Introduction
Lung cancer is one of the most common malignant
neoplasms worldwide. The majority of patients diagnosed with lung
cancer already have distant metastases, and are diagnosed as in an
advanced-stage disease (1). The
skeleton is one of the most frequent metastatic sites in patients
with advanced lung cancer (2,3), and the incidence of bone metastasis (BM)
is approximately 30–40%, when diagnosed in a clinical course of
lung cancer.
Cancer patients with BM should prevent skeletal
complications that increase suffer and may require high medical
costs (4). Such complications are
usually indicated as skeletal related events (SREs). However,
incidence of SREs in patients with lung cancer has varied across
several reports that investigated their clinical courses (5–7). While
SREs are also thought to exert a negative impact on quality of life
(QOL) in patients with lung cancer, few reports regarding the
association between SREs and QOL have been published (8–10).
In the previous work, we reported the incidence of
BM and SREs in patients with advanced lung cancer, as a prospective
study (11). The aim of the current
study was to prospectively investigate how QOL was affected by SRE
in patients with advanced lung cancer, as well as incidence of BM
and SRE.
Materials and methods
Patient enrollment and study
design
This study was a prospective multicenter cohort
study. The eligibility criteria included newly-diagnosed patients
with stage IIIB or IV non-small cell lung cancer (NSCLC) or small
cell lung cancer (SCLC) in any stage, whose ages were over 20-years
old, and who had provided written informed consent. All patients
were required to have not received chemotherapy or bisphosphonate
therapy.
Treatment for lung cancer and the administration of
zoledronate were at the discretion of the investigator, for the
enrolled patients. Denosumab was not approved during the study
period in Japan. The present study was approved by the
institutional review boards of the respective institutions, and was
conducted in compliance with international guidelines regulating
patient safety.
Evaluation of BM, SREs, and date
collection
The physicians and clinical research coordinators
(CRCs) collected data every four weeks during the six month periods
following enrollment, and every three months thereafter. The data
included Eastern Cooperative Oncology Group Performance Status
(ECOG-PS), body weight, blood sampling (to check hypercalcemia,
bone, or other metastases), use of zoledronate or analgesics, and
pain scale. We checked chest CT, bone scintigraphy, and
roentgenograms of the thoracic and lumbar bones at the time of
study enrollment. A chest CT was performed every three months and
bone scintigraphy was performed every six months. Treatment for
lung cancer and use of zoledronate were undertaken at the
discretion of the investigators. When bone metastases were
suspected, the patient underwent a CT to determine bone conditions
or an MRI and X-ray for the diagnosed BM sites.
QOL assessment and definition of
SREs
QOL was assessed via the EuroQOL-5 Dimension (EQ-5D)
(12) and Functional Assessment of
Cancer Therapy-General (FACT-G) (13). ADL was evaluated via the Barthel Index
(14). These QOL questionnaires were
conducted at the time of enrollment, at both three- and
twelve-months later, and also one month after the onset of SREs.
SREs are defined as pathologic fracture, radiation or surgery to
bone lesion, spinal cord compression, or hypercalcemia. Each QOL
questionnaire was analyzed only in patients from whom all answers
were collected.
Statistical analysis
The sample size has been described in the previous
report (11). The target number of
cases was set at 50, as a number of cases for which descriptive
statistics of some accuracy can be calculated, even for patients
with stage IIIB NSCLC, which is anticipated to have comparatively
small enrollment, for a total of roughly 400 cases from the
percentage of each cancer type. The sample size was determined to
answer another research question about the incidence of SREs, and
the present analysis was a secondary use of those data.
Background characteristics and types of SREs are
summarized by frequency and proportion. The mean values of the
EQ-5D, FACT-G, and Barthel index, among the baseline BM and SRE
status types, were drawn by box-whisker plot and compared by
one-way analysis of variance. In the patients who had SRE for the
first time within the follow-up period, the changes in EQ-5D,
FACT-G, and Barthel index by SRE occurrence were compared by paired
t-test. In addition, the changes in the subscales of the FACT-G
were evaluated in the same manner. P-value for comparisons between
SRE +/- and between BM +/- were calculated concurrently using
contrasts. At two-sided P<0.05 was considered to indicate a
statistically significant difference. All statistical analyses were
performed using SAS v9.2 (SAS Institute, Cary, NC, USA).
Results
Patients backgrounds at
enrollment
A total of 274 patients were enrolled in this study
from April 2007 to December 2009. The patients characteristics are
summarized in Table I. Patient ages
ranged from 35 to 89 years (median age, 68 years), and
male/female=193/81, ECOG-PS0/1/2/3=76/171/23/4, BM+/-=78/196,
SREs+/-=24/250, SCLC/NSCLC=77/197, and Stage IIIB/IV=73/124.
 | Table I.Patients characteristics at enrollment
(n=274). |
Table I.
