum calcium levels was in most cases mild,
recovered in a short time (within 2 weeks) with an increased in
oral calcium and vitamin D support and was not accompanied by
clinical complications. No ONJ events occurred. Patients
experiencing toothache (10.0%) did not develop ONJ, while two
patients (3.1%) reported dental infections in absence of
radiographic signs of bone remodeling.
 | Table VI.Class-related toxicy data. |
Table VI.
Class-related toxicy data.
|
| No. of patients
(n=90) (%) |
|---|
|
|
|
|---|
| Grade | Any grade | G3 | G4 |
|---|
| Acute phase
reactions | 2 (2.2) | – | – |
| Hypocalcemia | 17 (18.9) | 1 (1.1) | – |
| Hypercalcemia | 1 (1.1) | – | – |
| Fever | 12 (13.3) | – | – |
| Bone
pain/arthralgia | 24 (26.7) | – | – |
| Toothache | 9 (10.0) | – | – |
| Dental
infections | 2 (2.2) | – | – |
Discussion
Our ‘real life’ safety data confirmed the good
tolerability of denosumab. We reported 7.8% of flu-like symptoms
and no case of renal injury. We did not report cases of ONJ, even
in patients who experiencing toothache (12.5%). As previously
mentioned, all patients underwent orthopantomography and subsequent
maxillofacial visit before starting denosumab, to identify possible
risk factors such as recent dental alveolar surgery (<3 months),
dental-periodontal or peri-implant inflammatory disease,
incongruous removable dentures or poor oral hygiene, which could be
responsible for an increased risk of develop ONJ. In our study, G2
hypocalcemia occurred in 3.1% and G3 hypocalcemia in 1.6% of
patients. Almost all events of hypocalcemia occurred in the first 6
months of treatment, not related to clinical complications and a
more adequate support of calcium and vitamin D solved them within a
week.
We observed 17 first SREs; exclusively-osteolytic
type of metastases is the only factor significantly related to the
incidence of SREs and to median time to first SRE. As in most cases
(16 out of 17) the SRE was represented by radiotherapy, performed
with a palliative aim for bone pain, thus we could hypothesize that
exclusively-osteolytic bone metastases are associated with a
greater incidence of pain. Cancer-induced bone pain is a complex
syndrome, which involve inflammatory, neuropathic, ischemic and
cancer-specific mechanisms, and it is related to tumor growth,
release of pain mediators by the cancer cells and damage to the
sensory nerves caused by infiltration and/or compression by the
tumor cells (36). A different impact
on bone pain between osteolytic and osteoblastic lesions has never
been clearly demonstrated. Osteolytic metastases are caused by
proliferation and hypertrophy of osteoclasts. While inducing bone
remodeling, osteoclasts release various acidic and lytic enzymes,
causing bone degradation and a decrease in the pH of the tumor
microenvironment; also local acidosis could probably be involved in
the nociceptive stimulation of the primary afferents in the bone
(37).
The 95.6% of our patients had ‘luminal-like’
disease. In line with that, a recent prospective study, aimed at
identifying patterns of BC relapse according to the biological
subtype, Luminal A and Luminal B tumors demonstrated a predominant
rate of distant metastases to the bone, compared to HER2-enriched
and triple-negative tumors (38).
Multivariate survival analysis showed that just
ECOG-PS and bone-only disease result independent predictors for a
longer OS. Several other studies reported an improved prognosis for
bone-only MBC patients compared to patients with visceral or
central nervous system metastases (39–41). In a
recent retrospective analyses from the MD Anderson Cancer Center,
median OS of bone-only MBC patients was 4.9 years (42). However bone-only disease and ECOG-PS
were already been widely related with a longer OS (4,43).