Patients characteristics at enrollment
(n=274).
| Characteristics | Total |
|---|
| Sex |
|
| Male | 193 |
|
Female | 81 |
| Median age
(range) | 68 (35–89) |
| Histology |
|
|
NSCLC | 197 |
|
Stage IIIB | 73 |
|
Stage IV | 124 |
| SCLC | 77 |
|
LD | 30 |
|
ED | 47 |
| ECOG-PS |
|
| 0 | 76 |
| 1 | 171 |
| 2 | 23 |
| 3 or
4 | 4 |
| BM |
|
| + | 78 |
| − | 196 |
| SRE |
|
| + | 24 |
| − | 250 |
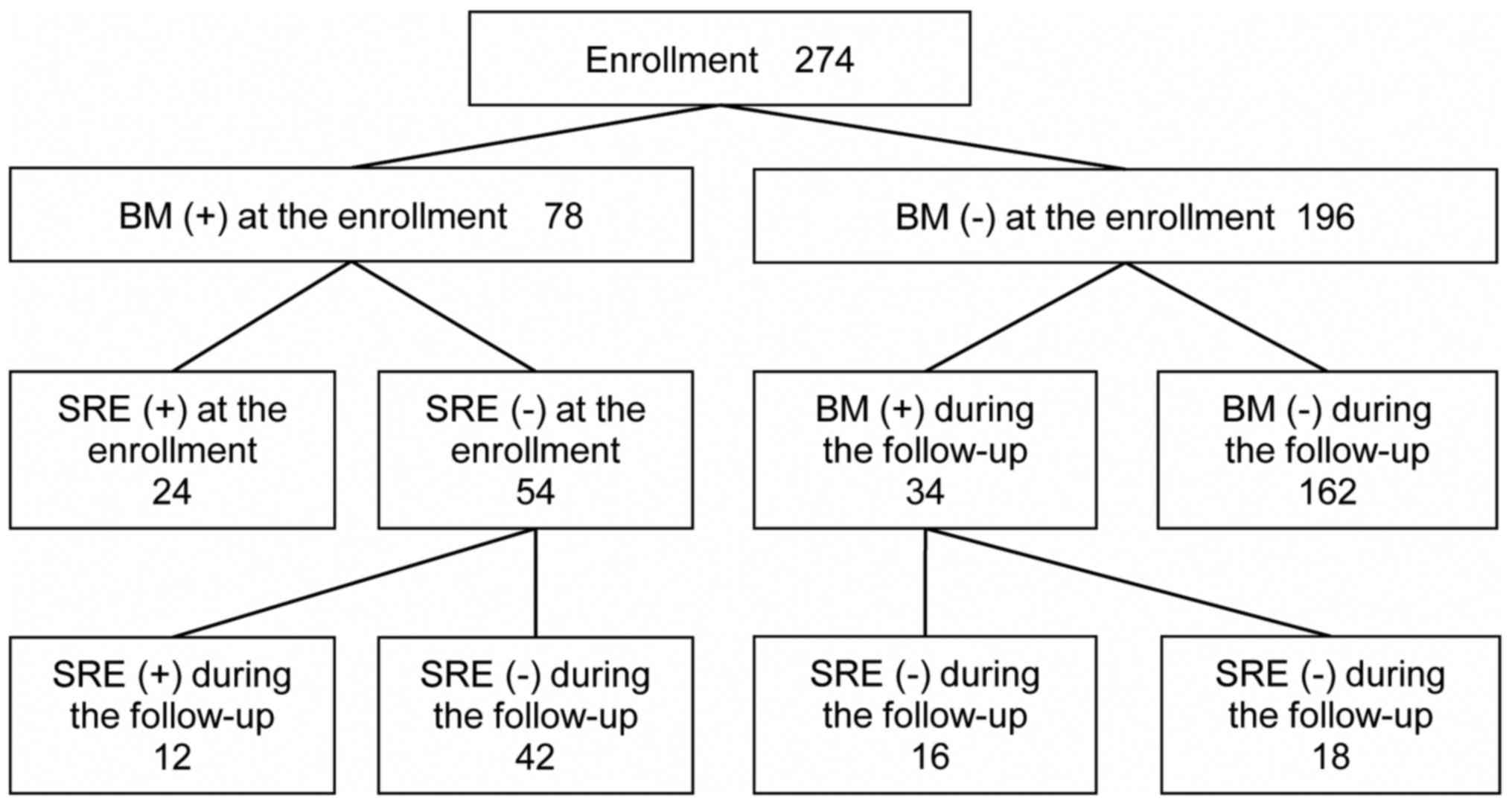
Incidence of BM and SREs
BM and SREs both at enrollment and during the
follow-up periods are summarized in Fig.
1. As previously reported (11),
the median follow-up period was 13.8 months (0–28.5 month). A total
of 78 patients (28% of all enrolled patients and 62% of stage IV
patients) already had BM at enrollment. Among these patients, 24
had accompanying SREs, while an additional 12 developed SREs during
the follow up. Among the 196 patients without initial BM, 34
developed BM. Among these 34 patients, 16 developed SREs during the
follow-up.
The types of SREs at enrollment were: Pathologic
fracture in nine patients; radiation to bone lesion in 22; spinal
cord compression in two; and hypercalcemia of malignancy in one.
The types of new SREs during the follow-up period were: Pathologic
fracture in five patients; radiation to bone lesion in 23; spinal
cord compression in one; and hypercalcemia of malignancy in
five.
QOL of the patients
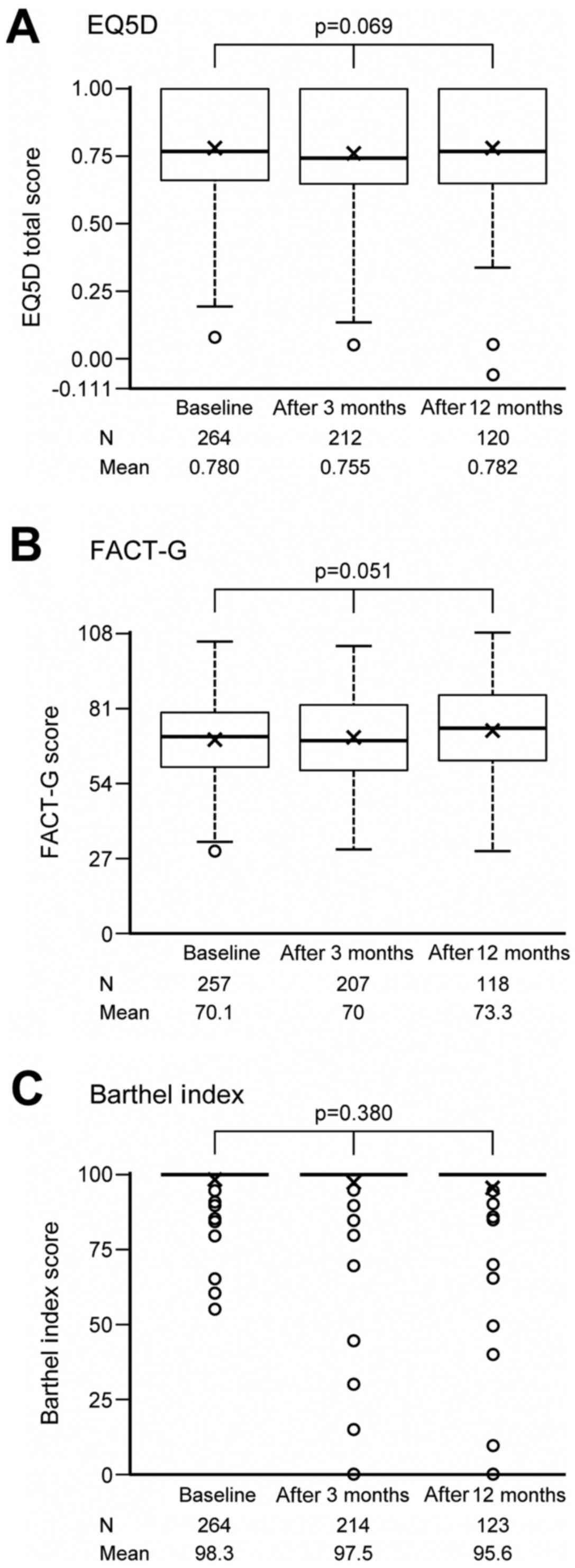
The EQ-5D, FACT-G, and Barthel Index scores at
enrollment were summarized according to three patient groups
classified by BM and SRE, and are respectively shown in Fig. 2A-C. QOL data were obtained from nearly
all patients at the enrollment. Among patients with BM at
enrollment, those who already had SREs had lower EQ-5D (mean=0.7)
and Barthel Index (mean=94.8) scores than those without SREs (mean
of EQ-5D=0.8, mean of Barthel Index=98.9), and the differences were
statistically significant (P=0.013 for EQ-5D, P=0.006 for Barthel
Index). Such significant lower scores in patients who already had
SREs were observed when compared to either those who did not have
BM or those had BM but no SREs. No significant difference was
observed for FACT-G between patients with (mean=66.8) and without
(mean=71.6) SREs at enrollment (P=0.225). In addition, none of the
EQ-5D, FACT-G, or Barthel Index scores showed a significant
difference between patients who did not have BM and those had BM
but no SREs.