Interestingly, regarding patients with metachronous
‘luminal-like’ disease, at univariate analysis we found that median
TSkR was significantly shorter for patients who were previously
exposed to AIs compared to those who were not (53.0 vs 102.0
months, respectively; P=0.0300), even if the significance was not
confirmed at multivariate analysis, where only a trend was
maintained. These findings are aligned with the abovementioned
‘seed and soil’ hypothesis (36),
suggesting that AI-induced osteoporosis could increase the risk of
developing bone metastases. Surely these data are not conclusive;
we must take into account the strong limitations of our analysis:
the small sample size (40 patients), the retrospective nature and
the selection biases. A certain fact is that tumor cells interfere
with the bone homeostasis by secreting growth factors, which
stimulate bone resorption; bone resorption, in turn, leads to
release of factors promoting tumor growth in a ‘vicious cycle’ of
tumor expansion and bone destruction (44). Bisphosphonates and denosumab can block
this cycle and prevent bone loss. In order to clarify if AIs could
have a certain role in developing bone metastases, we have already
planned a multicenter retrospective confirmatory study, which will
evaluate clinical pattern of disease progression in early
‘luminal-like’ BC patients who underwent adjuvant hormonal
treatments.
Denosumab have demonstrated to improve osteoporosis
progression in early BC postmenopausal patients receiving AIs.
Assuming that AIs adjuvant treatments could create a ‘bone-related’
risk conditions for developing bone metastases, could denosumab
have a role in prevention of this risk conditions?
The present study showed that denosumab in our hands
have a good safety profile, and a pro-active attitude let us to
treat in a ‘real life’ setting the majority of patients without
significant class-related toxicities and no ONJ events. The
majority of SRE were radiation therapy, so pain still remain the
clinical hallmark of bone metastases, particularly of osteolytic
ones. The suggestion that estrogen deprivation with AIs can favour
a ‘bone-related’ risk conditions for developing bone metastases
must be considered with caution and surely needs further
validations; our next multicenter confirmatory study will try to
shed light on this topic.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AC, KC, VC, DS, AI, GP, PB and PF and CF conceived
and designed the study. AP, FP, CDO, LV, OV, LZ, TS, and PLB
collected the data. AC, PB, PF and VC wrote the paper. KC, DS, CF
and GP reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All patients provided written informed consent to
treatment. The procedures followed were in accordance with the
ethical standards of the Local Responsible Committees on Human
Experimentation (Comitato etico per le province di L'Aquila e
Teramo).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karrison TG, Ferguson DJ and Meier P:
Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst.
91:80–85. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80 (8 Suppl):S1588–S1594. 1997. View Article : Google Scholar
|
|
4
|
Ahn SG, Lee HM, Cho SH, Lee SA, Hwang SH,
Jeong J and Lee HD: Prognostic factors for patients with bone-only
metastasis in breast cancer. Yonsei Med J. 54:1168–1177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacobson AF, Shapiro CL, Van den Abbeele
AD and Kaplan WD: Prognostic significance of the number of bone
scan abnormalities at the time of initial bone metastatic
recurrence in breast carcinoma. Cancer. 91:17–24. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gobbini E, Ezzalfani M, Dieras V, Bachelot
T, Brain E, Debled M, Jacot W, Mouret-Reynier MA, Goncalves A,
Dalenc F, et al: Time trends of overall survival among metastatic
breast cancer patients in the real-life ESME cohort. Eur J Cancer.
96:17–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Briasoulis E, Karavasilis V, Kostadima L,
Ignatiadis M, Fountzilas G and Pavlidis N: Metastatic breast
carcinoma confined to bone: Portrait of a clinical entity. Cancer.
101:1524–1528. 2014. View Article : Google Scholar
|
|
8
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bundred NJ, Walker RA, Ratcliffe WA,
Warwick J, Morrison JM and Ratcliffe JG: Parathyroid hormone
related protein and skeletal morbidity in breast cancer. Eur J
Cancer. 28:690–692. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vargas SJ, Gillespie MT, Powell GJ,
Southby J, Danks JA, Moseley JM and Martin TJ: Localisation of
parathyroid hormone-related protein mRNA expression in breast
cancer and metastatic lesions by in situ hybridisation. J Bone
Miner Res. 7:971–979. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geisler J, Lønning PE, Krag LE, Løkkevik
E, Risberg T, Hagen AI, Schlichting E, Lien EA, Ofjord ES, Eide GE,
et al: Changes in bone and lipid metabolism in postmenopausal women
with early breast cancer after terminating 2-year treatment with
exemestane: A randomised, placebo-controlled study. Eur J Cancer.