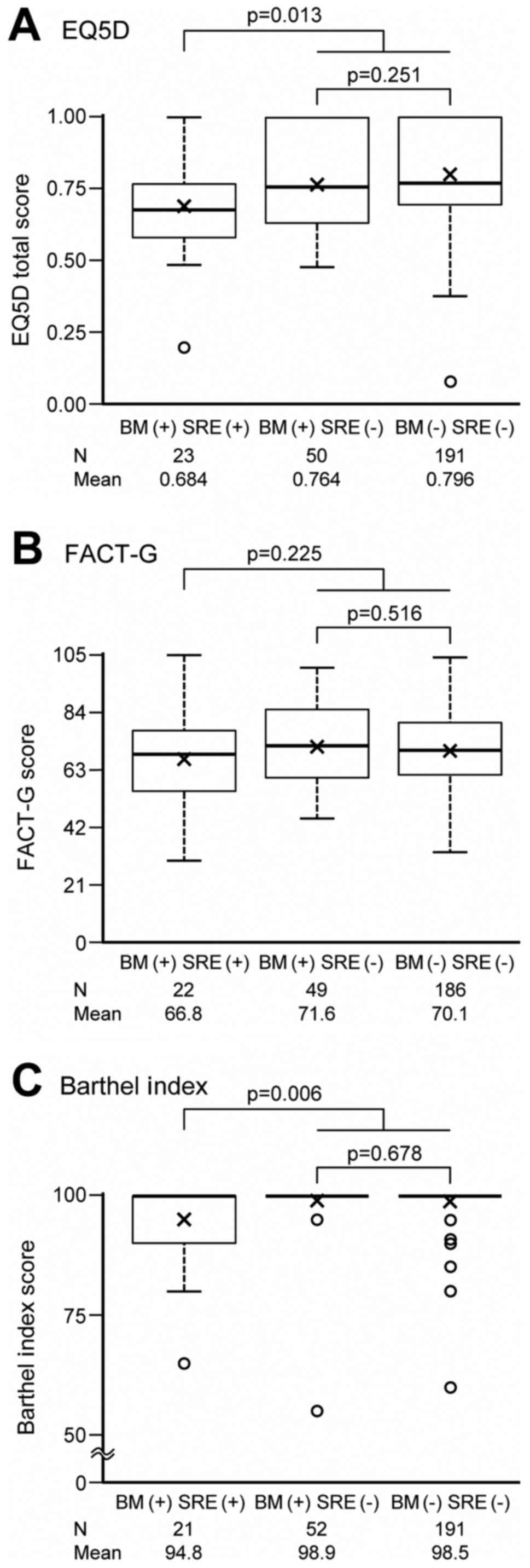 | Figure 2.(A) EQ-5D, (B) FACT-G and (C) Barthel
Index scores at enrollment, according to three patient groups
classified by BM and SREs using one-way analysis of variance.
P-value for comparisons between SRE +/- and between BM +/- were
calculated from post-hoc orthogonal contrast test of (2, −1, −1)
and (0,1, −1) in one-way analysis of variance. Multiplicity was not
adjusted because of exploratory analysis. Thick horizontal lines
delineate median values, while the box boundaries represent the
25th and 75th percentiles. ‘x’ represents mean values. Whiskers
outside the boxes delineate the maximum or minimum of 1.5 times the
interquartile range. BM, bone metastasis; EQ-5D, EuroQOL-5
Dimension; FACT-G, Functional Assessment of Cancer Therapy-General;
SREs, skeletal-related events. |
Changes in QOL and ADL in the patients during the
study period are summarized in Fig.
3A-C. A chronological analysis showed no statistically
significant change in the EQ-5D, FACT-G, or Barthel Index scores of
all patients for whom the QOL or ADL evaluation was performed.
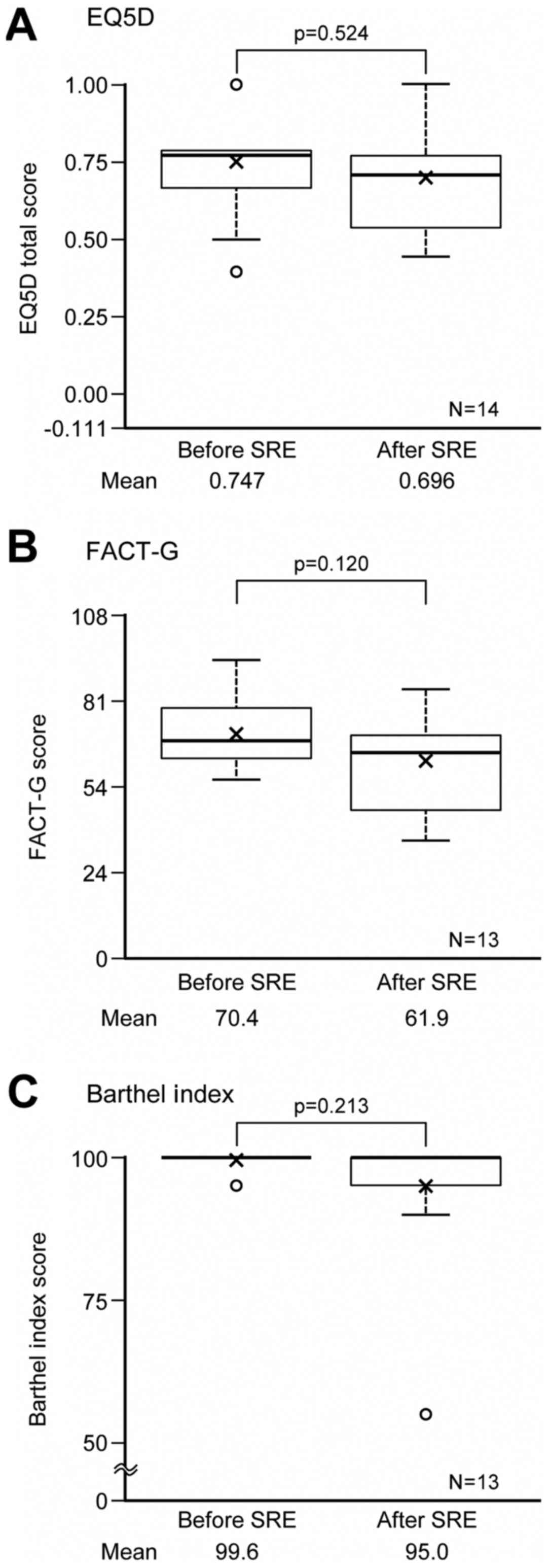
QOL data were collected for 14 (EQ-5D) or 13 (FACT-G
and Barthel Index) patients out of 28 who had SREs during the
follow-up. Changes in QOL after the SREs are summarized in Fig. 4A-C. For those 13 or 14 patients, QOL
and ADL scores changed by −0.05 in EQ-5D, by −8.5 in FACT-G, and by
−4.6 in Barthel Index. However, none of these decreases were
statistically significant.
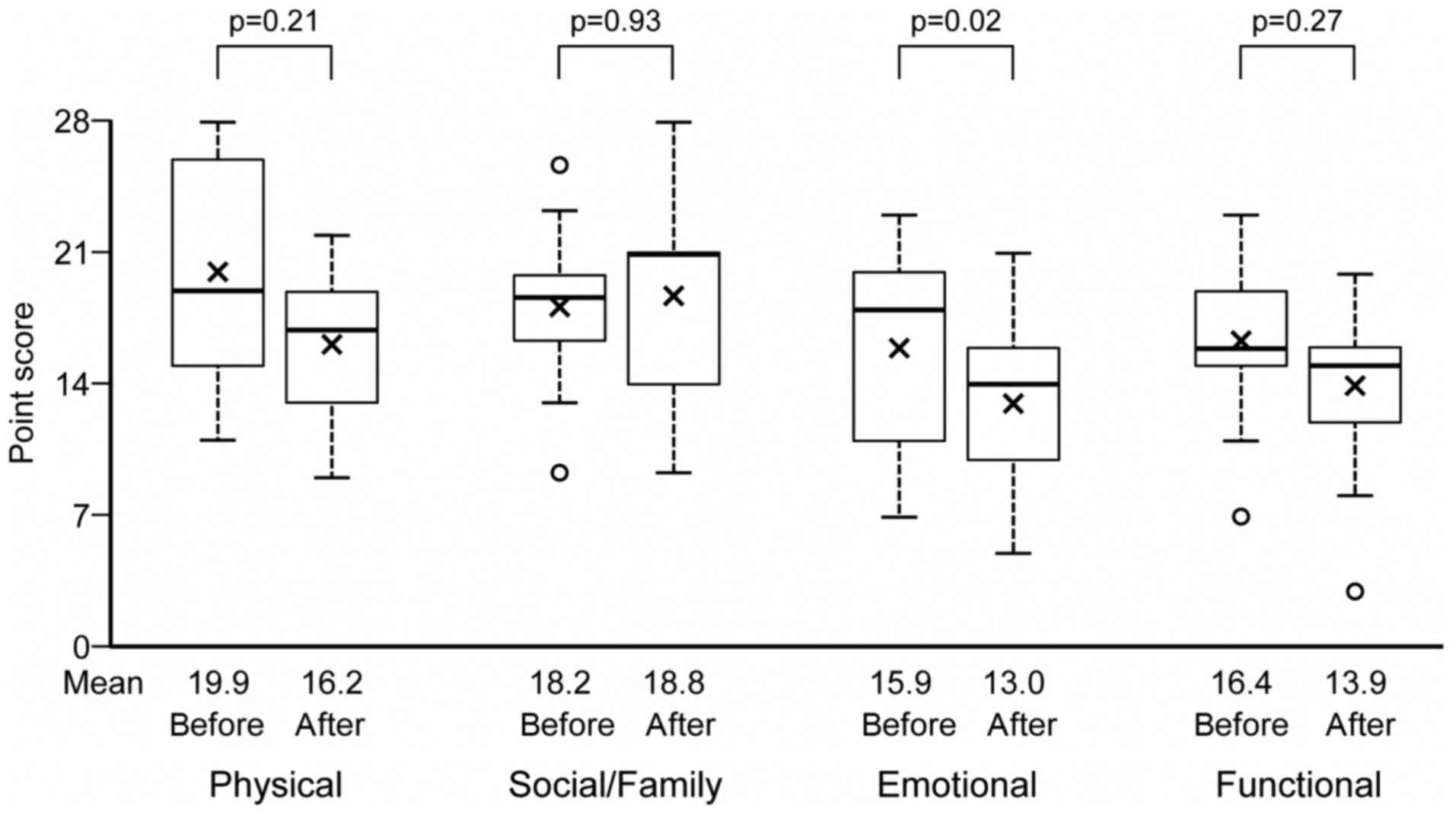
Changes in the QOL by the FACT-G after the onset of
SREs, for each functioning subscale are summarized in Fig. 5. An analysis of FACT-G by its four
subscales (physical, social/family, emotional, and functional)
showed that the emotional subscale decreased by 3.77 (95%CI: 6.83,
0.71), which was statistically significant (P=0.02). However,
physical, social/family, and functional subscales showed no
significant changes.
Discussion
This is another analysis which focuses on the QOL of
the patients with lung cancer, in whom the incidence of BM and SREs
were prospectively investigated. In the present study, QOL data of
the patients were also prospectively collected, according to the
methods. As shown in the results, we reported their incidence at
enrollment, as well as those occurred subsequently during the
follow-up.
The association between SRE and QOL has frequently
been studied from the point of an administration of zoledronate.
While a number of reports have shown that its administration
resulted in a decline of SRE, the accompanying effect on QOL
remains controversial (8–10,15,16). In
all three of the studies that reported a positive effect of
zoledronate on QOL in patients with cancer, the number of patients
was rather small and the studies were retrospective (6,17,18). To our knowledge, the present study is
the first that prospectively investigated the QOL of the patients,
by scheduled evaluation, including after the occurrence of SRE.
Several previous studies have been planned to