42:2968–2975. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perez EA, Josse RG, Pritchard KI, Ingle
JN, Martino S, Findlay BP, Shenkier TN, Tozer RG, Palmer MJ,
Shepherd LE, et al: Effect of letrozole versus placebo on bone
mineral density in women with primary breast cancer completing 5 or
more years of adjuvant tamoxifen: A companion study to NCIC CTGMA.
17. J Clin Oncol. 24:3629–3635. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paget S: The distribution of secondary
growths in cancer of the breast. Cancer Metastasis Rev. 8:98–101.
1889.
|
|
15
|
Chin AR and Wang SE: Cancer tills the
premetastatic field: Mechanistic basis and clinical implications.
Clin Cancer Res. 22:3725–3733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y and Cao X: Characteristics and
significance of the pre-metastatic niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lipton A, Chapman JW, Leitzel K, Garg A,
Pritchard KI, Ingle JN, Budd GT, Ellis MJ, Sledge GW, Rabaglio M,
et al: Osteoporosis therapy and outcomes for postmenopausal
patients with hormone receptor-positive breast cancer: NCIC CTG
MA.27. Cancer. 123:2444–2451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gnant M, Mlineritsch B, Stoeger H,
Luschin-Ebengreuth G, Heck D, Menzel C, Jakesz R, Seifert M,
Hubalek M, Pristauz G, et al: Adjuvant endocrine therapy plus
zoledronic acid in premenopausal women with early-stage breast
cancer: 62-month follow-up from the ABCSG-12 randomised trial.
Lancet Oncol. 12:631–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gnant M, Mlineritsch B, Luschin-Ebengreuth
G, Stoeger H, Dubsky P, Jakesz R, Singer C, Eidtmann H, Fesl C,
Eiermann W, et al: Long-term follow-up in ABCSG-12: Significantly
improved overall survival with adjuvant zoledronic acid in
premenopausal patients with endocrine-receptor-positive early
breast cancer. Cancer Res. 71 (3 Suppl):S1–S2. 2011. View Article : Google Scholar
|
|
20
|
Coleman RE, Marshall H, Cameron D, Stoeger
H, Dubsky P, Jakesz R, Singer C, Fest C, Eiermann W, Marth C, et
al: Breast cancer adjuvant therapy with zoledronic acid. N Engl J
Med. 365:1396–1405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan T, Yin W, Zhou Q, Zhou L, Jiang Y, Du
Y, Shao Z and Lu J: The efficacy of zoledronic acid in breast
cancer adjuvant therapy: A meta-analysis of randomised controlled
trials. Eur J Cancer. 48:187–195. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gnant M, Pfeile Gr, Dubsky PC, Hubalek M,
Greil R, Jakesz R, Wette V, Balic M, Haslbauer F, Melbinger E, et
al: Adjuvant denosumab in breast cancer (ABCSG-18): A multicentre,
randomised, double-blind, placebo-controlled trial. Lancet.
386:433–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coleman RE, Smith P and Rubens RD:
Clinical course and prognostic factors following bone recurrence
from breast cancer. Br J Cancer. 77:336–340. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cleeland CS, Body JJ, Stopeck A, von Moos
R, Fallowfield L, Mathias SD, Patrick DL, Clemons M, Tonkin K,
Masuda N, et al: Pain outcomes in patients with advanced breast
cancer and bone metastases: Results from a randomized, double-blind
study of denosumab and zoledronic acid. Cancer. 119:832–838. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stopeck AT, Lipton A, Body JJ, Steger GG,
Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA,
Viniegra M, et al: Denosumab compared with zoledronic acid for the
treatment of bone metastases in patients with advanced breast
cancer: A randomized, double-blind study. J ClinOncol.