observe QOL. However, the analyses were performed on a limited
population of patients, such as those who were undergoing adjuvant
chemotherapy (19), in the advanced
or recurrent stage (20,21), or who were undergoing maintenance
chemotherapy (22). None of those
studies reported a definite decline of QOL, and the present study
showed a similar result. With regard to QOL during the one year
after study enrollment, a definite change in QOL was not observed,
in spite of a slight decline indicated by an evaluation with the
Barthel index.
In the present study, the effect of SREs on QOL was
analyzed using two methods. First, QOL was compared at study
enrollment, between patients who already had and did not have SREs,
and the patients with SREs showed worse QOL or ADL scores. This
result suggests that SREs had a negative impact on QOL and ADL in
the patients, for whom various types of supportive care was not
sufficiently given.
In addition, the change in QOL and ADL was analyzed
by comparing values before and after the onset of SREs, however, a
significant decline was not observed after SRE onset. This result
may be due to the small number of cases in which an evaluation of
QOL was performed, and may also be due to the evaluation in
patients with some limited types of SREs. All of the fourteen
patients, in whom QOL was evaluated, had either radiation therapy
to skeletal lesion or hypercalcemia as SREs, which could be
relatively manageable to keep good QOL. On the other hand, QOL
could be evaluated in none of five patients, who had a pathologic
fracture after the enrollment and might cause functional
impairment.
There was a tendency for QOL or ADL to decline in
the fourteen evaluated patients. This result may suggest that SREs
exerted a more serious impact on QOL and ADL in the patients as a
whole, as it is possible that those who did not receive evaluations
had worse QOL or ADL compared to those for whom an evaluation was
performed due to the maintenance of relatively better
conditions.
One notable finding in the present study is that the
emotional score was declined after the onset of SREs, even though
the total FACT-G scores did not indicate a significant decline in
QOL. Although SREs would appear to be more associated with physical
function, the results did not match such expectations. It is
possible that the significant decline in FACT-G emotional score
could be due to the multiplicity of comparisons.
The present study has some limitations, the most
notable of which is that QOL could be evaluated in only fourteen
cases after the onset of SREs, although 28 patients experienced
various types of SREs. We could not evaluate nearly half of
patients with SREs after the enrollment. As previously mentioned,
this lack of QOL assignment might under-evaluate the decline in QOL
after the onset of SREs, by only evaluating patients in good health
and excluding patients who might have had more serious impairments
and could not have a QOL evaluation. It is possible that the 14
patients did not represent the whole population who newly had SREs
after the enrollment. Some patients might have multiple SREs, but
we could not analyze the association between QOL and each SRE,
although the association may differ according to type of SREs.
Such elimination might also result in
under-evaluation in the chronological analyses of QOL and ADL at
enrollment, and at three- and twelve-months later. It is possible
that a non-significant change in QOL or ADL was due to some
patients having serious impairments, so that they could not have
assignments.
Another limitation is that the selected population
of patients who were predominantly enrolled in this study, already
had the support of a palliative care team, including a clinical
research coordinator (CRC), as the enrollment was not consecutive.
It is possible that such support by various professionals resulted
in the maintenance of relatively good QOL even after the onset of
SREs, so that this population did not represent the whole
population of patients with lung cancer, although we do not have
concrete data of supportive care which the patients had taken
during the study period.
Finally, the present analysis was performed only in
Japanese population and could not be enough to discuss about
international relevancy.
In Conclusion, QOL and ADL in the patients with
advanced lung cancer was negatively affected by SREs just after the
diagnosis, when measured by the EQ-5D and the Barthel Index.
Although it was not proven that their QOL and ADL was affected by
the onset of SREs, when FACT-G was evaluated by the four factors,
emotional functioning significantly declined after SREs.
Acknowledgements
Not applicable.
Funding
Supported by Health Outcomes Research (CSP-HOR) of
the Public Health Research Foundation.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YO and KE conceived the study. HK, YS, YO, and KE
designed the study. HK, NK, KojT, KoiT, TS, HS, MH, SY, and KA
collected data. IY analyzed data. HK, IY, and NK wrote the
manuscript. All authors reviewed the manuscript and approved the
final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the institutional review
boards of the respective institutions (Yokohama Municipal Citizen's
Hospital, Institute of Biomedical Research and Innovation, Osaka
City General Hospital, Kyoto Prefectural University of Medicine,
Gifu Municipal Hospital, Aichi Cancer Center Aichi Hospital,
National Hospital Organization Hokkaido Cancer Center, and Toneyama
National Hospital) and was conducted in compliance with
international guidelines regulating patient safety.
Patient consent for publication
Patients provided written informed consent for data
collection and analysis, and publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stinchcombe TE, Lee CB and Socinski MA:
Current aproaches to advanced-stage non-small-cel lung cancer:
First-line therapy in patients with a good functional status. Clin
Lung Cancer. 7 Suppl 4:S111–S117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugiura H, Yamada K, Sugiura T, Hida T and
Mitsudomi T: Predictors of survival in patients with bone
metastasis of lung cancer. Clin Orthop Relat Res. 466:729–736.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Botteman M, Barghout V, Stephens J, Hay J,
Brandman J and Aapro M: Cost effectiveness of bisphosphonates in
the management of breast cancer patients with bone metastases. Ann
Oncol. 17:1072–1082. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuya A, Kurata T, Tamura K and Fukuoka M:
Skeletal metastases in non-small cell lung cancer: A retrospective
study. Lung Cancer. 57:229–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosen LS, Gordon D, Tchekmedyian S,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng
M, Urbanowitz G, et al: Zoledronic acid versus placebo in the
treatment of skeletal metastases in patients with lung cancer and
other solid tumors: A phase III, double blind, randomized trial-the
zoledoronic acid lung cancer and other solid tumors study group. J
Clin Oncol. 21:3150–3157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosen LS, Gordon D, Tchekmedyian NS,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng
M, Urbanowitz G, et al: Long-term efficacy and safety of zoledronic
acid in the treatment of skeletal metastases in patients with
nonsmall cell lung carcinoma and other solid tumors: A randomized,
phase III, double blind, placebo-controlled trial. Cancer.