28:5132–5139. 2010. View Article : Google Scholar
|
|
26
|
Steger GG and Bartsch R: Denosumab for the
treatment of bone metastases in breast cancer: Evidence and
opinion. Ther Adv Med Oncol. 3:233–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Parmelee PA, Thuras PD, Katz IR and Lawton
MP: Validation of the cumulative illness rating scale in a
geriatric residential population. J Am Geriatr Soc. 43:130–137.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Poznak CH, Temin S, Yee GC, Janjan NA,
Barlow WE, Biermann JS, Bosserman LD, Geoghegan C, Hillner BE,
Theriault RL, et al: American Society of Clinical Oncology
executive summary of the clinical practice guideline update on the
role of bone-modifying agents in metastatic breast cancer. J
ClinOncol. 29:1221–1227. 2011. View Article : Google Scholar
|
|
29
|
Tesarova P: Breast cancer in the
elderly-Should it be treated differently? Rep Pract Oncol
Radiother. 18:26–33. 2013. View Article : Google Scholar
|
|
30
|
Kaplan EL and Meier P: Nonparametric
estimation of incomplete observations. J Am Stat Assoc. 53:457–481.
1958. View Article : Google Scholar
|
|
31
|
Schemper M and Smith TL: A note on
quantifying follow-up in studies of failure time. Control Clin
Trials. 17:343–346. 1997. View Article : Google Scholar
|
|
32
|
Fisher RA: On the interpretation of
χ2 from contingency tables and the calculation of P. J
Royal Stat Soc. 85:87–94. 1922. View
Article : Google Scholar
|
|
33
|
Mantel N: Chi-square tests with one degree
of freedom: Extensions of the Mendel-Haenszel procedure. J Am Stat
Assoc. 58:690–700. 1963. View Article : Google Scholar
|
|
34
|
Peto R and Peto J: Asymptomatically
efficient rank invariant test procedures. J R Stat Soc A.
135:185–206. 1972. View Article : Google Scholar
|
|
35
|
Cox DR: Regression models and life tables
(with discussion). J Royal Stat Soc (Series B). 74:187–200.
1972.
|
|
36
|
Falk S and Dickenson AH: Pain and
nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol.
32:1647–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Julius D and Basbaum AI: Molecular
mechanisms of nociception. Nature. 413:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ignatov A, Eggemann H, Burger E and
Ignatov T: Patterns of breast cancer relapse in accordance to
biological subtype. J Cancer Res Clin Oncol. 144:1347–1355. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sherry MM, Greco FA, Johnson DH and
Hainsworth JD: Metastatic breast cancer confined to the skeletal
system. An indolent disease. Am J Med. 81:381–386. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leone BA, Romero A, Rabinovich MG, Vallejo
CT, Bianco A, Perez JE, Machiavelli M, Rodriguez R and Alvarez LA:
Stage IV breast cancer: Clinical course and survival of patients
with osseous versus extraosseous metastases at initial diagnosis.
The GOCS (Grupo Oncologico Cooperativo del Sur) experience. Am J
Clin Oncol. 11:618–622. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Perez JE, Machiavelli M, Leone BA, Romero
A, Rabinovich MG, Vallejo CT, Bianco A, Rodriguez R, Cuevas MA and
Alvarez LA: Bone-only versus visceral-only metastatic pattern in
breast cancer: Analysis of 150 patients. A GOCS study. Grupo
Oncológico Cooperativo del Sur. Am J Clin Oncol. 13:294–298. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Parkes A, Clifton K, Al-Awadhi A, Oke O,
Warneke CL, Litton JK and Hortobagyi GN: Characterization of bone
only metastasis patients with respect to tumor subtypes. NPJ Breast
Cancer. 4:22018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Niikura N, Liu J, Hayashi N, Palla SL,
Tokuda Y, Hortobagyi GN, Ueno NT and Theriault RL: Treatment
outcome and prognostic factors for patients with bone-only
metastases of breast cancer: A single-institution retrospective
analysis. Oncologist. 16:155–164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|