100:2613–2221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishiwata T, Hirose T, Hirama M, Miura K,
Iwakami S, Tominaga S, Adachi M and Takahashi K: A feasibility
study of zoledronic acid combined with carboplatin/nedaplatin plus
paclitaxel in patients with non-small cell lung cancer with bone
metastases. Tumori. 97:568–572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Addeo R, Nocera V, Faiola V, Vincenzi B,
Ferraro G, Montella L, Guarrasi R, Rossi E, Cennamo G, Tonini G, et
al: Management of pain in elderly patients receiving infusion of
zoledronic acid for bone metastasis: A single-institution report.
Support Care Cancer. 16:209–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Facchini G, Caraglier M, Santini D, Nasti
G, Ottaiano A, Striano S, Maiolino P, Ruberto M, Fiore F, Tonini G,
et al: The clinical response on bone metastasis from breast and
lung cancer during treatment with zoledronic acid is inversely
correlated to skeletal related events (SRE). J Exp Clin Cancer Res.
26:307–312. 2007.PubMed/NCBI
|
|
11
|
Katakami N, Kunikane H, Takeda K, Takayama
K, Sawa T, Saito H, Harada M, Yokota S, Ando K, Saito Y, et al:
Prospective study on the incidence of bone metastasis (BM) and
skeletal-related events (SREs) in patients (pts) with stage IIIB
and IV lung cancer-CSP-HOR 13. J Thorac Oncol. 9:231–238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsuchiya A, Ikeda S, Ikegami N, Nishimura
S, Sakai I, Fukuda T, Hamashima C, Hisashige A and Tamura M:
Estimating an EQ-5D population value set: The case of Japan. Health
Econ. 11:341–353. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cella DF, Bonomi AE, Lloyd SR, Tulsky DS,
Kaplan E and Bonomi P: Reliability and validity of the functional
assessment of cancer therapy-lung (FACT-L) quality of life
instrument. Lung Cancer. 12:199–220. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahoney FI and Barthel DW: Functional
evaluation; the Barthel Index. Md State Med J. 14:61–65.
1965.PubMed/NCBI
|
|
15
|
Kretzschmar A, Wiegel T, AI-Batran SE,
Hinrichs HF, Kindler M, Steck T, Illiger HJ, Heinemann V, Schmidt
K, Haus U, et al: Rapid and sustained influence of intravenous
zoledronic acid on course of pain and analgesics consumption in
patients with cancer with bone metastases: A multicenter open-label
study over 1 year. Support Cancer Ther. 4:203–210. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mystakidou K, Katsouda E, Parpa E,
Kouskouni E, Chondros C, Tsiatas ML, Galanos A and Vlahos L: A
prospective randomized controlled clinical trial of zoledronic acid
for bone metastases. Am J Hosp Palliat Care. 23:41–50. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chow E and Bottomley A: Understanding the
EORTC QLQ-BM22, the module for patients with bone metastases.
Expert Rev Pharmacoecon Outcomes Res. 9:461–465. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koizumi M, Yoshimoto M, Kasumi F, Iwase T
and Ogata E: Post-operative breast cancer patients diagnosed with
skeletal metastasis without bone pain had fewer skeletal-related
events and deaths than these with bone pain. BMC Cancer.
10:4232010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bezjak A, Lee CW, Ding K, Brundage M,
Winton T, Graham B, Whitehead M, Johnson DH, Livingston RB, Seymour
L and Shepherd FA: Quality-of-life outcomes for adjuvant
chemotherapy in early-stage non-small-cell lung cancer: Result from
a randomized trial, JBR. 10. J Clin Oncol. 26:5052–5059. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mills M, Murray LJ, Johnston BT, Cardwell
C and Donnelly M: Does a patient-held quality-of-life diary benefit
patients with inoperable lung cancer? J Clin Oncol. 27:70–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Efficace F, Bottomley A, Smit EF, Lianes
P, Legrand C, Debruyne C, Schramel F, Smit HJ, Gaafar R, Biesma B,
et al: Is a patient's self-reported health-related quality of life
a prognostic factor for survival in non-small-cell lung cancer
patients? A multivariate analysis of prognostic factors of EORTC
study 08975. Ann Oncol. 17:1698–1704. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Belani CP, Brodowicz T, Ciuleanu TE,
Krzakowski M, Yang SH, Franke F, Cucevic B, Madhavan J, Santoro A,
Ramlau R, et al: Quality of life in patients with advanced
non-small-cell lung cancer given maintenance treatment with
pemetrexed versus placebo (H3H-MC-JMEN): Result from a randomised,
double-blind, phase 3 study. Lancet Oncol. 13:292–299. 2012.
View Article : Google Scholar : PubMed/NCBI
|